Back to Journals » Biologics: Targets and Therapy » Volume 18
Novel Molecular Targets in Endometrial Cancer: Mechanisms and Perspectives for Therapy
Authors Soberanis Pina P, Lheureux S
Received 1 December 2023
Accepted for publication 22 February 2024
Published 21 March 2024 Volume 2024:18 Pages 79—93
DOI https://doi.org/10.2147/BTT.S369783
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shein-Chung Chow
Pamela Soberanis Pina, Stephanie Lheureux
Department of Medical Oncology, Princess Margaret Cancer Centre, Toronto, ON, Canada
Correspondence: Stephanie Lheureux, Princess Margaret Cancer Centre, 700 University Avenue, Toronto, M5G 1Z5, Ontario, Canada, Email [email protected]
Abstract: Endometrial cancer (EC) has a high epidemiological impact with incidence and mortality rising worldwide. In recent years, the integration of the pathologic and molecular classification has provided relevant information to understand the heterogeneity in the biology of EC, which led to the evolution in the management of patients. Currently, therapeutic breakthroughs have been made in advanced EC to improve oncologic outcomes, with efforts to include patient reported outcomes. Precision and personalized medicine are under way in EC exploring different combination approaches to target cross-talk pathways, cancer cell microenvironment, and metabolic vulnerabilities and improve drug delivery. Yet, collaborative efforts are needed to face the challenges in practice by refining patient selection, ideal biomarker identification, and de-escalation of therapies according to emerging molecular and genomic features of EC.
Keywords: endometrial cancer, targeted therapy, molecular targets, precision medicine
Introduction
Endometrial carcinoma (EC) is the fourth most common cancer among women worldwide, with both incidence and mortality on the rise, unlike other cancers.1,2 Approximately 10–15% of cases are diagnosed at an advanced stage and those who have advanced EC or experience recurrence, their survival outcomes are poor, with an estimated five-year overall survival (OS) of 17%.2,3 Current upfront treatment options for patients with EC include surgery, platinum-based chemotherapy and radiation. Recent data showed the benefit of the addition of immune checkpoint inhibitors (ICI) in first-line setting for advanced stage, in combination with carboplatin/paclitaxel, particularly in deficient mismatch repair (MMRd).4,5 Even though available options in the first-line setting are increasing, nearly half of the patients treated with platinum-based chemotherapy will ultimately progress or recur.2,3 Modest response rates have been observed with single-agent chemotherapy or endocrine therapy.3 For patients ICI naïve and MMRd, single-agent ICI have been approved.6,7 Meanwhile, the combination of pembrolizumab and lenvatinib for patients with recurrent EC has also been incorporated into management, changing the landscape for treatment.8,9
It is now well recognized that EC is not one disease and is characterized by different subtypes. Understanding the biology of ED has allowed the evolution of the classification of the different types of EC to guide management.10,11 Initially divided broadly into types 1 and 2 on the basis of pathology,12 The Cancer Genome Atlas (TCGA) has further classified EC into four molecular subtypes including POLE-mutant, MMRd, copy number low (CNL) and copy number high (CNH); POLE-mutant tumors being those with the best prognosis and CNH those with poor prognosis.13 Alternate algorithm based on immunohistochemistry (IHC) has been studied to implement in practice the TGCA classification.14,15 This deepen molecular characterization is opening a new area for research. This has led to an expansion and shift from the use of standard chemotherapy to promising targeted therapy. As the era of personalized oncology arises, EC has a great likelihood to benefit from novel agents and combinations currently under development that hit one or multiple druggable molecular alterations. The identification of potential targets that can be therapeutically exploited is a pressing need to improve patient selection and maximize timely drug development.16 Thus, this review will focus on new treatment opportunities and discuss potential implications.
EC Molecular Classification
The EC molecular classification has shown to impact outcomes and is currently used to guide the development of targeted therapies and thus personalized medicine.
TCGA classification
Four molecular subgroups, with a unique genetic profile.17
POLE-Mutated Group
This group is rare, representing approximately 5–10% of all EC patients,17,18 with a lower incidence in advanced EC19 and different dataset reporting excellent outcomes. This group is characterized by mutations in the exonuclease domain of POLE, a catalytic subunit of DNA polymerase epsilon, responsible for high fidelity replication.12,17 Eleven well-defined pathogenic POLE mutations have been associated with the mutated phenotype including P286R and V411L, which are the most common.17 Most of the patients have an endometrioid subtype and frequent mutations include alterations in PTEN (94%), PIK3CA (71%), PIK3R1 (65%), ARID1A (75%), TP53 (35%) and KRAS (50%). The expression of hormone receptors is variable within this group.12,17 Tumors possess an enhanced cytotoxic T-cell response and high neoantigen loads, suggesting they could be good candidates for ICI.17,20–22 Given favorable prognosis and sustained salvage rates, it raises the possibility to consider de-escalation therapy such as targeted therapy as a new option for these patients.18
MMRd Group
Mismatch repair (MMR) pathway is a system through which tumor cells repair DNA damage and maintain genome integrity. The acquisition of an alteration in this pathway can be by two mechanisms: mutation (somatic or germline (Lynch syndrome)) or epigenetic silencing (MLH1 gene promoter hypermethylation). The majority of cases of MMRd (75%) are caused by acquired promoter hypermethylation and the rest due to mutations.23 The MMR pathway comprises specific repair enzymes which are dependent on four main genes: MLH1, PMS2, MSH2 and MSH6. If one of the genes has a mutation, this results in the accumulation of multiple defects within the genome leading to tumorigenesis.17 EC patients with defects in the MMR pathway are known to harbor diverse somatic mutations, especially in regions of repetitive DNA (microsatellites) and their accumulation is known as microsatellite instability (MSI).12,20,21 Therefore, MSI (phenotype) refers to the cellular phenotype of hypermutability that is a consequence of MMRd (genotype).17
This group accounts for 20–30% of EC patients, mainly endometrioid, and has a ~10-fold greater mutation frequency than that of MMR proficient (MMRp) patients.12,20,21 Frequent reported mutations include PTEN (75–85%), PIK3CA (50–55%), PIK3R1 (30–40%), KRAS (35%) and ARID1A (35–40%). Few patients have alterations in TP53 (less than 5%). Up to 10% of patients can have ERBB2 alterations.12 Patients in this group are thought to have a large number of somatic mutations due to MMR defects, leading to high neoantigen loads and high infiltration of CD8+ T lymphocytes, which make them excellent candidates for ICI (immunogenic tumors) as demonstrated in clinical trials.17,20,21
CNL/Endometrioid-Like Group
CNL group is the most common accounting for 39% of cases and currently lacks a distinct molecular pattern (no specific molecular profile; NSMP). It has a low mutational burden, and its prognosis is more limited compared to MMRd and POLE-mutant tumors.12 Mutations especially involve CTTNB1 (>50%), followed by PTEN and PIK3, while TP53 mutation is unlikely present in this group of patients.12,20 Most tumors are low-grade and have a higher expression of estrogen and progesterone receptors, which could make them more responsive to endocrine therapy. It has also been identified an increased expression of RAD50, which is associated with DNA repair pathway.20 Given the diverse landscape, this is the most heterogeneous group that requires specific targeted therapies and further characterization.17 For instance, this group may benefit from combination therapies including mammalian target of rapamycin (mTOR) inhibitors, endocrine therapy and inhibition of WNT/beta catenin pathway.20
CNH/Serous-Like Group
Almost one quarter of EC patients is part of this group, including non-endometrioid subtypes (serous) and grade 3 endometrioid cases. They are characterized by extensive copy number alterations and few DNA methylation changes. Invariably, TP53 mutation is present among them (>90%) and could require escalation of treatment to improve outcomes.12,20 In CNH group, patients have also mutations in FBXW7, PIK3 and PPP2R1A. PI3K/AKT/mTOR pathway is affected in 50–60% and ERBB2 alterations are observed in 25% of the patients, which can be potential targets for new combinations.12,20
Classification Implementation in Clinic
