Back to Journals » Journal of Blood Medicine » Volume 14
Multiply Relapsed Secondary CNS Non-Germinal Center Diffuse Large B-Cell Lymphoma Successfully Treated with CNS-Centric Therapy
Authors Fournier LL , Kimbrough EO , Alhaj Moustafa M , Li K, Iqbal M, Gupta V , Tun HW
Received 21 January 2023
Accepted for publication 16 July 2023
Published 16 August 2023 Volume 2023:14 Pages 455—461
DOI https://doi.org/10.2147/JBM.S405521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Lyndsey L Fournier,1 ErinMarie O Kimbrough,1 Muhamad Alhaj Moustafa,1 Ke Li,2 Madiha Iqbal,1 Vivek Gupta,3 Han W Tun1
1Division of Hematology and Medical Oncology, Mayo Clinic, Jacksonville, FL, USA; 2Department of Pathology, Mayo Clinic, Jacksonville, FL, USA; 3Department of Radiology, Mayo Clinic, Jacksonville, FL, USA
Correspondence: Han W Tun, Division of Hematology and Oncology, Mayo Clinic, 4500 San Pablo Road S, Jacksonville, FL, 32224, USA, Tel +1 904 953 2693, Fax +1 904 953 2315, Email [email protected]
Abstract: Secondary central nervous system involvement by systemic diffuse large B-cell lymphoma (DLBCL) carries a very poor prognosis. We present a female patient who had two episodes of intracerebral central nervous system (CNS)-only relapse of systemic non-germinal center diffuse large B-cell lymphoma (NGC-DLBCL). Her treatment at initial diagnosis consisted of induction with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and intrathecal (IT) - methotrexate (MTX) followed by consolidation with autologous stem cell transplant (ASCT) after high-dose carmustine, etoposide, cytarabine, and melphalan (BEAM) chemotherapy. She had the first CNS-only relapse 1.5 years post-ASCT and received whole brain radiation therapy (WBRT). She developed the second intracerebral CNS-only relapse 2 years post-WBRT. A CNS-centric therapeutic approach with salvage chemoimmunotherapy incorporating rituximab, high-dose methotrexate (HD-MTX), high-dose cytarabine (HiDAC), and ibrutinib was utilized for her second CNS-only relapse. She underwent consolidation with a second ASCT following high-dose carmustine (BCNU) and thiotepa chemotherapy. Given her high risk of CNS recurrence, she was started on maintenance ibrutinib. To date, she has remained in complete remission for 3 years. In our experience, multiply relapsed secondary CNS lymphoma (SCNSL) with this response is very rare. We suggest one CNS-centric therapeutic approach that can potentially salvage patients with SCNSL who have not had prior exposure to adequate CNS-directed therapies but acknowledge that additional research is necessary to validate our findings.
Keywords: SCNSL, relapsed CNS lymphoma, CNS lymphoma, secondary CNS DLBCL
Introduction
Secondary central nervous system diffuse large B-cell lymphoma (SCNS-DLBCL) represents central nervous system (CNS) dissemination and involvement by a systemic DLBCL at the time of initial diagnosis (de novo SCNSL; DN-SCNSL) or relapse (R-SCNSL).1–6 R-SCNSL include CNS-only or concomitant CNS-systemic relapse. R-SCNSL develops early in the disease course with a median time to relapse of 5.4 months from diagnosis. Approximately 80% of patients relapse while receiving chemotherapy or within 6 months of completion of therapy.1
Among the seventy-five patients enrolled in the largest clinical trial of secondary CNS DLBCL (MARIETTA trial), DN-SCNSL represented 43% of cases, while CNS-only R-SCNSL accounted for 20%, and CNS-systemic R-SCNSL 37% of cases.5 The neuroanatomical localization of SCNSL included intracerebral (45%), leptomeningeal (11%), intracerebral/leptomeningeal (17%), intracerebral/ocular (13%), intracerebral/leptomeningeal/ocular (8%), ocular (3%), or spinal cord (3%) disease.5 Based on Han’s criteria for cell of origin, 59% of the evaluable patients had non-germinal center (NGC)-DLBCL.5 The neurological manifestations of SCNSL included motor impairment (49%), sensory impairment (33%), cognitive impairment (20%), sensorial impairment (16%), and language impairment (9%).5
SCNSL is extremely uncommon, and there is limited data available regarding optimal treatment. Two of the largest studies reported an incidence of 1.05% for DN-SCNSL and 2.2–2.8% for relapse central nervous system lymphoma (R-CNSL).1,7 While others have suggested that CNS relapse of DLBCL occurs in 3% to 5% of patients with a median overall survival (mOS) of less than 7 months.6,8–10 In the studies in which the treatment consisted of intensive CNS-directed therapy and consolidation with autologous stem cell transplant (ASCT), the mOS was less than 10 months with a 3-year overall survival (OS) of 22% for the population as a whole and 42% for patients who underwent consolidation with ASCT has been reported.2,11,12 The survival outcome for multiply relapsed SCNSL is not known but likely very poor.
