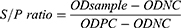Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Molecular and Serological Investigation of Infectious Bronchitis Virus in the East Shewa, Central Ethiopia
Authors Hirbaye G, Tola EH , Moje N, Sori T
Received 7 December 2023
Accepted for publication 4 March 2024
Published 11 March 2024 Volume 2024:15 Pages 81—90
DOI https://doi.org/10.2147/VMRR.S452153
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Gemachu Hirbaye,1 Eyob Hirpa Tola,2 Nebyou Moje,2 Teshale Sori2
1School of Veterinary Medicine, Wollega University, Nekemte, Ethiopia; 2College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu, Ethiopia
Correspondence: Eyob Hirpa Tola, Email [email protected]
Background: Infectious bronchitis (IB) is an economically important disease in poultry with worldwide distribution. The occurrence of IB has been reported both in commercial and backyard poultry in Ethiopia, although comprehensive information lacks available prevalence of the disease and the circulating serotypes.
Methods: A cross-sectional study was conducted from November 2021 to June 2022 in seven commercial farms found in East Shewa, Central Ethiopia. Serological assay using indirect ELISA, virus isolation techniques in embryonated eggs, and molecular techniques such as one-step reverse transcriptase polymerase chain reaction (RT-PCR) and nested polymerase chain reaction (PCR) targeting a 466 bp S1 gene were employed.
Results: A total of 196 blood samples, 7 pools (35) of swab samples, and 5 pools of tracheal samples were investigated. The results of serological analysis revealed that 97.96% (192/196; 95% CI: 94.86– 99.44) of the sera samples were found to be positive for antibodies against IBV. Out of the 7 pools of swab and 5 pools of tracheal tissue samples analyzed using RT-PCR 33.3% (4/12) of them gave positive results all from swab samples. The RT-PCR-positive samples were subjected to a nested PCR yielding 295bp and 154bp indicating the circulation of Mass and 793/B (4/91) strains of IBV, respectively. The 12 pools of samples inoculated into embryonated egg showed cytopathic changes such as congestion, bleeding, and deformation only after three passages.
Conclusion: Two serotypes of IBV are circulating in Ethiopian chickens, and molecular identification of the Massachusetts serotype is the first report in Ethiopia. Further epidemiological investigation is needed in order to devise effective control measures.
Keywords: chickens, Central Ethiopia, IBV, nested-PCR, RT-PCR, serology
Introduction
Poultry farming is estimated to account for 40% of the world’s animal protein. However, poultry farms are vulnerable to infectious diseases such as infectious bronchitis (IB).1 It infects chickens of all ages, but young chicks are more susceptible as resistance increases with age.2 IB causes major economic losses in the chicken industry because of poor performance, decreased egg production and quality, and mortality, which can be high in presence of nephropathogenic strains or when secondary infections occur.3 IB is estimated to account for the third-highest losses among all livestock diseases, following avian influenza and echinococcosis.4
Infectious bronchitis virus (IBV) is a pathogen of domestic fowl (Gallus gallus), causing respiratory, renal and reproductive damages in commercial poultry farms worldwide. In addition, IBV suppresses the immune system, thereby paving the way for secondary infections. The combined effects of tissue damage caused by IBV and secondary infections contribute to higher mortality and loss of production.5,6
The IBV genome is a single positive-sense RNA strand about 27.6 Kb in length encoding the RNA-dependent RNA polymerase as well as the structural nucleocapsid (N), membrane (M), envelope (E) and spike (S) proteins. As with other coronaviruses, S is separated into the subunits S1 and S2 with approximately 535 and 625 amino acids, respectively. S1 glycoprotein is necessary for adsorption to the cellular receptor, and S2 is critical for virus entry into the host cell. The S1 gene is highly diverse among different viral lineages/strains, highlighting three hypervariable regions (HVR1/2/3) along the nucleotide sequences. S1 protein is also directly related to IBV antigenicity and the induction of neutralizing antibodies in the host.7–11
IBV presents a high genetic heterogeneity, having previously been divided into 6 genetic types (GI-GVI) and 32 (1–32) lineages spread worldwide. The distribution and diversity of IBV varies remarkably among genotypes. The distribution of some of the lineages has been extensively studied. For example, IBV genetic type I lineage 23 (GI-23) has been increasingly detected in severe respiratory infections from poultry flocks worldwide including Europe, Africa and Brazil in the last few decades.12 Due to its huge economic losses to the poultry industry, vaccination programs targeting IBV GI-23 have been implemented in commercial farms.