Different groups have validated an equivalent molecular analysis to TCGA whole genome sequencing using feasible techniques of IHC and Sanger or next-generation sequencing (NGS) analysis.17 For example, Proactive Molecular Risk Classifier for Endometrial Cancer (PRoMisE) classification is a system based on the TCGA genomic subgroups that assigns patients to 1 of 4 groups based on a combination of mutation and protein expression (single-classifier). This system makes use of surrogate markers to generate a stepwise path to stratify patients molecularly and looks at POLE alterations, MMR status and p53 status (Figure 1).14,15,24–28 Approximately, 3–6% of EC exhibit more than one molecular classifying signature, which are known as multiple-classifier.14 Within a cohort of 3518 tumors, 3% were multiple-classifier, with most of them being MMRd (MMRd/p53 abnormal), followed by POLE-mutated (POLE-mutated/p53 abnormal) and the minority had the three aberrations. The first two groups were mostly grade 3 endometrioid EC and had morphological features of MMRd or POLE-mutated. P53 overexpression was found to be subclonal in the majority of cases and similar mutational changes to single-classifier MMRd or POLE-mutant were noted, suggesting TP53 was a late and passenger event acquired during progression. Clinical outcomes of patients with MMRd/p53 abnormal and POLE-mutated/p53 abnormal were superior to those with single-classifier p53 abnormal.29
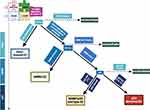 |
Figure 1 ProMisE classification for endometrial cancer. The Proactive Molecular Risk Classifier (ProMisE) is a decision tree analysis and the order of stratification differs from TCGA analysis. Analyses are sequentially ordered from POLE status to MMR status and finally p53 immunohistochemistry. The first step is to assess for POLE pathogenic mutations, if present, the tumor is classified as POLE-mutated. If absent (POLE wild type) or non-pathogenic mutations are detected, tumors are evaluated for MMR by IHC and classified as deficient (MMRd) or intact (MMR proficient; MMRp). Then, p53 status is evaluated. If p53 IHC is marked as 0 or 2+ is then classified as p53 abnormal and p53 wild type/NSMP if p53 IHC shows normal pattern (1+). This system has been confirmed and validated previously.28 Adapted from Jamieson A, Barroilhet L M, McAlpine J N. (2022). Molecular classification in endometrial cancer: Opportunities for precision oncology in a changing landscape. Cancer, 128(15), 2853–2857. © 2022 American Cancer Society.28 Created with Biorender.com. |
Other EC Characterization
As the data are evolving, new biomarkers are identified including HER2, ER/PR status, CCNE1 amplification and will be further developed below. In addition, efforts are on-going to characterize the different potential targets beyond the tumor tissue, with data emerging on circulating tumor (ctDNA) as another tool to identify potential targets in a timely fashion. A correlation between the mutational landscape of primary EC with ctDNA levels was analyzed in 38 patients with newly diagnosed EC. Mutations detected in plasma were representative of those present in primary tumor, with 92% of primary tumor mutations detected in the ctDNA at diagnosis.30 Efforts are under way to define the role of ctDNA for molecular monitoring, detection of progression and possible impact on treatment approaches.31 Depending on the technique, detection of ctDNA varies between cohorts from 18% to more than 65% in EC patients.32,33 Longitudinal ctDNA samples from 25 patients on ICI, including 12 relapsed EC, showed that ctDNA dynamics predicted clinical benefit. The rate of ctDNA detection while on-treatment was 68% and all 7 patients with increased ctDNA had consistently progressive disease, with an average time of 2.5 months prior to imaging.34 A second cohort also showed that ctDNA detected recurrence and progression earlier than imaging studies with a median lead time of 2.5 months in 13 EC patients and ctDNA levels accurately mirrored the radiological response in 3 patients.32 Serial measurements of ctDNA were shown as useful to monitor response to treatment and identification of early relapses in a cohort of 16 serous EC and carcinosarcoma patients. During the longitudinal tumor ctDNA monitoring, they found that one patient lost genetic mutations originally identified in the primary tumor, while others remained, suggesting that clonal evolution under the selective pressure of treatment may modify the genomic landscape in EC and can be detected by ctDNA.35 Therefore, ctDNA represents a promising tool that could closely mirror the clinical and genetic course in EC and could guide treatment decisions.31,35 Further studies are on-going to assess this promising tool in EC.
Novel Molecular Targets
As knowledge of molecular characterization expands, additional markers and targets with potential treatment implications are being identified for specific subgroups of EC (Figure 2).17 Overlapping and crosstalk between the different pathways involved in EC makes the combination of novel agents feasible in order to develop effective therapies.16
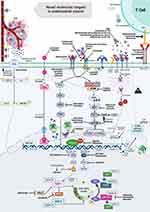 |
Figure 2 Novel molecular targets in endometrial cancer (EC). Numerous pathways are involved in EC and they have become targets of interest in the development of current therapies. Different agents have been studied alone or in combination approaches to target cross-talk pathways including PI3K/AKT/mTOR, HER2, DNA damage response pathway, hormone receptors, cancer microenvironment (ie immune cells, cytokines, angiogenic factors, growth factors), and metabolic vulnerabilities and improve drug delivery by leveraging cancer cell surface antigens. A deepened understanding of the molecular subtypes of EC, genomic diversity and oncogenic drivers will improve translational research and derive in recognition of mechanisms of resistance and molecularly driven therapy.16 Created with Biorender.com. |
Targeting the Cancer Cell Microenvironment
ICI as a Game Changer in EC
The MMRd identification subgroup has led to a significant paradigm change in the landscape of therapy. MMRd tumors have a high neoantigen load, and as a result, there is high infiltration of CD8+ T lymphocytes.
Pembrolizumab and dostarlimab have been approved as treatment for patients with metastatic, MMRd solid tumors that have progressed following prior platinum-based chemotherapy. KEYNOTE-158 study demonstrated a compelling objective response rate (ORR) of 48% for the EC cohort (n = 79) and the median duration of response (DOR) was not reached.6 Consistently, the GARNET trial showed an overall ORR of 43.5% including 11 patients with complete response in 108 MMRd EC patients that received dostarlimab.7 A third trial also showed an ORR of 48% in 36 patients treated with durvalumab.36 Thus, current evidence supports the adoption of ICI in patients with recurrent and advanced endometrial cancer with MMRd after failure of first-line chemotherapy, ICI naïve.37
In the MMRp group, single-agent ICI post-platinum-based chemotherapy have shown limited ORR between 3 and 15%; yet, MMRp are a very heterogeneous group with specific genomic features and represent the majority of EC patients (75%).37 Leveraging ICI’s activity, combination approaches have been explored including antiangiogenic therapy, poly ADP ribose polymerase inhibitors (PARPi) and chemotherapy. All of them can induce multiple changes within the tumor microenvironment and cell population enhancing the immune response. Antiangiogenic agents can reduce T-regulatory cell activity, reverse immunosuppressive effects of vascular endothelial growth factor (VEGF) and improve T-cell trafficking and infiltration of CD-8+ T cells.38 PARPi can enhance DNA damage and increase CD8+ T cells as well. This is mediated by the STING pathway.39 Chemotherapy agents can enhance immunogenic cell death, induce an effective presentation of tumor-specific antigens and increase T-cell activation by dendritic cells.40
Pembrolizumab in combination with lenvatinib has changed the landscape for treatment in MMRp EC patients after they have progressed on platinum-based chemotherapy. Initial evaluation of this combination was a single-arm phase 2 trial that enrolled 94 patients with MMRp EC, the ORR was 37%, and median progression-free survival (PFS) was 7.4 months.9,37 Later on, Study-309/KEYNOTE-775, a phase 3 trial (n = 827 with recurrent EC), confirmed a significant benefit in MMRp (n = 697) in PFS (HR 0.60; 95%CI, 0.50–0.72) and overall survival (OS) (HR 0.70; 95%CI, 0.58–0.83) with this combination. Benefit in survival in all comers was also demonstrated.8 A translational randomized phase II study of cabozantinib with or without nivolumab in heavily pretreated recurrent EC reported an ORR of 25% with the combination (Arm A, n = 36) and ORR of 11.1% with nivolumab (Arm B, n = 18), suggesting a benefit by combining antiangiogenic agents and ICI as previously noted in KEYNOTE-775.41 The combination of atezolizumab and bevacizumab in patients with recurrent EC (1–2 prior lines) similarly yielded durable responses with an ORR of 30% (n = 57) and for those patients with MMRp tumors of 33%. The median DOR was 15 months in the overall population.42