We report a case of multiply relapsed intracerebral SCNSL associated with systemic NGC-DLBCL who was successfully salvaged with CNS-centric therapy with a sustained complete remission (CR) for 3 years to date.
Case
A 38-year-old Asian female presented with a 6-week history of progressive abdominal discomfort and distention, fatigue, 10-pound weight loss, and night sweats. Computed tomography (CT) of the abdomen and pelvis showed extensive soft tissue masses throughout the peritoneal cavity and retroperitoneum. Positron emission tomography-computed tomography (PET-CT) demonstrated generalized hypermetabolic bulky nodal masses including an 8.5 × 6.9 centimeter (cm) peritoneal mass, a 7.5 × 6.4 cm pelvic mass, and a 6.3 × 1.7 cm anterior mediastinal mass. CT guided biopsy of an abdominal mass was performed and demonstrated lambda light chain restricted monoclonal B-cells expressing CD19, CD20, CD45, BCL2, and MUM1. The Ki67 proliferation rate was greater than 90%. Fluorescence in situ hybridization (FISH) showed the MYC gene region was within normal limits. The peripheral blood lactate dehydrogenase (LDH) was reportedly elevated at an outside facility. Bone marrow biopsy and cerebrospinal fluid (CSF) evaluations were negative. She was diagnosed with stage IV NGC-DLBCL with a high-intermediate risk age-adjusted international prognostic index (IPI) score given stage IV disease and an elevated LDH at diagnosis. She received 6 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with 4 doses of intrathecal (IT) -methotrexate (MTX) with resolution of her symptoms. Restaging evaluation was consistent with complete remission (CR). After completion of induction therapy, there were concerns among the care team members at the time regarding the high risk of rapid relapse based on aggressiveness of the disease on presentation. The decision was made to proceed with ASCT. She underwent consolidation with high-dose carmustine, etoposide, cytarabine, and melphalan (BEAM) followed by ASCT.
Unfortunately, she developed blurred vision and gait imbalance and was found to have an intracerebral CNS-only relapse 1.5 years following ASCT. Magnetic resonance imaging (MRI) brain showed a 1.7 cm mass in the right foramen of Luschka invading the medulla and right cerebellar tonsil. MRI brain was negative for leptomeningeal enhancement and CSF cytology was negative for lymphoma. CT soft tissue neck, chest, abdomen, and pelvis with contrast showed no evidence of systemic disease. She received whole brain radiation therapy (WBRT) for a total of 2340 cGy with a brain stem boost of 2340 cGy local radiation to the tumor. One month following WBRT, a repeat MRI brain showed a CR.
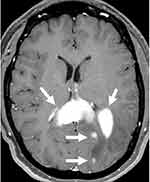
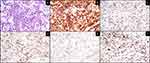
Two years following WBRT, she presented to our institution with word finding difficulty and mental status changes. MRI of the brain revealed intracerebral, multifocal, enhancing mass lesions with one of the larger components centered within the splenium of the corpus callosum, measuring 1.8 × 3.5 × 1.6 cm (Figure 1). A PET-CT demonstrated a hypermetabolic focus in the left parieto-occipital region suggesting recurrent lymphoma. An excisional biopsy of the left parietal tumor showed large lymphoma cells (Figure 2A). The lymphoma cells were positive for CD20, BCL6, BCL2, and MUM1 by immunohistochemistry (IHC) (Figure 2B–E). They were negative for CD10, CD30, and TdT. Epstein-Barr encoding region (EBER) ish was negative. The Ki67 proliferation index was 90% (Figure 2F). The CSF cytology was negative. These findings were consistent with relapsed CNS NGC-DLBCL according to Hans’ algorithm.13 Unfortunately, additional testing could not be performed due to the small biopsy.