In Africa, IB is considered one of the most important viral diseases of chicken. The occurrence of vaccine genotypes (Mass and 793B strains) and several other non-vaccine types have been reported, although the lineage types and their distribution have not been defined. However, a number of IBV serotypes, antigenic variants, and field strains have been isolated. In contrast, only limited published information is available on IB in Ethiopia. To the best knowledge of the authors, only very few molecular studies have been conducted to identify the genotype of IBV. Bande et al13 and Hutton et al14 reported the circulation of the 793B genotype in commercial chicken farms in central Ethiopia with a 92–95% identity with the French isolate FR-94047-94 strain. The later authors also reported a high (94.5%) seroprevalence of IB. The circulation of IBV 793B (GI-13) strains was detected in backyard flocks.15 The serological evidence of IB has been documented from two studies, Birhan et al1 who reported a seroprevalence of 23.96% in Northwest Ethiopia and Tesfaye et al16 who observed a seroprevalence of 74.88% and 68.75% in unvaccinated backyard and commercial farms, respectively.
Commercial poultry farms are escalating in Ethiopia as an integral part of attaining food security and providing economical source of quality protein to the ever growing population. However, there is a setback when there is prevalence of diseases such as IB that poses considerable economic loses to poultry production. The occurrence of the virus has been confirmed as described above. As a result of lack of cross protection among various strains, it is difficult to use the commercial vaccines without the knowledge of identification of field strains of IBV. In Ethiopia, there are only few diagnostic laboratories which can confirm the actual identity of the virus and diagnosis is only made by postmortem examination. Imported vaccine prepared from Massachusetts-41 (M-41) strain of IBV used although outbreaks of IB are continuously reported in commercial flocks including vaccinated ones and backyard chicken. However, repeated failure of various IBV vaccines, including those against the Mass-41, 4/91, D-274, and D-1466 strains, suggests the circulation of IBV variants that have not yet been discovered.17 Since only very few researches have been carried out in Ethiopia to the best of the authors’ knowledge it is crucial to estimate the prevalence of IB and identify the genotypes circulating in the field. This will contribute to the understanding of the epidemiology of the diseases and effective design of prevention and control. Therefore, the objective of this study is to ascertain the serological and molecular evidence on the variants of the virus circulating in commercial chicken in central Ethiopia.
Material and Methods
Study Area
East Shewa is one of the administrative zones of the National Regional State of Oromia Ethiopia. It is situated between 8°9’N–8°15’N latitude and 38°49’E–38°817’E longitude having an altitude of 1707 meters above sea level (Figure 1). The area is known for its poultry production. The total poultry population in East Shewa zone is 14,39,821 of which 131,406 are roosters; 87,765 are cockerels; 148,214 are pullets; 66,555 are non-laying hens; 509,499 are chicks and 496,381 are laying hens.18
 |
Figure 1 Map indicating study site prepared with GIS software. |
Study Design
A cross-sectional study design was employed from November 2021 to June 2022 in two selected districts (Liben and Ada’a) and one city administration (Bishoftu) of East Shewa zone.