PARPi have also shown positive signal of response in this context when given in combination with ICI. A phase 2 trial reported an ORR of 11.4% (n = 35) in MMRp EC patients with avelumab and talazoparib. They found that those patients with homologous recombination repair (HRR) pathway alterations had a better PFS (p = 0.03) compared with non-HRR-altered tumors.43 Conversely, DOMEC trial explored the role of durvalumab and olaparib in 50 recurrent EC patients, 20% of them with MMRd tumors and reported an ORR of 16%, which did not reach the threshold for significance, with no difference according to molecular subtype in PFS (p = 0.67).44
In line with this, a non-randomized phase 2 study (NEC trial) also showed similar outcomes in 22 recurrent EC patients that received dostarlimab and niraparib with a modest activity with the combo (ORR 14%).45 Given the limited benefit, the incorporation of additional agents has been investigated. A recent preliminary report showed that combining bevacizumab, atezolizumab and rucaparib was feasible by demonstrating a clinically meaningful improvement in response in 30 patients (ORR 39%) with acceptable toxicity.46 Furthermore, incorporating this strategy earlier on the patient journey has been explored in the DUO-E trial (n = 718). It reported positive results with the upfront combination of platinum-based chemotherapy ± durvalumab, followed by maintenance therapy with either durvalumab plus olaparib or durvalumab alone or placebo in advanced or recurrent EC. It elicited a statistically significant improvement in median PFS in the durvalumab + olaparib arm for the intent-to-treat population (HR 0.55; 0.43 to 0.69; P<0.0001) vs control. An exploratory analysis showed that 97 of 718 had HRR mutations (defined as a sample with a pathogenic mutation in any of the following pre-specified genes: ATM, BRCA1, BRCA2, BARD1, BRIP1, CDK12, CHEK1, CHEK2, FANCL, PALB2, RAD51B, RAD51C, RAD51D, RAD54L). HRR-mutated patients that received olaparib and durvalumab showed a positive signal in PFS (HR 0.30; 0.15 to 0.58) compared to non-HRRm, even though, it was a very small sample and further data is needed.47
Lastly, the combination of ICI with platinum-based chemotherapy has recently been explored in phase 3 randomized studies given their immunogenic synergistic effect. RUBY study included 494 patients with advanced or recurrent (if chemotherapy-free interval was at least 6 months) EC that were randomly assigned to receive either dostarlimab or placebo, plus carboplatin and paclitaxel for 6 cycles, followed by dostarlimab or placebo for up to 3 years. In the MMRd population (118/494), median PFS at 24 months was 61.4% in the dostarlimab group and 15.7% in the placebo group (HR 0.28; 95% CI: 0.16–0.50; P < 0.001). In the overall population, there was also a benefit in PFS (HR 0.64; 95% CI: 0.51–0.80; P < 0.001). OS at 24 months for all comers was 71.3% with dostarlimab and 56.0% with placebo (HR 0.64; 95% CI: 0.46–0.87).4 The second trial (NRG-GY018) included 846 patients with advanced or recurrent (if chemotherapy free-interval was at least 12 months) EC (carcinosarcoma excluded). They had a consistent benefit in PFS at 12 months with the addition of pembrolizumab to carboplatin and paclitaxel compared to placebo in the MMRd cohort (74% vs 38%, HR 0.30; 95% CI: 0.19–0.48; P<0.001), and in the MMRp cohort as well (HR 0.54; 95% CI: 0.41–0.71; P < 0.001).5 Preliminary results from the combination of atezolizumab with chemotherapy (Attend/ENGOT-EN7) showed benefit in PFS as well (HR 0.74, 95%CI: 0.61–0.91; p = 0.0219) in all comers.48 Interestingly, exploratory molecular analyses were performed on 400 of 495 patients of RUBY study, of which 1.3% had POLE mutations, 22.8% MMRd, 22% TP53 mutations and 54% NSMP. PFS according to molecular subtypes was better in the MMRd (HR 0.31; 95%IC: 0.17–0.56) and TP53 mutated (HR 0.41; 95%IC: 0.2–0.82) with dostarlimab and chemotherapy, yet these results are exploratory and require additional investigation.19 Ongoing clinical trials studying ICI with different combinations and settings in EC will continue paving the treatment pathway for these patients.
Antiangiogenic Therapy
Antiangiogenics have been actively explored alone or in combination with other agents in EC. Translational research demonstrated that proangiogenic gene expression and high microvessel density could predict poor outcomes according to histologic and molecular groups in EC. Modest efficacy (10–15%) was reported across different single-agent antiangiogenic agents.49 Bevacizumab is well known in gynecological cancer and is a monoclonal antibody against VEGF-A. In 52 recurrent EC patients with 1–2 prior lines of therapy, a phase 2 trial showed that single-agent bevacizumab yielded an ORR of 13.5% and median PFS of 4.2 months.50 This agent has also been investigated along with chemotherapy. GOG86P, a phase 2 trial of 349 EC patients evaluated bevacizumab in combination with chemotherapy, chemotherapy plus temsirolimus or ixabepilone, carboplatin and bevacizumab. PFS was not significantly increased in any of the arms compared to historical controls.51 Of note, patients with TP53 mutations (44%) had a striking treatment effect favoring bevacizumab-containing arm (PFS HR 0.46, 95% CI, 0.26–0.88; OS HR: 0.31, 95% CI, 0.16–0.62) compared to temsirolimus. TP53 mutations included missense or p53 null tumor; however, the sample was too small to segregate both alterations, so whether which one affects outcomes was not clear. Regardless of the type of alteration, the regulation of the cell cycle is dysregulated, and angiogenesis may be enhanced in EC cells.52 MITO END-2 trial (n = 108) demonstrated a PFS of 13.7 months and ORR of 74.4% in patients with recurrent EC and no more than 1 prior line of treatment that received chemotherapy plus bevacizumab.53
Small-molecule multitargeted tyrosine kinase inhibitors (TKI) have been considered as well in this population. In a phase 2 trial, patients with recurrent or advanced EC or carcinosarcoma had an ORR of 18.1% (n = 33) with sunitinib; 7 of them had control of the disease for over a year. Toxicity was a limitation as 52% of patients required dose reductions.54 Further studies with single-agent TKI showed ORR of 9.4% with sunitinib (n = 32), 14.3% with lenvatinib (n = 133), 14% with cabozantinib in endometrioid subtype (n = 36), 12% with cabozantinib in serous subtype (n = 34) and with cediranib an ORR of 12.5% in 48 patients with recurrent EC after 1–2 prior regimens.20,55 Combining antiangiogenic agents with other targeted therapies is an active area of current research.
Leveraging the Cancer Cell Pathways Alterations
Endocrine Therapy
Given the favorable safety profile, endocrine therapy is an appealing option for treatment, and it has been used for several years; yet, large randomized trials are lacking as well as validated biomarkers (definition of estrogen and progesterone receptor (ER and PR) status).49 Endocrine therapy is well tolerated and has ORR of 9 to 33%.56 For unselected population, progestins used as first-line therapy for recurrent and advanced EC showed an ORR of 23.3% and PFS of 2.9 months.57 A subset of patients may benefit in the long term but further investigation is needed for decision-making and to identify predictive factors for response beyond hormone receptors and low-grade tumors.49 High response rates have been seen especially among tumor grade 1 (ORR 38%) as reported by GOG-153 that evaluated tamoxifen alternated with megestrol acetate.58 Estrogen receptor agents and aromatase inhibitors (AI) have exhibited limited ORR from 9 to 17%.56 As such, combination therapies are preferred. AI have been found to be complimentary with therapies that target downstream molecular pathways, where there is a crosstalk between PI3K/AKT/mTOR pathway and endocrine signaling.49,56 Letrozole and everolimus have been evaluated in small studies showing an ORR of 22–32%, with the greatest benefit in chemo-naïve patients, and manageable toxicity.56 Recently, in a phase I/II study of 49 heavily pretreated patients, vistusertib (mTOR inhibitor) plus anastrozole showed an ORR of 24.5% and median PFS of 5.2 months.59
In addition to combination with mTOR inhibitors, cyclin-depending kinase (CDK) 4/6 inhibitors are being tested in EC given its elevated expression in 35 to 77% of endometrioid EC.49,56 The combination of these inhibitors with endocrine therapy has been widely explored in breast cancer. One of the first studies in EC assessed the combination of letrozole and ribociclib (third-generation CDK4/6) and showed that 55% of patients (n = 20) with ER+ EC were on treatment at 12 weeks and 35% at 24 weeks. However, up to 60% had at least one grade 3 adverse event. A second phase II trial evaluated letrozole with abemaciclib in 30 heavily pretreated ER+ patients and the ORR was 30% with a median DOR of 7.4 months. Palbociclib has also been assessed in combination with letrozole (n = 36), which demonstrated an improved PFS (8.3 months) compared to letrozole alone (3 months; HR 0.56; 95%CI: 0.32–0.98).56 Continued research efforts to target hormone receptor positive tumors may focus on defining the hormone receptor status threshold, combining diverse agents and overcoming escape pathways in a selected group of patients like CNL group.