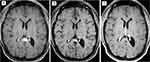
Salvage chemotherapy with rituximab, high-dose methotrexate (HD-MTX), and high-dose cytarabine (HiDAC) was initiated with improvement in her neurologic symptoms. She developed severe dermatitis which was thought to be secondary to HiDAC. She was transitioned to rituximab, HD-MTX, and Ibrutinib for cycles 2–4 of treatment. Ultimately, the rash was due to acyclovir, and she completed cycles 5–6 with a combination of rituximab, HD-MTX and HiDAC. Subsequent MRI demonstrated a near CR (Figure 3A and B).
She was consolidated with a second ASCT following high-dose carmustine (BCNU) and thiotepa. Her day 100 work-up including a PET scan, MRI brain and bone marrow biopsy showed a CR (Figure 3C). She was started on maintenance Ibrutinib 560 mg daily due to her high risk of relapse. She has remained in CR for 3 years. She currently enjoys excellent quality of life with full-time employment. A summary of her treatment is provided in Table 1.
 |
Table 1 Case Presentation Treatment Summary |
Discussion
This case highlights the importance of utilizing CNS-penetrating agents for SCNSL. The patient developed her first CNS-only relapse despite R-CHOP, IT-MTX, and consolidation with ASCT following high dose BEAM chemotherapy. This suggests that the regimen was adequate for her systemic disease but insufficient for the CNS disease. Her second CNS-only relapse occurred after WBRT. This suggests that WBRT alone without systemic CNS-directed therapy is also inadequate. She was successfully salvaged with a combination of CNS-penetrating therapy followed by consolidation with ASCT following CNS-penetrating high dose conditioning chemotherapy. She was then placed on maintenance ibrutinib due to her high risk of CNS relapse. She has been in CR for 3 years to date. The findings in our case indicate that CNS-centric therapy can potentially salvage multiply relapsed SCNSL if the prior systemic treatments do not adequately penetrate the CNS.
Our patient received CNS-directed therapy without systemic disease-directed therapy as she had late-onset CNS-only SCNSL. Most commonly patients with R-SCNSL have early relapse; however, our patient’s relapse was more atypical and presented late.1 Her treatment resembled regimens commonly used for primary CNS lymphoma (PCNSL). In a Phase II clinical trial evaluating patients with SCNSL, induction regimens consisted of CNS-directed as well as systemic disease-directed therapies.4,5,14,15 This approach is well suited if the SCNSL has both a CNS and systemic components or if the patient has an early CNS relapse. Therapy directed at systemic disease may not be necessary for CNS-only SCNSL with late relapse. In addition, CNS-directed therapy also impacts systemic disease. Further research is necessary to determine the optimal therapeutic approach for CNS-only SCNSL with late relapse.
There have been four phase II clinical trials for SCNSL.4,5,14,15 These trials incorporated CNS and systemic disease directed induction followed by consolidation with ASCT/CNS-penetrating high-dose chemotherapy with promising survival outcomes.4,5,14,15 The 5-year OS was 41% in the entire study group and 68% in patients who received ASCT.4 This strategy was not as effective for R-SCNSL. These patients had a 2-year progression free survival (PFS) of 28% compared to 71% for DN-SCNSL.5 Maintenance therapy following ASCT has not been evaluated. A summary of key clinical trials in SCNSL and associated outcomes are included in Table 2.