Study Population and Sampling
Seven commercial poultry farms rearing Sasso breed were purposively selected based on the accessibility and willingness of the farm owners for this study. All the farms included in this study did not vaccinated chicken against IBV. Healthy chickens older than three weeks were selected from each farm for blood collection for serological testing. Accordingly, sera samples were collected from 196 chickens. Additionally, chickens died as a result of suspected cases of IB particularly with history of coughing, sneezing, tracheal rattles for 10–14 days, conjunctivitis, dyspnea, and decreased egg production were sampled for molecular diagnosis. Hence, pooled swab samples were collected from trachea and oropharyngeal areas from 35 chickens having respiratory signs suggestive of IB. Tissue samples were also collected from five chickens per farm that died during the study period from five of the seven farms for virus isolation. Age classification was carried out based on the owners’ record. Young chickens aged between 3 and 6 months and adult chickens over 6 months old were classified based on the criteria set by Belayheh et al.19
Blood Sample Collection and Serum Preparation
Blood samples were collected aseptically from wing veins of chickens as per WOHA guidelines.20 Approximately 2–3 mL of blood was collected from each chicken using sterile syringes and needles. The blood was allowed to clot overnight at room temperature. After clotting, sera were separated by centrifugation at 1500 g and transferred to labeled cryovials and stored at −20°C until testing. Relevant information on chickens like age, breed, sex, farm type, etc., was gathered through interviews. Serological testing was done at the National Veterinary Institute, Ethiopia.
Swab and Tissue Sample Collection
Oropharyngeal and tracheal swabs (n = 35) were collected from chickens exhibiting respiratory signs from 7 commercial farms. A pool of swab samples was made from five chickens per farm, resulting in a total of 7 pools. The swabs were put into containers containing virus transport medium. In the laboratory, the pooled swab samples were subjected to gentile centrifugation and transferred to phosphate buffered saline (PBS). Additionally, tracheal tissues were collected from affected chickens into a pool of five. The pool of tracheal tissues were homogenized in PBS, centrifuged to collect supernatants, and shipped on dry ice in viral transport medium to National Veterinary Institute, Virology Laboratory. Both the swab and tracheal tissue samples were stored at −80°C until further analyzed (Figure 2).
 |
Figure 2 Pooled swab sample and tissue Sample. |
Laboratory Procedures
Serological Analysis
Indirect ELISA was used for the detection of antibodies against IBV in chicken sera (ID Screen®, IDvet, 310, rue Louis Pasteur-Grabels, France) according to the manufacturer’s instructions.16 Test kit was validated and verified before analyzing the samples. The antibody titer was estimated from sample to positive ratio (S/P) using the Equation below as described by the manufacturer. Those serum samples with S/P value of greater than 0.2 were considered positive.
Virus Isolation and Propagation
Virus isolation and propagation were performed following WOHA protocols.20,21 All samples were homogenized in a laboratory using mortar after mixing one part of tissue with two parts of 0.9% saline. The homogenate was centrifuged at 1500 g for 20 minutes at 4°C. The supernatant was filtered through a 0.22 μm filter, supplemented with 1000 IU/mL penicillin and 10 mg/mL streptomycin. Then, 200 μL of the filtered supernatant was inoculated into the allantoic cavity of 10-day-old specific pathogen free (SPF) embryonated eggs (3 eggs per sample). The inoculated eggs were incubated at 37°C and candled daily to assess embryonic viability. After 5–6 days, the chorioallantoic fluid was harvested and inoculated into SPF eggs. This procedure was repeated three times, collecting the fluid 5–6 days post-inoculation. The eggs were examined for characteristic gross changes such as curling and dwarfing of embryos with feather dystrophy [clubbing] as described previously.21 The allantoic fluid was aspirated from the eggs showing the characteristic embryonic changes and preserved at −80°C.20
Molecular Identification
The 7 pooled swab samples and the 5 pooled tracheal tissues were analyzed by RT-PCR. RNA extraction from the samples was done using QIAampi® RNA micro kit [Qiagen Ltd., Hilden, Germany] as per the manufacturer’s instructions. The pools were tested for IBV presence using the SuperScript™ III Platinum™ One-Step RT-PCR Kit (Thermo Fisher, Waltham, MA, USA), amplifying a 464 bp hyper-variable region of the S1 gene using the method described by Adzhar et al,22 while a method described by Jahantigh et al23 was used for Nested PCR (Table 1).
 |
Table 1 Primers to Identify IBV Serotypes in Reverse Transcription Nested PCR |
Data Management and Analysis
The data collected in this study were thoroughly checked for input errors, coded, and then imported into IBM SPSS version 20 for descriptive analysis. Disease prevalence was determined using descriptive statistics such as frequency and percentage. Logistic regression was used to investigate the association between IB seropositivity and risk factors including age, gender, production goals, farm type, and ventilation and disinfection of farm shoes. A P-value of less than 0.05 was considered statistically significant.
