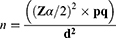Back to Journals » Pathology and Laboratory Medicine International » Volume 15
Observational Assessment of Pre-Analytical Errors in Request Format and Phlebotomy Practice in Hematology Tests at Hawassa University Comprehensive Specialized Hospital in Sidama Zone, Southern Ethiopia
Authors Addisu B, Kelem A, Hirigo AT
Received 30 September 2023
Accepted for publication 12 December 2023
Published 19 December 2023 Volume 2023:15 Pages 83—89
DOI https://doi.org/10.2147/PLMI.S439227
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Paul Zhang
Bedasa Addisu,1 Amanuel Kelem,2 Agete Tadewos Hirigo3
1Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Berhan University, Debre Berhan, Amhara, Ethiopia; 2Department of Medical Laboratory Sciences, Asrat Woldeyes Health Science Campus, Debre Berhan University, Debre Berhan, Ethiopia; 3School of Medical Laboratory Science, College of Medicine and Health Sciences, Hawassa University, Hawassa, Ethiopia
Correspondence: Bedasa Addisu, Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Berhan University, PO Box 445, Debre Berhan, Ethiopia, Tel +251912496933, Email [email protected]
Background: The significance of quality in laboratories necessitates the implementation of comprehensive quality management throughout the entire laboratory process, encompassing the pre-analytical to post-analytical phases. The pre-analytical phase, which plays a crucial role in laboratory services, is particularly significant, as it is responsible for approximately 70% of errors. Enhancing the quality of the pre-analytical phase is essential to ensure the effectiveness of laboratory services and safeguard patient safety.
Objective: The objective of this study was to assess errors made in the request format and phlebotomy procedures during the collection of blood samples for hematology laboratory tests between April and June 2019.
Methods: A cross-sectional study was carried out at Hawassa University College of Medicine and Health Sciences on 393 samples with their respective request forms. The study employed a systematic sampling technique and utilized well-structured checklists to gather all the necessary data for hematology laboratory tests, including a thorough evaluation of the requested form. Data analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 21.
Results: A total of 393 hematological samples from their respective request forms were analyzed in this study. Generally, the request forms did not contain all the necessary information, and each form had at least one error. The study also revealed the occurrence of pre-analytical errors in phlebotomy practice. These errors included prolonged tourniquet application (26.2%), improper identification (22.9%), inadequate cleaning of the vein puncture site (62.8%), unsafe needle removal and blood transfer to test tubes (6.6%), and improper mixing of collected blood samples (41.5%).
Conclusion: The findings of this study revealed a substantial occurrence of pre-analytical errors in the hematology laboratory department. Therefore, it is crucial to prioritize the correct execution and utilization of phlebotomy procedures as well as request format to enhance quality assurance in hematology laboratory units.
Keywords: pre-analytical errors, phlebotomy practices, laboratory quality management
Introduction
Laboratory diagnostics plays a crucial role in clinical decision-making, providing valuable support in the disease prevention, diagnosis, and therapeutic monitoring of a wide range of health problems. It is estimated that around 60–70% of the most critical medical decisions heavily rely on laboratory test results. Consequently, the utilization of laboratory diagnostics becomes imperative to guarantee the reliability and accuracy of outcomes derived from clinical laboratories.1–3
Despite significant advancements in scientific innovations aimed at enhancing the laboratory environment and ensuring the accuracy of clinical laboratory test results, errors have continued to persist.4 These errors can be categorized into pre-analytical, analytical, and post-analytical errors, with pre-analytical errors being the most prevalent and posing a significant risk to patient diagnosis and treatment.3,4
The pre-analytical phase of the laboratory diagnostic process is susceptible to errors that can occur from the moment a test is ordered by a physician until the sample is prepared for analysis.3 These errors are responsible for a significant proportion of the errors encountered during the entire process, with up to 70% of them occurring during sample collection, handling, transportation, and storage.5,6 The occurrence of pre-analytical errors before sample collection can be attributed to various factors. Notably, the incomplete request format information form plays a crucial role in this regard, as it lacks essential details such as the physician's name, patient misidentification, and the requested test format.3,6 These omissions significantly contribute to the overall prevalence of pre-analytical errors. In addition, the incorrect labeling of specimen collection materials further exacerbates the situation, as it heightens the risk of erroneous patient sample collection and subsequent analysis.3,6
Hemolysis, a common pre-analytical error, can arise during the collection of blood samples due to various factors. These factors may include the application of a tourniquet for an extended period,7 the use of a fine needle to force blood, and the excessive shaking of test tubes. The analysis of such blood samples can lead to erroneous results, such as a decrease in red blood cell counts and an increase in platelet count.4,8,9 Hematological tests also can be adversely affected by a decrease in cell count7 due to clotting or dilution of blood specimens caused by inadequate mixing with additives after collection. Furthermore, pre-analytical errors may occur as a result of laboratory personnel’s insufficient training and understanding of the blood collection process.10,11 Moreover most significant factor that affects the laboratory results of patients from blood specimens is the collection of venous blood during the pre-analytical phase. However, if phlebotomy is not appropriately regulated, it can have adverse effects on patient safety and health. The most common problems encountered during phlebotomy are related to patient identification and improper skin puncturing practices.12 Furthermore, of utmost importance is the fact that errors stemming from this stage can lead to unjustified investigations, increased care expenses, and inappropriate modifications to therapy.2
Therefore, this study aimed to evaluate the occurrence of pre-analytical errors in the request format and phlebotomy practices in hematology tests at Hawassa University Comprehensive Specialized Hospital (CHUCSH) in the Sidama Zone, Southern Ethiopia.