 |
Table 2 Summary of Clinical Trials in SCNSL and Reported Outcomes |
In regard to WBRT, a retrospective study evaluated response in 25 SCNSL patients who received consolidative versus palliative WBRT. Thirteen patients received consolidative WBRT with a mOS of 24 months and 2-year OS of 64%. The 12 patients who received palliative WBRT had a mOS of 3 months and 2-year OS of 8%. The results of this small study sample suggest that consolidative WBRT may improve long-term survival in SCNSL and could be a potential option for transplant ineligible patients or patients with CNS-only relapse.16 Additionally, WBRT has been used as the stand-alone treatment in PCNSL with ORR of 90% and median OS of 11.6 months.17
At the time of first CNS-only relapse, our patient was treated at an outside institution and underwent WBRT. It would have been reasonable to consider treating with CNS-directed systemic therapy instead of WBRT. This is especially true when taking into consideration the neurotoxicity that can result from WBRT including decreased attentiveness and executive function.18
Our patient has been maintained on continuous ibrutinib therapy due to high risk of relapse. Ibrutinib has been shown to be efficacious in systemic NGC-DLBCL and provides excellent CNS penetration.19,20 It has been used successfully in newly diagnosed and relapsed PCNSL.21–23 In a Phase I clinical trial, seven patients with R-SCNSL were treated with ibrutinib with an overall response rate of 71%. Approximately 57% of patients experienced a CR, and the median PFS was 7.4 months.21 Our patient has been tolerating maintenance ibrutinib quite well.
There is limited data regarding the optimal treatment of multiply relapsed CNS-only SCNSL. This represents just one case and potential treatment strategy. We acknowledge that additional research is necessary to determine the optimal treatment regimen and to assess the role of maintenance therapy.
Conclusion
CNS-directed therapy is critical in the management of SCNSL. Our patient achieved a long-term CR after salvage CNS-directed treatment for multiply relapsed intracerebral CNS-only SCNSL. The therapeutic success in our patient is likely due to high-dose BCNU/Thiotepa chemotherapy with ASCT and maintenance ibrutinib. This case demonstrates the benefit of CNS-centric salvage therapy in multiply relapsed SCNSL when the patient has previously received inadequate systemic CNS-directed therapy. For eligible SCNSL patients, induction therapy and high-dose chemotherapy consisting of CNS-penetrating agents should be considered. Current therapies for R-SCNSL are limited and are often ineffective. Novel therapies are urgently needed for elderly and unfit patients who are not eligible for currently available intensive therapies. In addition, the role of maintenance therapy needs to be further explored. Additional research is necessary to assess the therapeutic approach which was undertaken in our patient with multiply relapsed SCNSL.
Abbreviations
ASCT, autologous stem cell transplant; BCNU, carmustine; BEAM, carmustine, etoposide, cytarabine, melphalan; cm, centimeter; CNS, central nervous system; CR, complete remission; CSF, cerebrospinal fluid; CT, computed tomography; DN-SCNSL, de novo secondary CNS lymphoma; DLBCL, diffuse large b-cell lymphoma; EBER, Epstein-Barr encoding region; FISH, fluorescence in situ hybridization; HiDAC, high-dose cytarabine; HD-MTX, high-dose methotrexate; IHC, immunohistochemistry; IPI, international prognostic index; IT, intrathecal; LDH, lactate dehydrogenase; MRI, magnetic resonance imaging; MTX, methotrexate; mOS, median overall survival; NGC, non-germinal center; NGC-DLBCL, non-germinal center DLBCL; OS, overall survival; PET-CT, positron emission tomography-computed tomography; PCNSL, primary CNS lymphoma; PFS, progression free survival; R-CNSL, relapse central nervous system lymphoma; R-SCNSL, relapse secondary CNS lymphoma; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; SCNSL, secondary CNS lymphoma; SCNS-DLBCL, secondary CNS diffuse large B-cell lymphoma; WBRT, whole brain radiation therapy.