Results
Results of Serological Analysis
Out of a total of 196 sera samples analyzed using indirect ELISA 192 (97.96%; 95% CI: 94.86–99.44) of them tested positive. The seroprevalence IB ranges from 88.899% at Adulala2 farm to 100% at nearly all of the rest of the farms. The prevalence of IB was found to be higher in farms which did not practice footbath and disinfections of boots than those which practice boot disinfection. This difference was statistically highly significant (OR: 1.091; CI: 1.002–1.188; P = 0.003). In contrast, no statistically significant difference was observed in seroprevalence among other variables such as sex chicken, purpose of production, age of chicken, history of clinical disease, and farms. The results of logistic regression analysis on the effects of various variables on the seroprevalence of IB are given in Table 2.
 |
Table 2 Infectious Bronchitis Seropositive Rates Based on Risk Factors |
Results of Virus Isolation
Seven pools of swab and five pools of tracheal tissue samples inoculated in to 9–11 day old embryonated chicken egg allantoic sac were passaged three times. After the third passage, all of the inoculated embryonated eggs showed evidence of virus growth (infection) such as congestion, bleeding, and deformation. After day 15, following the third passage, gross changes, such as dwarfing, curling of embryo and feather dystrophy, were observed (Figure 3).
 |
Figure 3 Gross changes in chicken embryos caused by IBV at 15 days age, Dwarfing and curling of embryo at 15 days of age and also feather dystrophy. |
Results of Molecular Analysis
A one step RT-PCR was carried out on 17 pools of samples (12 pools of swab and 5 pools of trachea) employing a pair of primers designated XCE1+ and XCE2 targeting a 466bp the S1 gene of IBV. The results showed that 4 (33.33%) of the 12 pools yielded positive desired amplification product (Figure 4). The positive samples were all from the pooled swab samples.
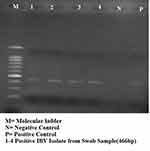 |
Figure 4 IBV detected with general primer using RT-PCR. |
The four RT-PCR positive samples were further analyzed by a nested PCR for identification of the serotypes of IBV. The nested PCR yielded 295bp and 154bp of gene in all of the four samples belonging to Mass and 793/B (4/91) strains of IBV, respectively. That is, there was a co-infection of chickens in the study area with strains Mass and 4/91 strains of IBV. The results of nested PCR are depicted in Figure 5.
 |
Figure 5 IBV serotypes detected by species specific primers using nested PCR. |
Discussion
Infectious bronchitis is incriminated in causing severe respiratory and renal damages in broiler and layer chickens, leading to high mortality and economic losses. The IBV is highly mutating, and disease has been reported in vaccinated flocks.2 The continual loss due to IB raised the need for an epidemiological study in order to find out the prevalence of the disease and identify the circulating serotypes in commercial poultry farms. In this study, we demonstrated the seroprevalence of IB in unvaccinated commercial chickens and identified two serotypes, which are crucial for the livestock and veterinary authorities in their endeavor to devise prevention and control measures.
In this study, very high overall seroprevalence (97.95%) of IB is reported in non-vaccinated chickens in seven commercial farms in central Ethiopia. This suggests the widespread occurrence of IBV among poultry farms in the area. Since the chickens were not vaccinated, the seroprevalence observed indicates natural exposure of chickens and circulation of IBV in the area. Our results are in consent with previous reports of seroprevalence in Ethiopia14,24 and Spain.25 However, our findings are higher than seroprevalence reported from Northwestern Ethiopia1 and elsewhere in the world such as the Sudan,26 Ghana,27 Nigeria,28 and Central America.29 The difference observed in seroprevalence between the current study and the previous reports could be attributed to variation in the farm management practices, the level of farm hygiene, level of awareness of the farm owners and implementation of preventive measures. Additionally, the poultry farms in the study area are very close to each other and the main roads. As a result, there is a high risk of spread of the virus among the farms.