PI3K/AKT/mTOR Pathway
Among molecular targeted therapies that have been studied in EC, PI3K/AKT/mTOR pathway inhibitors have been one of the main agents extensively studied. This pathway is involved in proliferation, metabolism, migration, and survival of tumor cells. It is a frequent activated pathway in EC, especially in endometrioid tumors that harbor more than 90% of mutations within this pathway. Single-agent inhibitor targeting one of the genes involved in this pathway has reported a modest response rate in early phase trials.12,20 Temsirolimus, a mTORC1 inhibitor, has been one of the most studied drugs with response rates between 4% and 24% and median PFS up to 24% at 6 months.20 Novel inhibitors of PI3K pathway have been studied, including pilaralisib, which showed ORR of 6% and PFS at 6 months of 11.9% in recurrent EC patients with up to 2 prior lines of therapy. Another PI3K inhibitor has been explored, BKM120, in a phase 2 trial that reported a median PFS of 3.4 months; yet, accrual was stopped due to a high proportion of grade 3/4 toxicities.12,60 One of the limitations of this group of inhibitors has been diverse signaling feedback loops and cross-talk between different pathways including RAS/RAF/MEK, MAPK and estrogen receptor pathways.20 Moreover, they are considered to be poor inhibitors of the pathway with more a cytostatic effect than cytotoxic, and KRAS mutations could also play a role in this regard.12,61
AKT inhibitors have also been explored as a way to inhibit this pathway more efficiently upstream. MK-2206 was tested in 36 patients showing only 1 partial response and almost 40% of patients required dose reduction. Following this, AZD5363, a pan-AKT kinase inhibitor, was given to heavily pretreated patients with gynecologic tumors with AKT1 E17K mutations (present in 2% of EC) and achieved a median PFS of 6.6 months.60 As part of the NCI-MATCH study, 32 patients with the same molecular alteration received ipatasertib, a pan-AKT inhibitor, and achieved an ORR of 22% including one patient with endometrioid EC.62 This pathway is an attractive target in EC; yet, it has been challenging to develop agents given toxicity, cross resistance as monotherapy, but further innovative drug design, patient selection and alternative combinations are an ongoing field of interest.
HER2 Pathways
Human epidermal growth factor receptor 2 (HER2) is a receptor tyrosine-protein kinase encoded by ERBB2 which has also been studied as a therapeutic target for EC treatment. ERBB2 is amplified or overexpressed in 17–33% of uterine carcinosarcomas, serous EC and a subset of high-grade endometrioid EC. Its activation leads to signal transduction through PI3K and MAPK pathways which in turn induces proliferation, angiogenesis and survival of EC cells.20 Trastuzumab, a humanized-monoclonal-antibody targeting Her2/Neu, has been studied in 61 advanced/recurrent serous EC in combination with carboplatin and paclitaxel in a phase II trial showing clinical benefit.63 Patients with recurrent EC and ERBB2/3 amplification, overexpression or mutations were enrolled in TAPUR study (n = 28), a pragmatic basket trial, and treated with pertuzumab and trastuzumab. This combination showed preliminary activity with an ORR of 7%, disease control of 37% and median PFS of 16 weeks.64 A phase 2/3 trial (NCT05256225) is currently looking at the combination of pertuzumab, trastuzumab and chemotherapy in serous EC and carcinosarcoma overexpressing HER2.
HER2/neu is also an interesting target for antibody drug conjugate (ADC) development. Ado-trastuzumab emtansine (T-DM1) was the first ADC against HER2 linked with emtansine (microtubule inhibitor) to become available and was studied as part of NCI-MATCH study in 38 patients with HER2-amplified tumors. Stable disease was achieved in 47% including 8/10 with ovarian and uterine carcinomas; however, it did not meet the primary endpoint for ORR (5.6% for entire cohort).65 Thereafter, the therapeutic landscape of trastuzumab-drug conjugates continues evolving from TDM-1 to novel next-generation ADC which have shown positive results. Trastuzumab-deruxtecan (T-Dxd) is a next-generation ADC composed of human antibody against HER2, a cleavable linker and a highly potent 11qatopoisomerase I inhibitor (DXd). Early results from the STATICE trial showed a response rate of 55% in recurrent HER2-positive uterine carcinosarcoma (n = 22). Interestingly, it also showed activity in HER2-low positive tumors (n = 10) with an ORR of 70%. Toxicity was consistent with previous reports of breast cancer, including grade 3 interstitial lung disease in one patient.66 Destiny-PanTumor02 study included 40 patients with EC not eligible for curative therapy with HER2 positive expression (IHC 3+ or 2+). ORR in EC cohort was 57.5% with T-Dxd, those with expression IHC 3+ had an ORR of 84.6% while those with IHC2+ of 47.1%.67 Based on this promising early activity, this agent is currently being explored in other prospective studies. DB1303, another ADC consisting of a humanized anti-HER2 antibody covalently linked to a topoisomerase I inhibitor (P1003), has shown preliminary antitumor activity (ORR 44.2%, 23/52) including EC (ORR 33.3%, 1/3) in a phase I/II.68 Current evidence from ADC against HER2 is encouraging and further development is in progress to improve personalized care.
Targeting the DNA Damage and Cell Cycle Pathway
Given the initial good response to platinum-based chemotherapy in some patients, targeting DNA repair pathway is an interesting approach. Recently, the phase IIb UTOLA trial explored the role of olaparib vs placebo as maintenance therapy after achieving control of the disease with first-line platinum-based chemotherapy in advanced EC, showing no benefit in the intent to treat population. However, in the exploratory analyses, a benefit in PFS was observed (n = 73; PFS 5.4 vs 3.6 months, p = 0.02) in those with HR-deficient tumors (defined by number of large genomic events) with olaparib.69 Of note, the reports about HR deficiency rates in EC are so variable, from 15 to 53%, without a clear definition of this phenotype. They are more frequent in serous subtype and TP53-mutated tumors.17 Future research is needed to clarify the clinical implication of HRR mutations and/or HR deficiency in EC and to establish a standardized definition.
Targeting replication stress (RS) and cell cycle control is also of special interest in EC, particularly in the serous subtype.45 Elevated levels of RS trigger DNA damage response sensors including WEE1-like protein kinase (WEE1), protein kinase membrane associated tyrosine/threonine 1 (PKMYT1), ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related kinase (ATR), which promote cell cycle arrest while allowing cell reparation and survival. Thus, the inhibition of this pathway induces mitotic catastrophe and cell death. WEE1 kinase is a key regulator of the S and G2/M phase cell-cycle checkpoints and inactivates cyclin E1 (CCNE1) to control DNA replication. CCNE1 amplification is frequent in gynecologic tumors including EC and carcinosarcoma.17 A non-randomized phase II study of WEE1 inhibitor adavosertib showed promising clinical activity (ORR 29.4%) and PFS at 6 months of 47.1% in 34 evaluable patients with recurrent serous EC.70 Recently, ADAGIO phase 2b study showed an ORR of 26% and median DOR of 4.7 months with adavosertib as single agent in 104 heavily pretreated serous EC patients. However, tolerance was a limitation with 68.8% of patients with > grade 3 adverse events.71 Tumors with ATM or p53 deficiency are highly dependent on the ATR/CHK1/WEE1 pathway to be able to repair DNA. ATR plays a key role in the DNA damage response as well, and its inhibition could affect genomic instability. Confirmed partial responses were observed in 3 patients with EC and 1 with anal cancer (n = 49) with ART0380 (ATR inhibitor) in a phase I study.72 CHK1/2 participate in the cell cycle by inhibiting S and G2/M checkpoints and allowing repair of DNA. GDC-0575, a CHK1 inhibitor, was tested alone or in combination with gemcitabine in a phase 1 trial in refractory solid tumors (n = 90) including 4 EC patients and showed clinical benefit in 66%, but overall tolerability with gemcitabine was limited.73 PKMYT1 inhibition disrupts the G2/M checkpoint leading to premature mitosis and catastrophic DNA damage. In a phase I trial (MYTHIC), lunresertib (PKMYT1 inhibitor) was tested alone or in combination with camonsertib (ATR inhibitor) in resistant solid tumors with CCNE1-amplified, deleterious FBXW7 or PPP2R1A, of which 40 had EC. Preliminary response with the combination was seen in gynecologic cancer.74 Further studies are needed in a selected population.