Consent for Publication
The study participant has given written informed consent to participate as well as written informed consent to publish the case details and accompanying images. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bernstein SH, Unger JM, LeBlanc M, et al. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 – the southwest oncology group. J Clin Oncol. 2009;27(1):114–119. doi:10.1200/JCO.2008.16.8021
2. Bromberg JE, Doorduijn JK, Illerhaus G, et al. Central nervous system recurrence of systemic lymphoma in the era of stem cell transplantation--an international primary central nervous system lymphoma study group project. Haematologica. 2013;98(5):808–813. doi:10.3324/haematol.2012.070839
3. El-Galaly TC, Cheah CY, Bendtsen MD, et al. Treatment strategies, outcomes and prognostic factors in 291 patients with secondary CNS involvement by diffuse large B-cell lymphoma. Eur J Cancer. 2018;93:57–68. doi:10.1016/j.ejca.2018.01.073
4. Ferreri AJ, Donadoni G, Cabras MG, et al. High doses of antimetabolites followed by high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation in patients with systemic B-cell lymphoma and secondary CNS involvement: final results of a multicenter phase II trial. J Clin Oncol. 2015;33(33):3903–3910. doi:10.1200/JCO.2015.61.1236
5. Ferreri AJM, Doorduijn JK, Re A, et al. MATRix-RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): an international, single-arm, Phase 2 trial. Lancet Haematol. 2021;8(2):e110–e121. doi:10.1016/S2352-3026(20)30366-5
6. Klanova M, Sehn LH, Bence-Bruckler I, et al. Integration of cell of origin into the clinical CNS international prognostic index improves CNS relapse prediction in DLBCL. Blood. 2019;133(9):919–926. doi:10.1182/blood-2018-07-862862
7. Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol. 2007;18(1):149–157. doi:10.1093/annonc/mdl327
8. Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182–2188. doi:10.1182/blood-2015-10-676700
9. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34(26):3150–3156. doi:10.1200/JCO.2015.65.6520
10. Sehn LH, Salles G. Diffuse large B-cell lymphoma. N Engl J Med. 2021;384(9):842–858. doi:10.1056/NEJMra2027612
11. Kasamon YL, Jones RJ, Piantadosi S, et al. High-dose therapy and blood or marrow transplantation for non-Hodgkin lymphoma with central nervous system involvement. Biol Blood Marrow Transplant. 2005;11(2):93–100. doi:10.1016/j.bbmt.2004.09.009
12. Williams CD, Pearce R, Taghipour G, et al. Autologous bone marrow transplantation for patients with non-Hodgkin’s lymphoma and CNS involvement: those transplanted with active CNS disease have a poor outcome--a report by the European bone marrow transplant lymphoma registry. J Clin Oncol. 1994;12(11):2415–2422. doi:10.1200/JCO.1994.12.11.2415
13. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
14. Doorduijn JK, van Imhoff GW, van der Holt B, et al. Treatment of secondary central nervous system lymphoma with intrathecal rituximab, high-dose methotrexate, and R-DHAP followed by autologous stem cell transplantation: results of the HOVON 80 phase 2 study. Hematol Oncol. 2017;35(4):497–503. doi:10.1002/hon.2342
15. Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica. 2013;98(3):364–370. doi:10.3324/haematol.2012.077917
16. Walburn T, Grover NS, Shen CJ, et al. Consolidative or palliative whole brain radiation for secondary CNS diffuse large B-Cell lymphoma. Leuk Lymphoma. 2021;62(1):68–75. doi:10.1080/10428194.2020.1821014
17. Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23(1):9–17. doi:10.1016/0360-3016(92)90538-S
18. Ferreri AJM, Cwynarski K, Pulczynski E, et al. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. 2022;36(7):1870–1878. doi:10.1038/s41375-022-01582-5
19. Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell. 2017;31(6):833–843 e5. doi:10.1016/j.ccell.2017.04.012
20. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–926. doi:10.1038/nm.3884
21. Grommes C, Pastore A, Palaskas N, et al. Ibrutinib Unmasks Critical Role of Bruton Tyrosine Kinase in Primary CNS Lymphoma. Cancer Discov. 2017;7(9):1018–1029. doi:10.1158/2159-8290.CD-17-0613
22. Kuhlman JJ, Alhaj Moustafa M, Jiang L, et al. Long-term survival with ibrutinib therapy in elderly patients with newly diagnosed primary central nervous system lymphoma. Blood Lymphat Cancer. 2022;12:23–29. doi:10.2147/BLCTT.S360442
23. Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–130. doi:10.1016/j.ejca.2019.05.024
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.