The chicken type and breed could also contribute to variation in seroprevalence. To this end, higher prevalence was observed in layers than broilers, although the difference was not statistically significant. Previous studies demonstrated higher seroprevalence of IB in layers and twin flocks than in broiler flocks.2,30 The higher prevalence in laying hens could be attributed to the fact that they spend more time on the farm, thus increasing their likelihood of exposure to the virus when no effective prevention strategy is in place.27 This observation conforms to the explanation described by previous authors regarding the effects of prolonged exposure on the seroprevalence of IB.1
Statistically significant difference was observed in seroprevalence of IB between farms which practice boot disinfection and footbath facilities and those which do not practice. This finding suggests the importance of implementing biosecurity measures such as boot disinfection or use of appropriated footbath in preventing the entrance and spread of IBV. The importance of rigorous biosecurity in preventing the introduction and spread of IBV has already been described. Additionally, there is a strong positive relationship between loose biosecurity and a high prevalence of diseases.12
This study identified two genotypes of IBV in central Ethiopia, namely 793B and Mass using molecular techniques. The 793B genotype was detected in unvaccinated backyard poultry in Ethiopia15 using the same molecular methods. Serological evidence of circulation of 793B genotype was also reported earlier by Tesfaye et al,16 although the reliability of serological identification of genotypes is obscured as a result of cross-reactions. The detection of 793B is not surprising, considering its widespread use as a vaccine. However, in the considered Ethiopian farms, no IBV vaccination was implemented. Therefore, the positive results were likely due to natural infection. Tegegne et al15 previously confirmed the identity of 793B from the high genetic distance compared to commonly applied vaccines. In the absence of strict biosecurity measures, several spreading sources could be involved, including wild birds, wherein IBV-like viruses have been identified from time to time. In addition, the role of fomites (feed/slaughter contacts) and human movements (company technician, veterinarian, and shared farm workers) could be linked to introduction of IBV.12
The second genotype identified was Mass. Although molecular confirmation of this genotype is the first one in Ethiopia, the co-occurrence of Mass with 793B genotypes has been reported elsewhere in the world.31 It is also one of a widely used vaccine strain, although the chickens sampled in this study were unvaccinated. Previously, the use of live attenuated vaccine was hypothesized to impact IBV genetic population structure.11
The paragraph compares the development of IBV in different organ cultures such as chick kidney and trachea, and highlights that IBV develops well in the developing chick embryo, with the highest virus titer reached at 1 to 2 days after inoculation. The paragraph goes on to explain that there may be negative consequences to the embryo, with increased embryo mortality and dwarfism after the second passage. The study being referred to allowed the embryo to develop for at least 15 days to observe any changes that might indicate the presence of IBV and observed dwarfing, curling of embryo, and feather dystrophy at 15 days of age.
However, embryonic changes induced by Infectious Bronchitis Virus (IBV) are not specific to the virus and can be seen in other conditions. Lentogenic variants of Newcastle disease virus, for instance, may also cause stunted embryonic lesions during development.32 In addition, management errors and other biological agents could also lead to similar effects.21 To confirm the presence of IBV, further investigation was conducted, and allantoic fluid was collected from suspected embryonated eggs and analyzed using RT-PCR.
In conclusion, this study investigated the presence and risk factors of avian infectious bronchitis virus (IBV) in chickens in Ethiopia. It revealed the circulation of the Massachusetts and 793B serotype using molecular methods. The study reported a comprehensive and valuable findings on IBV in Ethiopia, which the livestock and veterinary authorities should take into account while devising prevention and control measures.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study design and method have been evaluated and endorsed by the Ethics Committee of Wollega University in compliance with relevant regulations, humane animal care standards, and the research protocol, as evidenced by the certification letter labeled with reference number WUSVMRE022/21. Consent Statement: Informed and written consent was obtained from all animal owners prior to involve in the study for the use of their cattle in this study.