Metabolic Vulnerabilities in EC
Metabolic reprogramming is defined as a complex process in which rewiring of amino acid, glucose and lipid leads to tumor cell growth and metastasis; an area of study in EC. Cancer metabolic process is modulated by multiple factors and pathways, including PI3K/AKT signaling network that possesses various downstream impacts on the metabolic process. Diverse adipokines have direct effects on tumor proliferation and metastasis in patients with EC and insulin resistance; the latter has been identified as a potential link to EC. It has been hypothesized that estrogen along with insulin favor proliferation of endometrial tumor cells via PI3K/AKT and MEK/ERK signaling.75 Based on these changes, metformin has been explored in vitro, which blocks the proliferation of EC cells through activation of AMPK, upregulation of Hippo pathway, suppression of PD-L1 and subsequent inhibition of mTOR pathway.75,76 Metformin has been evaluated in different solid tumors, with often disappointed outcomes.76 A phase II study assessed the combination of metformin, letrozole and everolimus in patients with endometrioid EC and less than 2 prior lines, and showed an ORR of 28% (15/54) and stable disease of 50% (12/54), with no differences in outcomes according to PTEN, PI3KCA or KRAS alterations.77 ENDOLA study, a prospective phase I/II study, assessed the efficacy of olaparib combined with metformin and metronomic cyclophosphamide in 31 pre-treated EC patients and found an ORR of 20.8% and disease-control rate of 66.6%.78 Metformin may have a synergistic activity with PARPi through direct mechanisms (insulin-independent) and PIK3CA/AKT/mTOR pathway (indirect).76
GLP-1R agonists have been recently studied in oncology, and they might exert an effect on cancer growth and progression and reverse chemoresistance in different solid tumors.79–81 They might induce the expression of GLP-1R in EC cells and inhibit cancer cell growth, via the AMPK signaling pathway.79 SGLT2 inhibitors have been associated with an anti-cancer effect representing an advantageous option for the development of novel therapies.82 Further clinical evidence will clarify the role of the antihyperglycemic drugs in EC and the potential role if tested earlier in the patient journey.
Other Potential Targets in Recurrent EC
Epidermal growth factor receptor (EGFR) activation transduces multiple-signaling pathways and its complex interplay with them impact the function of relevant downstream elements. The interaction with PI3K pathway is of critical importance in EC, and given EGFR is overexpressed between 43 and 67%, previous trials have tried to inhibit its activity.83 However, among EGFR inhibitors, erlotinib, cetuximab and lapatinib reported discouraging results.12,20
MEK activation can lead to the phosphorylation of ERK1, induce proliferation and survival of tumor cells. Selumetinib, a selective MEK1/2 inhibitor, was studied in the treatment of recurrent EC but had a very limited ORR of 6%.20 Given poor response, the combination of an MEK inhibitor (trametinib) with AKT inhibitor (GSK2141795) was explored in a phase I trial but showed high levels of toxicity.61
The karyopherin protein exportin-1 (XPO-1) is responsible for the nuclear export of multiple proteins including p53 and growth and cell cycle proteins (p21 and p16). Selinexor is a selective inhibitor of nuclear export compound, which blocks XPO1 activity, leading to nuclear accumulation and reactivation of p53. This agent was tested as maintenance after response to platinum-based chemotherapy in EC and showed a median PFS of 5.7 vs 3.8 months with placebo (HR 0.76, 95%CI: 0.54–1.08; p = 0.126). Patients with TP53-wild type EC had a median PFS of 13.7 and 3.7 months with selinexor and placebo (HR 0.41, 95%CI: 0.23–0.72, p = 0.002), which was a prespecified exploratory subgroup analysis.84 A phase 3 study is ongoing to assess selinexor as maintenance treatment for patients with recurrent TP53-wild type EC (NCT05611931).
Leveraging the Cancer Cell Surface Antigen to Improve Drug Delivery
Antibody-drug conjugates (ADC) combine the tumor-targeting capabilities of monoclonal antibodies with cytotoxic agents and have been explored in advanced or recurrent EC. Different tumor antigens have been identified as potential targets.
Tisotumab vedotin is a monoclonal antibody targeting tissue factor (TF) combined to the microtubule-disrupting agent monomethyl auristatin E. TF is overexpressed in around 50% (14–100%) of patients with EC, and preliminary results have been reported as part of the phase 1/2 basket InnovaTV trial. An ORR of 7% (1/14) in the EC cohort was outlined with a manageable toxicity profile.85 Folate receptor (FR) α is expressed in approximately 64% of EC, and anti-FRα ADC have been developed including mirvetuximab soravtansine.86 In a phase I study of FRα-positive solid tumors, 2 of 11 endometrial cancer patients at a dose of 5 mg/kg showed a response.87 Ongoing trials are evaluating mirvetuximab soravtansine in EC in this context (NCT03835819 and NCT03748186). Luveltamab tazevibulin is a novel anti-FRα ADC and initial efficacy data showed an ORR of 37.5% (6/16) in recurrent EC with acceptable toxicity.88
Trophoblast Cell Surface Antigen-2 (Trop2) is a tumor-associated calcium signal transducer that is overexpressed in over 90% of endometrioid and 65% serous EC. Sacituzumab govitecan is an ADC targeting Trop2 recently developed. In a phase I/II basket trial, within the EC cohort with Trop2 overexpression (n = 21), an ORR of 33% and median PFS of 5.7 months were reported.89 B7-H4 is a B7 immune checkpoint ligand upregulated in EC in up to 70% of patients and especially among those with CNL or NSMP tumors.90 SGN-B7H4V is a vedotin ADC targeting this marker, and the first results from Part A of an ongoing phase I study showed that 1 of 16 patients with EC had an ORR to this agent irrespective of B7-H4 expression.91
ADC represents a rapidly evolving field of targeted therapy which have shown early clinical benefit with acceptable toxicity and larger trials are planned. Future generation of ADC, including bispecific ADC, dual or immune stimulating payload and radionucleotide ADC, and recognition of new surface targets, offer novel perspectives for investigation.92
Perspectives for Targeted Therapy
The landscape of EC is changing at great speed with more options available for patients based on disease characteristics. As we lean towards precision medicine, it is important to ensure that this benefit is observed for everyone; yet, trials inclusion do not necessarily represent the population diversity. For example, efforts are needed to understand why Black women have significantly higher rates of mortality compared with White women.93 Aggressive molecular subtypes from TCGA such as CNH are more common in Black women which conferred a worse PFS.94 Black patients are also more likely to have TP53-mutated tumors that are associated with poor survival.95 Similarly, a significantly higher prevalence of high-risk histologic (serous and carcinosarcoma, p < 0.001) and molecular (CNH/TP53 abnormal, p < 0.001) subtypes of EC in self-identified Black compared with White patients has been noted. CN-H/TP53abnormal EC in Black patients more frequently harbored CCNE1 amplification (21% vs 11%, q < 0.1).96 Real-world data (Endometrial Cancer Molecularly Targeted Consortium) from 994 patients with recurrent/advanced EC who had NGS testing reported that Black patients were more likely to have tumors with TP53 alterations (41.6 vs 26.6%, p < 0.001), and less likely to have PI3K/PTEN alterations (p < 0.001) and tumors with high tumor mutational burden (3.1 vs 7%, p = 0.001).97 Therefore, there is a critical need to address the importance of diversity and ensure population representation in clinical trials.
While interrogation of biological processes can inform new treatment paradigms, the identifiable target may not demonstrate oncogenic addiction, leading to weak interactions between the target and the drug, thus, may not provide therapeutic benefit. In addition to tumor characterization, functional assessment is important as the target needs to be druggable and translate into clinical actionability to circumvent tumor progression and control disease.98 Effective drug engineering requires a comprehensive knowledge of the biology and genomics of EC, identification of druggable/oncogenic targets, effective drug delivery system, targeting specificity of the ligand and tumor tissue targeting (drug accumulation in tumor tissue while reducing uptake in normal tissues).99 In addition to drug development, understanding the mechanisms of intrinsic and acquired resistance in EC is also part of the process to optimize personalized therapy.