Methods and Materials
Study Setting and Study Population
The study was conducted at Hawassa University’s comprehensive specialized hospital (HUCSH) in Hawassa City. The town of Hawassa is 275 km from Addis Ababa. The hospital provides diverse outpatient and inpatient services for approximately 15 million people from all over the Southern Ethiopia population and its neighboring regions. HUCSH currently operates with a capacity of over 400 beds, delivering exceptional patient care across a wide range of services. Annually, the hospital attends to a significant number of individuals, including more than 90,200 outpatients, 18,116 inpatients, and 1092 emergency cases. This research, conducted within the hospital’s hematology laboratory unit, was an observational cross-sectional study carried out from April to June 2019. The study focused on venous blood collection for laboratory tests, specifically examining the process of phlebotomy. All requests for blood collection were considered for inclusion in the study, while any blood samples and request forms referred to the laboratory outside of duty hours were excluded. Furthermore, laboratory technicians and technologists who were not involved in the sample collection section during the study period were also excluded from the study. They were 2 out of 16 laboratory technicians and technologists who were involved in the sample collection section during the study period.
Sample Size and Sampling Technique
The sample size estimation was based on 41.6% of pre-analytical errors in Hematology tests, which was conducted in Central Oromiya, Ethiopia.13 The required sample size was calculated by a single population proportion formula at a confidence interval (CI) of 95%.
Where P= proportion of pre-analytical errors in Hematology tests, Zα/2= Critical value at 95% level of confidence (Z =1.96), q= (1-P), d= margin of error (5%), n= the required sample size, which is 374. With considering a 5% non-response rate, the final sample size was calculated to be 393. To include the study participants, the direct patient flow for hematological tests over one month was examined, and the logbook of patients from the preceding month was further evaluated to validate the findings. Thus, the data indicated an average monthly patient flow of 1492. Ultimately, a systematic random sampling technique was employed to select every fourth patient with a request paper for hematological tests.
Data Collection Tools and Procedure
Since the laboratory information system was not available, data was entered manually. To assess compliance with the standard operating procedure for the pre-analytical phase of patient blood sample collection for hematological tests, a standard checklist was created. This checklist was employed during data collection to ensure adherence to the procedure. Data collectors assessed the performance of phlebotomists in a blinded manner by observing their technique during the pre-analytical procedure, using a standardized checklist to gather information.14 This evaluation was conducted to address whether or not the phlebotomists adhered to the standard operating procedure.
Operational Definitions
Pre-analytical error during blood collection: the occurrence of pre-analytical errors during blood collection is characterized by the collection of blood specimens by laboratory phlebotomists with incorrect or missing information on the container, inadequate blood volume, unsuitable quality, from the wrong patient, unsuitable container, or incorrect specimen types.
Data Quality Management and Analysis
The structured inspection sheets underwent a pre-test to guarantee their clarity, acceptability, and consistency. Any necessary adjustments were made following the pre-test examination. Daily checks were conducted to ensure the completeness of the collected data. Each team member meticulously carried out the data collection through observation and by checking the request format. The data were then coded, cleaned, entered, and analyzed using Statistical Package for Social Sciences (SPSS). Frequency and percentage calculations were performed, and the data were summarized using frequencies, and percentages, Figure 1 and Table 1.
 |
Table 1 Pattern of Pre-Analytical Errors Related to Laboratory Request Forms for Hematological Tests |
 |
Figure 1 The frequency of pre-analytical errors in phlebotomy practice for Hematology laboratory tests. |
Results
Pre-Analytical Errors of the Request Formats
Of the 393 enrolled patients with their corresponding request formats, it revealed that the patient request forms lacked essential information, such as patient’s age, hospital identification number, and sex, with a percentage of 2.5%, 6.4%, and 3.8%, respectively. In addition, the request forms were found to be missing crucial details that included clinical diagnoses, clinician name, and signature, with a percentage of 76.08% and 72.8%, respectively (Table 1).