Acknowledgments
We express our sincere gratitude to Addis Ababa University College of Veterinary Medicine and Wollega University School of Veterinary Medicine for their invaluable support, guidance, and assistance in the preparation of this manuscript. We also acknowledge the National Veterinary Institute for their provision of laboratory reagents, kits, and facilities support, without which this work would not have been possible. The authors extend special thanks to all the staff members who provided helpful suggestions and feedback on the manuscript.
Funding
Authors declare there is no funding assigned/allocated for this Study.
Disclosure
The authors declare that they have no conflicts of interest to declare for this work.
References
1. Birhan M, Temesgen M, Shite A, et al. Seroprevalence and associated risk factors of infectious bronchitis virus in chicken in Northwest Ethiopia. Hindawi: 2021.
2. Fayyaz A, Saleemi MK, Gul ST, Gilani MM, Irshad H. Sero-epidemiology and pathology of infectious bronchitis in commercial poultry from faisalabad division. Pak Vet J. 2023;43(1):146–152. doi:10.29261/pakvetj/2021.065
3. TAFS-Forum. World Livestock Disease Atlas a Quantitative Analysis of Global Animal Health Data [2006–2009]. Washington, DC, USA: The World Bank; 2011.
4. Toro H, Pennington D, Gallardo RA; World Bank. World Livestock Disease Atlas. A Quantitative Analysis of Global Animal Health Data (2006–2009) World Bank; Washington, DC, USA: 2011. Infectious bronchitis virus subpopulations in vaccinated chickens after challenge. Avian Dis. 2012;56(3):501–508. doi:10.1637/9982-110811-Reg.1
5. Gallardo R. Infectious bronchitis virus variants in chickens: evolution, surveillance, control and prevention. Austral J Vet Sci. 2021;53(1):55–62. doi:10.4067/S0719-81322021000100055
6. Cook JKA, Jackwood M, Jones RC. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41(3):239–250. doi:10.1080/03079457.2012.680432
7. Fan W, Tang N, Dong Z, et al. Genetic analysis of avian coronavirus infectious bronchitis virus in yellow chickens in southern China over the past decade: revealing the changes of genetic diversity, dominant genotypes, and selection pressure. Viruses. 2019;11(10):898. doi:10.3390/v11100898
8. Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet Res. 2007;38(2):281–297. doi:10.1051/vetres:2006055
9. Cavanagh D, Davis PJ, Cook JK, Li D, Kant A, Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21(1):33–43. doi:10.1080/03079459208418816
10. Cornelis J, Vermeulen, Remco Dijkman J, de Wit JJ (Sjaak), Bosch B-J, Heesterbeek JAP (Hans), van Schaik G. Genetic analysis of infectious bronchitis virus (IBV) in vaccinated poultry populations over a period of 10 years. Avian Pathol. 2023;52(3):157–167. doi:10.1080/03079457.2023.2177140
11. Ikuta N, Kipper D, Freitas DSSD, Fonseca ASK, Lunge VR. Evolution and Epidemic Spread of the avian infectious bronchitis virus (IBV) GI-23 in Brazil. Viruses. 2023;15(6):1229. doi:10.3390/v15061229
12. Bande F, Arshad SS, Omar AR, Hair-Bejo M, Mahmuda A, Nair V. Global distributions and strain diversity of avian infectious bronchitis virus Animal. Health Res Rev. 2019;18:70–83.
13. Hutton S, Bettridge J, Christley R, Habte T, Ganapathy K. Detection of infectious bronchitis virus 793B, avian metapneumovirus, Mycoplasma gallisepticum and Mycoplasma synoviae in poultry in Ethiopia. Trop Anim Health Prod. 2016;49(2):317–322. doi:10.1007/s11250-016-1195-2
14. Tegegne D, Deneke Y, Sori T, et al. Molecular epidemiology and genotyping of infectious bronchitis virus and avian metapneumovirus in backyard and commercial chickens in Jimma zone, southwestern Ethiopia. Veter Sci. 2020;7(4):187. doi:10.3390/vetsci7040187
15. Tesfaye A, Kassa T, Mesfin S. Four serotypes of infectious bronchitis virus are widespread in unvaccinated backyard chicken and commercial farms in Ethiopia. World J Veter Sci. 2019;1:1001.