With the development of new therapies and the will to cure, combination therapy is often used to maximize dose/intensity to the target. This evaluation needs to be developed alongside patient-reported outcomes to ensure patient benefit and quality of life improvement. As the management of EC is rapidly changing, the question of treatment sequence has not yet been elucidated, including ICI after ICI, impact on further treatment and the need for long-term follow-up.
Conclusion
Whilst EC treatment remains challenging, a better understanding of the genomic diversity, tumor microenvironment and oncogenic drivers has led to innovative therapeutic approaches towards personalized medicine. Emerging areas of therapeutic interest lie in selected patients (molecularly driven therapy) and combination approaches. Additional efforts are needed with robust biomarker identification, resistance mechanism recognition, and further translational research to improve patient’s outcome in every stage of EC. Special interest should be driven towards the importance of clinical trial design, including diversity and patient-reported outcomes.
Authorship credit
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Stephanie Lheureux reports grants or contracts to their institution from Merck, AstraZeneca, Regeneron, Roche, Repare Therapeutics, GSK, and Seagen; consulting fees from Novocure, Merck, AstraZeneca, Roche, Schrodinger, GSK, Eisai, and Shattuck Labs; payment or honoraria for lectures, presentations, speakers bureaus, or educational events from AstraZeneca, GSK, and Eisai; and participation on a data safety monitoring board or advisory board from AstraZeneca. She has been principal investigator or co-investigator of different trials with PARPi and DDR agents. The other author declares no conflicts of interest in this work.
References
1. Liu L, Habeshian TS, Zhang J, et al. Differential trends in rising endometrial cancer incidence by age, race, and ethnicity. JNCI Cancer Spectr. 2023;7(1). doi:10.1093/jncics/pkad001
2. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
3. Tronconi F, Nero C, Giudice E, et al. Advanced and recurrent endometrial cancer: state of the art and future perspectives. Crit Rev Oncol Hematol. 2022;180:103851. doi:10.1016/j.critrevonc.2022.103851
4. Mirza MR, Chase DM, Slomovitz BM, et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med. 2023;388(23):2145–2158. doi:10.1056/NEJMoa2216334
5. Eskander RN, Sill MW, Beffa L, et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N Engl J Med. 2023;388(23):2159–2170. doi:10.1056/NEJMoa2302312
6. O’Malley DM, Bariani GM, Cassier PA, et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: results From the KEYNOTE-158 Study. J Clin Oncol. 2022;40(7):752–761. doi:10.1200/JCO.21.01874
7. Oaknin A, Gilbert L, Tinker AV, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-A phase I, single-arm study. J Immunother Cancer. 2022;10(1). doi:10.1136/jitc-2021-003777
8. Makker V, Colombo N, Casado Herraez A, et al. Lenvatinib Plus Pembrolizumab in Previously Treated Advanced Endometrial Cancer: updated Efficacy and Safety From the Randomized Phase III Study 309/KEYNOTE-775. J Clin Oncol. 2023;41(16):2904–2910. doi:10.1200/JCO.22.02152
9. Makker V, Colombo N, Casado Herraez A, et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N Engl J Med. 2022;386(5):437–448. doi:10.1056/NEJMoa2108330
10. Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer. 2021;31(1):12–39. doi:10.1136/ijgc-2020-002230
11. Berek JS, Matias-Guiu X, Creutzberg C, et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet. 2023;162(2):383–394. doi:10.1002/ijgo.14923
12. Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet. 2016;387(10023):1094–1108. doi:10.1016/S0140-6736(15)00130-0
13. Arciuolo D, Travaglino A, Raffone A, et al. TCGA Molecular Prognostic Groups of Endometrial Carcinoma: current Knowledge and Future Perspectives. Int J Mol Sci. 2022;23(19):19. doi:10.3390/ijms231911684
14. Loukovaara M, Pasanen A, Butzow R. Molecular classification of endometrial carcinoma: a clinically oriented review. J Clin Pathol. 2022;75(11):731–738. doi:10.1136/jclinpath-2022-208345
15. Walsh CS, Hacker KE, Secord AA, et al. Molecular testing for endometrial cancer: an SGO clinical practice statement. Gynecol Oncol. 2023;168:48–55. doi:10.1016/j.ygyno.2022.10.024
16. Westin SN, Broaddus RR. Personalized therapy in endometrial cancer: challenges and opportunities. Cancer Biol Ther. 2012;13(1):1–13. doi:10.4161/cbt.13.1.18438
17. Corr B, Cosgrove C, Spinosa D, et al. Endometrial cancer: molecular classification and future treatments. BMJ Med. 2022;1(1):e000152. doi:10.1136/bmjmed-2022-000152
18. McAlpine JN, Chiu DS, Nout RA, et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: an individual patient data meta-analysis. Cancer. 2021;127(14):2409–2422. doi:10.1002/cncr.33516
19. Herrstedt J, Shahin MS, et al. Dostarlimab + chemotherapy for the treatment of primary advanced or recurrent endometrial cancer (pA/rEC): analysis of progression free survival (PFS) and overall survival (OS) outcomes by molecular classification in the ENGOT-EN6-NSGO/GOG-3031/RUBY trial. Ann Oncol. 2023;34:S507. doi:10.1016/j.annonc.2023.09.1919
20. Mitamura T, Dong P, Ihira K, et al. Molecular-targeted therapies and precision medicine for endometrial cancer. Jpn J Clin Oncol. 2019;49(2):108–120. doi:10.1093/jjco/hyy159
21. Howitt BE, Shukla SA, Sholl LM, et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1(9):1319–1323. doi:10.1001/jamaoncol.2015.2151
22. Mehnert JM, Panda A, Zhong H, et al. Immune activation and response to pembrolizumab in POLE-mutant endometrial cancer. J Clin Invest. 2016;126(6):2334–2340. doi:10.1172/JCI84940
23. Antill Y, Buchanan DD, Scott CL. Mismatch repair and clinical response to immune checkpoint inhibitors in endometrial cancer. Cancer. 2022;128(6):1157–1161. doi:10.1002/cncr.34024
24. Ruscelli M, Maloberti T, Corradini AG, et al. Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma. J Pers Med. 2023;13(5):723. doi:10.3390/jpm13050723
25. Talhouk A, McConechy MK, Leung S, et al. Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer. 2017;123(5):802–813. doi:10.1002/cncr.30496
26. Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113(2):299–310. doi:10.1038/bjc.2015.190
27. Singh N, Piskorz AM, Bosse T, et al. p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol. 2020;250(3):336–345. doi:10.1002/path.5375
28. Jamieson A, Barroilhet L M, McAlpine J N. (2022). Molecular classification in endometrial cancer: Opportunities for precision oncology in a changing landscape. Cancer, 128(15), 2853–2857. 10.1002/cncr.34328
29. Leon-Castillo A, Gilvazquez E, Nout R, et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J Pathol. 2020;250(3):312–322. doi:10.1002/path.5373
30. Ashley CW, Selenica P, Patel J, et al. High-Sensitivity Mutation Analysis of Cell-Free DNA for Disease Monitoring in Endometrial Cancer. Clin Cancer Res. 2023;29(2):410–421. doi:10.1158/1078-0432.CCR-22-1134
31. Grant BM, Pugh TJ, Oza AM. Molecular Monitoring in Endometrial Cancer-Ready for Prime Time? Clin Cancer Res. 2023;29(2):305–308. doi:10.1158/1078-0432.CCR-22-2781
32. Moss EL, Gorsia DN, Collins A, et al. Utility of Circulating Tumor DNA for Detection and Monitoring of Endometrial Cancer Recurrence and Progression. Cancers (Basel). 2020;12(8):2231. doi:10.3390/cancers12082231
33. Lee YJ, Kim SW, et al. 591P Feasibility of longitudinal monitoring of whole blood-based circulating tumour DNA (ctDNA) in patients with endometrial cancer. Ann Oncol. 2022;33:S816–S817. doi:10.1016/j.annonc.2022.07.719
34. Sj CK, Chae YK, Sindhu H, et al. Immunotherapy response monitoring using personalized circulating tumor DNA analysis in patients with relapsed gynecologic malignancies. Int j Gynecological Cancer. 2022;32:A410.