Pre-Analytical Errors of the Phlebotomy and Its Procedure
The labeling error rate before phlebotomy collection was recorded at 2.3% (9.0), while 56.7% (223) of errors were attributed to failure to verify patient identity. Improper mixing of blood samples accounted for 41.5% (163) of errors, and 62.8% (247) of errors were due to inadequate cleaning of the vein puncture site (Figure 1).
Discussions
Our study revealed that the test request forms sent to the laboratory were incomplete, and lacked essential patient identification parameters such as hospital ID number (6.4%), age (2.5%), and sex (3.8%). Furthermore, the majority of the papers did not include the clinician’s name and signature or clinical diagnosis (72.8% and 76.08% respectively).
The patient address and sample collection time were not found in any of the request forms, which is an interesting observation. Conversely, the hematology request formats had a well-documented patient name, as confirmed by a study conducted at St. Paul’s Hospital.15 In our study, it was observed that approximately 76% of the request formats lacked appropriate clinical details of the patient. This rate was higher than the study conducted at St. Paul’s Hospital, which reported a rate of 70.1%.15 The study findings revealed that the omission rates for age and sex in the forms were 2.5% and 3.8% respectively. These rates were comparatively lower than the study conducted in Ghana, where 25.6% of patient age and 67.3% of sex were omitted.16 In addition, the investigation discovered that clinical diagnosis information was present in just 23.97% of the request forms, which is a substantial difference from the Ghana study results which reported a 77.3% inclusion rate.16 This result suggests that clinicians may not be accustomed to documenting clinical details. The results of the present study indicated that 72.8% of the requested papers did not include the name of the clinician. In comparison to the previous study conducted at Central Oromiya, where 22.5% of the requested papers did not mention the clinician’s name,13 the rate observed in this study was higher. However, it was still lower than the rate reported in the study conducted at St. Paul’s Hospital Millennium Medical College, where 85% of the requested format did not mention the clinician’s name.15 The difference in attention given by requesting physicians and the workload may account for the variation in these parameters.
The findings of the present study indicated the prevalence of pre-analytical error in phlebotomy practice, encompassing prolonged tourniquet application (26.2%), inaccurate identification of vein puncture site (22.9%), and inadequate mixing of blood samples (41.5%). The findings are consistent with the study conducted in Pakistan,17 which revealed a 20.8% occurrence of vein misidentification.
It is recommended by the WHO blood drawing guidelines and Dhingra et al18 that the puncture site must be cleaned in a circular motion from the puncture site towards the periphery. However, a significant number of patients (62.8%) in the present study did not receive proper cleaning of the vein puncture site. The cleaning procedure was done improperly by rubbing vigorously and irregularly, sometimes in a zigzag pattern, and repeating the cleaning process with a dirty swab. However, the finding was lower than the study reported from Brazil (85%).4
In this study, a significant proportion of samples, 41.5%, were not mixed properly. This finding was lower than the previous studies conducted at Addis Ababa Public Hospital in 201719 and in Brazil,4 where 60% and 83% of samples were not mixed properly.
In this study, phlebotomists collected 26.2% of blood samples with incorrect tourniquet application which differed from the findings of the study conducted at Addis Ababa Public Hospital.19 This variation could potentially be attributed to differences in the target population and the design of the study. Further, in this study, the labeling of test tubes was conducted in 97.7% of blood samples before blood collection and patient assessment, which was significantly greater than the 51% rate observed in a comparable study conducted at Addis Ababa Public Hospital.19 This inconsistency may be due to the large sample size used in our study.
Limitations of the Study
The study only included phlebotomists present during the study period, excluding those who were not at the working site. Additionally, the study did not assess factors associated with errors in the pre-analytical phase of the testing process. Lastly, the study solely focused on vein blood collection practices and did not consider other specimen collections or handling up to the result report.
Conclusions
This study revealed the presence of various pre-analytical errors in the laboratory. Errors in using test request forms and phlebotomy practices were common in the assessed unit. To ensure accurate patient results, it is important to focus on improving phlebotomy practice by training on pre-analytical errors during blood collection. Additionally, refreshing clinicians/physicians is also essential to provide all required information on the request paper to limit errors. Further, well-designed studies are required to investigate factors related to pre-analytical errors.
Abbreviations
SNNP, Southern Nations, Nationalities, and Peoples; SOP, Standard Operating Procedure; SPSS, Statistical Package for the Social Sciences; HU, Hawassa University; ID, identification; IV, intravenous; HUCSH, Hawassa University Comprehensive Specialized Hospital.