16. Bhuiyan MSA, Amin Z, Bakar AMSA, et al. Factor influences for diagnosis and vaccination of avian infectious bronchitis virus (Gammacoronavirus) in chickens. Vet Sci. 2021;8(3):47. doi:10.3390/vetsci8030047
17. EShZADO. East shawa zone agriculture development office: livestock population data; 2021.
18. Belayheh G, Moses N, Melese K. Seroprevalence of Newcastle disease virus antibodies in village chickens in kersana-kondalaity District, Ethiopia. GlobalVeterinaria. 2014;12:426–430.
19. WOHA. OIE Terrestrial Manual, CHAPTER 3.3.2. Avian Infectious Bronchitis; 2018.
20. Cavanagh D, Gelb J. Infectious Bronchitis. Iowa State Press; 2008:117–135.
21. Adzhar A, Gough RE, Haydon D, Shaw K, Britton P, Cavanagh D. Molecular analysis of the 793/B serotype of infectious bronchitis virus in Great Britain. Avian Pathol. 1997;26(3):625–640. doi:10.1080/03079459708419239
22. Jahantigh M, Salari S, Hedayati M. Detection of infectious bronchitis virus serotypes by reverse transcription polymerase chain reaction in broiler chickens. Springer Plus. 2013;2(1):36. doi:10.1186/2193-1801-2-36
23. Jirata S, Tamiru D, Misgana T, Yobsan T. Seroprevalence of infectious bronchitis virus in broiler and layer farms of central Ethiopia. Hindawi. 2022;2022:5.
24. Cortes V, Sandra SN, Cristina G, Clara M, Pablo CG. Seroprevalence and prevalence of Infectious Bronchitis Virus in broilers, laying hens and broiler breeders in Spain. Poultr Sci. 2022;101(5):101760. doi:10.1016/j.psj.2022.101760
25. Ballal A, Karrar AE, El-Hussein AM. Serosurveillance study on avian infectious bronchitis virus in Sudan. J Anim Vet Adv. 2005;4:908–909.
26. Ayim-Akonor M, Owusu-Ntumy DD, Ohene-Asa HE. Serological and molecular surveillance of infectious bronchitis virus infection in free-range chickens and Guinea fowls in the Ga-east district of Ghana. J Vet med A. 2018;2018:6.
27. Emikpe BO, Ohore OG, Olujonwo M, Akpavi SO. prevalence of antibodies to Infectious Bronchitis Virus (IBV) in chickens in Southwestern Nigeria. Afr J Microbiol Res. 2010;4:92–95.
28. Sabarinath A, Sabarinath GP, Tiwari KP, Kumthekar SM, Tomas D, Sharma RN. Seroprevalence of infectious bronchitis virus in birds of Grenada. Int J Poult Sci. 2011;10(4):266–268. doi:10.3923/ijps.2011.266.268
29. Shettima YM, El-Yuguda AD, Zanna MY, Abubakar MB, Hamisu TM, Maina MM. Serological evidence of infectious bronchitis virus among some poultry species in Maiduguri, Nigeria. Alexandria J Vet Sci. 2016;20:135–139.
30. Ghalyanchil AA, Hosseini H, Kafi ZZ, Sadri N, Rajeoni AH, Najafi H. New insights on epidemiology of infectious bronchitis virus in Iran by comparing two genotyping methods. Turkish J Veter Animal Sci. 2023;47:12. doi:10.55730/1300-0128.4272
31. Han Z, Sun C, Yan B, et al. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect Genet Evol. 2011;11(1):190–200. doi:10.1016/j.meegid.2010.09.002
32. Roussan DA, Totanji WS, Khawaldeh GY. Molecular subtype of infectious bronchitis virus in broiler flocks in Jordan. Poultr Sci. 2008;87(4):661–664. doi:10.3382/ps.2007-00509
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.