35. Bellone S, McNamara B, Mutlu L, et al. Monitoring Treatment Response, Early Recurrence, and Survival in Uterine Serous Carcinoma and Carcinosarcoma Patients Using Personalized Circulating Tumor DNA Biomarkers. Int J Mol Sci. 2023;24(10):56.
36. Antill Y, Kok PS, Robledo K, et al. Clinical activity of durvalumab for patients with advanced mismatch repair-deficient and repair-proficient endometrial cancer. A nonrandomized phase 2 clinical trial. J Immunother Cancer. 2021;9(6):e002255. doi:10.1136/jitc-2020-002255
37. Di Dio C, Bogani G, Di Donato V, et al. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol Oncol. 2023;169:27–33. doi:10.1016/j.ygyno.2022.11.031
38. Ciciola P, Cascetta P, Bianco C, et al. Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents. J Clin Med. 2020;9(3):675. doi:10.3390/jcm9030675
39. Wu Z, Cui P, Tao H, et al. The Synergistic Effect of PARP Inhibitors and Immune Checkpoint Inhibitors. Clin Med Insights Oncol. 2021;15:1179554921996288. doi:10.1177/1179554921996288
40. Bailly C, Thuru X, Quesnel B. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer. 2020;2(1):zcaa002. doi:10.1093/narcan/zcaa002
41. Lheureux S, Matei DE, Konstantinopoulos PA, et al. Translational randomized phase II trial of cabozantinib in combination with nivolumab in advanced, recurrent, or metastatic endometrial cancer. J Immunother Cancer. 2022;10(3):e004233. doi:10.1136/jitc-2021-004233
42. Rd FK, Arend R, Mccourt C, et al. Atezolizumab and bevacizumab in recurrent endometrial cancer: a phase II multi institutional trial. Int J Gynecol Cancer. 2022;32:A8.
43. Konstantinopoulos PA, Gockley AA, Xiong N, et al. Evaluation of Treatment With Talazoparib and Avelumab in Patients With Recurrent Mismatch Repair Proficient Endometrial Cancer. JAMA Oncol. 2022;8(9):1317–1322. doi:10.1001/jamaoncol.2022.2181
44. Post CCB, Westermann AM, Boere IA, et al. Efficacy and safety of durvalumab with olaparib in metastatic or recurrent endometrial cancer (phase II DOMEC trial). Gynecol Oncol. 2022;165(2):223–229. doi:10.1016/j.ygyno.2022.02.025
45. Madariaga A, Garg S, Tchrakian N, et al. Clinical outcome and biomarker assessments of a multi-centre phase II trial assessing niraparib with or without dostarlimab in recurrent endometrial carcinoma. Nat Commun. 2023;14(1):1452. doi:10.1038/s41467-023-37084-w
46. Bradley HM, Taylor N, Rader JS, et al. An open label, nonrandomized, multisite phase II trial combining bevacizumab, atezolizumab, and rucaparib for the treatment of previously treated recurrent and progressive endometrial cancer. J Clin Oncol. 2022;40(16_suppl):5510. doi:10.1200/JCO.2022.40.16_suppl.5510
47. Westin SN, Moore K, Chon HS, et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: the Phase III DUO-E Trial. J Clin Oncol. 2023;101200JCO2302132.
48. Hk CN, Hudson E, Galli F, et al. LBA49-Phase III double-blind randomized placebo controlled trial of atezolizumab in combination with carboplatin and paclitaxel in women with advanced/recurrent endometrial cancer. Ann Oncol. 2023;34:S1254–S1335.
49. MacKay HJ, Freixinos VR, Fleming GF. Therapeutic Targets and Opportunities in Endometrial Cancer: update on Endocrine Therapy and Nonimmunotherapy Targeted Options. Am Soc Clin Oncol Educ Book. 2020;40:1–11. doi:10.1200/EDBK_280495
50. Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259–2265. doi:10.1200/JCO.2010.32.6397
51. Aghajanian C, Filiaci V, Dizon DS, et al. A phase II study of frontline paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus, or ixabepilone/carboplatin/bevacizumab in advanced/recurrent endometrial cancer. Gynecol Oncol. 2018;150(2):274–281. doi:10.1016/j.ygyno.2018.05.018
52. Thiel KW, Devor EJ, Filiaci VL, et al. TP53 Sequencing and p53 Immunohistochemistry Predict Outcomes When Bevacizumab Is Added to Frontline Chemotherapy in Endometrial Cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol. 2022;40(28):3289–3300. doi:10.1200/JCO.21.02506
53. Lorusso D, Ferrandina G, Colombo N, et al. Carboplatin-paclitaxel compared to Carboplatin-Paclitaxel-Bevacizumab in advanced or recurrent endometrial cancer: MITO END-2 - A randomized phase II trial. Gynecol Oncol. 2019;155(3):406–412. doi:10.1016/j.ygyno.2019.10.013
54. Castonguay V, Lheureux S, Welch S, et al. A phase II trial of sunitinib in women with metastatic or recurrent endometrial carcinoma: a study of the Princess Margaret, Chicago and California Consortia. Gynecol Oncol. 2014;134(2):274–280. doi:10.1016/j.ygyno.2014.05.016
55. Dhani NC, Hirte HW, Wang L, et al. Phase II Trial of Cabozantinib in Recurrent/Metastatic Endometrial Cancer: a Study of the Princess Margaret, Chicago, and California Consortia (NCI9322/PHL86). Clin Cancer Res. 2020;26(11):2477–2486. doi:10.1158/1078-0432.CCR-19-2576
56. Wagner VM, Backes FJ. Do Not Forget about Hormonal Therapy for Recurrent Endometrial Cancer: a Review of Options, Updates, and New Combinations. Cancers (Basel). 2023;15(6):1799. doi:10.3390/cancers15061799
57. Ethier JL, Desautels DN, Amir E, et al. Is hormonal therapy effective in advanced endometrial cancer? A systematic review and meta-analysis. Gynecol Oncol. 2017;147(1):158–166. doi:10.1016/j.ygyno.2017.07.002
58. Fiorica JV, Brunetto VL, Hanjani P, et al. Phase II trial of alternating courses of megestrol acetate and tamoxifen in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92(1):10–14. doi:10.1016/j.ygyno.2003.11.008
59. Heudel P, Frenel JS, Dalban C, et al. Safety and Efficacy of the mTOR Inhibitor, Vistusertib, Combined With Anastrozole in Patients With Hormone Receptor-Positive Recurrent or Metastatic Endometrial Cancer: the Victoria Multicenter, Open-label, Phase 1/2 Randomized Clinical Trial. JAMA Oncol. 2022;8(7):1001–1009. doi:10.1001/jamaoncol.2022.1047
60. Avila M, Grinsfelder MO, Pham M, et al. Targeting the PI3K Pathway in Gynecologic Malignancies. Curr Oncol Rep. 2022;24(12):1669–1676. doi:10.1007/s11912-022-01326-9
61. Westin SN, Sill MW, Coleman RL, et al. Safety lead-in of the MEK inhibitor trametinib in combination with GSK2141795, an AKT inhibitor, in patients with recurrent endometrial cancer: an NRG Oncology/GOG study. Gynecol Oncol. 2019;155(3):420–428. doi:10.1016/j.ygyno.2019.09.024
62. Zw KK, McCourt C, Mitchell EP, et al. Ipatasertib in Patients with Tumors with AKT Mutations: results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) Sub-protocol Z1K. Eur J Cancer. 2022;174:S8–9. doi:10.1016/S0959-8049(22)00824-3
63. Fader AN, Roque DM, Siegel E, et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): updated Overall Survival Analysis. Clin Cancer Res. 2020;26(15):3928–3935. doi:10.1158/1078-0432.CCR-20-0953
64. Rm AE, Mangat PP, Garrett-Mayer E, et al. Pertuzumab Plus Trastuzumab in Patients With Endometrial Cancer With ERBB2/3 Amplification, Overexpression, or Mutation: results From the TAPUR Study. J Clin Oncol Precision Oncol. 2023;7:e2200609.
65. Jhaveri KL, Wang MV, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. 2019;30(11):1821–1830. doi:10.1093/annonc/mdz291
66. Nishikawa T, Hasegawa K, Matsumoto K, et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): the STATICE Trial. J Clin Oncol. 2023;41(15):2789–2799. doi:10.1200/JCO.22.02558
67. Oaknin A, Oh D, et al. LBA34-Trastuzumab deruxtecan (T-Dxd) for pretreated patients (pts) with HER2-expressing solid tumors: primary analysis from the DESTINY-PanTumor02 (DP-02) study. Ann Oncol. 2023;34:S1254–S1335.