Ethical Clearance and Consent to Participate
Ethical clearance was obtained from the institutional review board of Hawassa University College of Medicine and Health Sciences. A permission letter with an ethical clearance letter obtained from Hawassa University was again submitted to Hawassa University's comprehensive specialized hospital laboratory to get permission for data collection. All methods to conduct this study were carried out in accordance with relevant guidelines and regulations in Helsinki the declaration. Written informed consent was obtained from all participants and the protocol of the study was explained to each participant. Any information obtained during the study was kept at strict confidentiality.
Acknowledgment
We would like to thank the laboratory staff working in the Hawassa University comprehensive and specialized hospital laboratory for their assistance during data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors disclose that there is no competing interest.
References
1. Saurav Patra M, Mukherjee B, Das AK. Pre-analytical errors in the clinical laboratory and how to minimize them. Int J Bioassays. 2013;2(3):551–553.
2. Naz S, Mumtaz A, Sadaruddin A. Preanalytical errors and their impact on tests in clinical laboratory practice. Pak J Med Res. 2012;51(1):27.
3. Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44(6):750–759. doi:10.1515/CCLM.2006.123
4. Lima-Oliveira G, Guidi GC, Salvagno GL, et al. Is phlebotomy part of the dark side in the clinical laboratory struggle for quality? Lab Med. 2012;43(5):172–176. doi:10.1309/LMZ7YARD6ZSDIID
5. Adiga U, Preethika A. Errors in clinical biochemistry laboratory. Br J Med Med Res. 2016;14(8):1–6. doi:10.9734/BJMMR/2016/25012
6. Rana SV. No Preanalytical Errors in Laboratory Testing: A Beneficial Aspect for Patients. Springer; 2012:319–321.
7. Usman U, Siddiqui JA, Lodhi J. Evaluation & control of pre analytical errors in required quality variables of clinical lab services. IOSR J Nurs Health Sci. 2015;4(3):54–71.
8. Sholademi BA. Identification and Reduction of Pre-Analytical Errors in Clinical Chemistry Through Expert Advice. Sheffield Hallam University; 2017.
9. Chawla R, Goswami B, Tayal D, Mallika V. Identification of the types of preanalytical errors in the clinical chemistry laboratory: 1-year study at GB Pant Hospital. Lab Med. 2010;41(2):89–92. doi:10.1309/LM9JXZBMLSVJT9RK
10. Neogi SS, Mehndiratta M, Gupta S, Puri D. Pre-analytical phase in clinical chemistry laboratory. J Clin Sci Res. 2016;5(3):171–178. doi:10.15380/2277-5706.JCSR.15.062
11. Cuhadar S. Preanalytical variables and factors that interfere with the biochemical parameters: a review. OA Biotechnol. 2013;2(2):19.
12. Serdar MA, Kenar L, Hasimi A, Kocu L, Türkmen YH, Kurt I. Tourniquet application time during phlebotomy and the influence on clinical chemistry testing; is it negligible. Turk J Biochem. 2008;33(3):85–88.
13. Wondimagegn MW, Yallew WW, Anijajo TT. Assessment of pre-analytical error on blood specimens referred for CD4 and haematology tests in central Oromiya, Ethiopia. Am J Lab Med. 2016;1(3):58–64.
14. BIDMC. Procedure for the collection of diagnostic blood specimens by venipuncture; 2012.
15. Tadesse H, Desta K, Kinde S, Hassen F, Gize A. Errors in the hematology laboratory at St. Paul’s hospital millennium medical college, Addis Ababa, Ethiopia. BMC Res Notes. 2018;11(1):1–5. doi:10.1186/s13104-017-3088-5
16. Olayemi E, Asiamah-Broni R. Evaluation of request forms submitted to the haematology laboratory in a Ghanaian tertiary hospital. Pan Afr Med J. 2011;8(1). doi:10.4314/pamj.v8i1.71148
17. Zehra N, Malik AH, Arshad Q, Sarwar S, Aslam S. Assessment of preanalytical blood sampling errors in clinical settings. J Ayub Med Coll Abbottabad. 2016;28(2):267–270.
18. Dhingra N. Safe Injection Global Network. In: WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy. World Health Organization; 2010.
19. Mekonon WL, Abebe AT, Haile EL, Misganaw AS. Evaluation of phlebotomy service in clinical laboratory setting in Addis Ababa Public Hospitals, Addis Ababa, Ethiopia. Am J Lab Med. 2017;2(3):24–33. doi:10.11648/j.ajlm.20170203.11
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.