68. Sd MKN, Du Y, Li X, et al. Safety and efficacy of DB-1303 in patients with advanced/metastatic solid tumors: a multicenter, open-label, first-in-human, phase 1/2a study. J Clin Oncol. 2023;41:abst 3023.
69. La LFJ, Ray-Coquard IL, Asselain B, et al. LBA42-Olaparib vs placebo as maintenance therapy after platinum-based chemotherapy in advanced/metastatic endometrial cancer patients: the GINECO randomized phase IIb UTOLA trial. Ann Oncol. 2023;34:S1254–S1335.
70. Liu JF, Xiong N, Campos SM, et al. Phase II Study of the WEE1 Inhibitor Adavosertib in Recurrent Uterine Serous Carcinoma. J Clin Oncol. 2021;39(14):1531–1539. doi:10.1200/JCO.20.03167
71. Oza A. ADAGIO: a phase IIb, open-label, single-arm, multicenter study, assessing the efficacy and safety of adavoosertib (AZD1775) as treatment for recurrent or persistent uterine serous carcinoma. Gynecol Oncol. 2023;176:S33. doi:10.1016/j.ygyno.2023.06.512
72. Kn PS M, Flachook GS, Fontana E, et al. 680P-First results from the phase I trial of the ATR inhibitor, ART0380, in advanced solid tumors. Ann Oncol. 2023;34:S458–S497.
73. Italiano A, Infante JR, Shapiro GI, et al. Phase I study of the checkpoint kinase 1 inhibitor GDC-0575 in combination with gemcitabine in patients with refractory solid tumors. Ann Oncol. 2018;29(5):1304–1311. doi:10.1093/annonc/mdy076
74. Lee EK, Simpkins F, et al. MYTHIC: first-in-human biomarker-driven phase I trial of first-in-class PKMYT1 lunresertib alone and with ATR inhibitor camonsertib in solid tumors with CCNE1 amplification or deleterious alterations in FBXW7 or PPP2R1A.
75. Huang P, Fan X, Yu H, et al. Glucose metabolic reprogramming and its therapeutic potential in obesity-associated endometrial cancer. J Transl Med. 2023;21(1):94. doi:10.1186/s12967-022-03851-4
76. Madariaga A, Goodwin PJ, Oza AM. Metformin in Gynecologic Cancers: opening a New Window for Prevention and Treatment? Clin Cancer Res. 2020;26(3):523–525. doi:10.1158/1078-0432.CCR-19-3645
77. Soliman PT, Westin SN, Iglesias DA, et al. Everolimus, Letrozole, and Metformin in Women with Advanced or Recurrent Endometrioid Endometrial Cancer: a Multi-Center, Single Arm, Phase II Study. Clin Cancer Res. 2020;26(3):581–587. doi:10.1158/1078-0432.CCR-19-0471
78. Rodrigues M, Follana P, et al. Safety and efficacy of olaparib combined to metronomic cyclophosphamide and metformin in recurrent advanced/metastatic endometrial cancer patients: ENDOLA trial. Cancer Res. 2022;82:CT005. doi:10.1158/1538-7445.AM2022-CT005
79. Kanda R, Hiraike H, Wada-Hiraike O, et al. Expression of the glucagon-like peptide-1 receptor and its role in regulating autophagy in endometrial cancer. BMC Cancer. 2018;18(1):657. doi:10.1186/s12885-018-4570-8
80. Wr ZH, Wang L, Tian Q, et al. Activation of glucagon-like peptide-1 receptor inhibits growth and promotes apoptosis of human pancreatic cancer cells in a cAMP-dependent manner. J Physiol Endocrinol Metab. 2014;306:E1431–E1441. doi:10.1152/ajpendo.00017.2014
81. Zhao WZ, Zhou X, Sun Z. Liraglutide inhibits the proliferation and promotes the apoptosis of MCF-7 human breast cancer cells through downregulation of microRNA-27a expression. Mol Med Rep. 2018;17(4):5202–5212. doi:10.3892/mmr.2018.8475
82. Scafoglio C, Hirayama BA, Kepe V, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A. 2015;112(30):E4111–4119. doi:10.1073/pnas.1511698112
83. Reyes HD, Thiel KW, Carlson MJ, et al. Comprehensive profiling of EGFR/HER receptors for personalized treatment of gynecologic cancers. Mol Diagn Ther. 2014;18(2):137–151. doi:10.1007/s40291-013-0070-3
84. Vergote I, Perez-Fidalgo JA, Hamilton EP, et al. Oral Selinexor as Maintenance Therapy After First-Line Chemotherapy for Advanced or Recurrent Endometrial Cancer. J Clin Oncol. 2023;JCO2202906.
85. de Bono JS, Concin N, Hong DS, et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(3):383–393. doi:10.1016/S1470-2045(18)30859-3
86. Karpel HC, Powell SS, Pothuri B. Antibody-Drug Conjugates in Gynecologic Cancer. Am Soc Clin Oncol Educ Book. 2023;43(43):e390772. doi:10.1200/EDBK_390772
87. Moore KN, Borghaei H, Dm O, et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor alpha-targeting antibody-drug conjugate, in patients with solid tumors. Cancer. 2017;123(16):3080–3087. doi:10.1002/cncr.30736
88. Oaknin B, Martin LP, O’Malley D, et al. 741M0-Luveltamab tazevibulin (STRO-002), an anti-folate receptor alpha (FOLRa) antibody drug conjugate (ADC), demonstrate clinical activity in recurrent/progressive epithelial endometrial cancer (EEC): STRO-002-GM1 phase I dose expansion. Ann Oncol. 2023;34:S507–S542.
89. Santin A, Siegel SR, Harold J, et al. Preliminary results of a phase III triaal with sacituzumab govitecan-hziy in patients with recurrent endometrial carcinoma overexpressing Trop-2. J Clin Oncol. 2023;41:5599. doi:10.1200/JCO.2023.41.16_suppl.5599
90. Zong L, Yu S, Mo S, et al. B7-H4 Further Stratifies Patients With Endometrial Cancer Exhibiting a Nonspecific Molecular Profile. Arch Pathol Lab Med. 2023;147(11):1288–1297. doi:10.5858/arpa.2022-0182-OA
91. Lakhani N, Call JA, et al. 660M0-First-in-human study of SGN-B7H4V, a B7-H4-directed vedotin ADC, in patients with advanced solid tumors: preliminary results of a phase I study (SGNBH4V-001). Ann Oncol. 2023;34:S458–497.
92. Tarantino P, Carmagnani Pestana R, Corti C, et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72(2):165–182. doi:10.3322/caac.21705
93. Makker V, MacKay H, Ray-Coquard I, et al. Endometrial cancer. Nat Rev Dis Primers. 2021;7(1):88. doi:10.1038/s41572-021-00324-8
94. Dubil EA, Tian C, Wang G, et al. Racial disparities in molecular subtypes of endometrial cancer. Gynecol Oncol. 2018;149(1):106–116. doi:10.1016/j.ygyno.2017.12.009
95. Whelan K, Dillon M, Strickland KC, et al. TP53 mutation and abnormal p53 expression in endometrial cancer: associations with race and outcomes. Gynecol Oncol. 2023;178:44–53. doi:10.1016/j.ygyno.2023.09.009
96. Weigelt B, Marra A, Selenica P, et al. Molecular Characterization of Endometrial Carcinomas in Black and White Patients Reveals Disparate Drivers with Therapeutic Implications. Cancer Discov. 2023;13(11):2356–2369. doi:10.1158/2159-8290.CD-23-0546
97. Pb SAA, Backes F, Thaker P, et al. Genomic alterations, molecularly targeted therapy and race: real world data from the Endometrial Cancer Molecularly Targeted Therapy Consortium (LBA 8). Gynecol Oncol. 2022;166:S52–S53. doi:10.1016/S0090-8258(22)01301-4
98. Urick ME, Bell DW. Clinical actionability of molecular targets in endometrial cancer. Nat Rev Cancer. 2019;19(9):510–521. doi:10.1038/s41568-019-0177-x
99. Chen W, Sun Z, Lu L. Targeted Engineering of Medicinal Chemistry for Cancer Therapy: recent Advances and Perspectives. Angew Chem Int Ed Engl. 2021;60(11):5626–5643. doi:10.1002/anie.201914511
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
