Back to Journals » Medical Devices: Evidence and Research » Volume 16
Observational Study to Assesses the Efficacy and Safety of Microcurrent Therapy with a Portable Device in Patients Suffering from Chronic Back Pain, Skeletal System Pain, Fibromyalgia, Migraine or Depression
Received 18 September 2023
Accepted for publication 25 October 2023
Published 7 December 2023 Volume 2023:16 Pages 261—280
DOI https://doi.org/10.2147/MDER.S436667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Peter Marmann,1 Werner Wiatrek2
1Clinical Research, Healy World GmbH, Isaac-Fulda-Allee 1, Mainz, 55124, Germany; 2WQS Management Consultants GmbH, Hamm, 59075, Germany
Correspondence: Peter Marmann, Clinical Research Department, Healy World GmbH, Isaac-Fulda-Allee 1, Mainz, 55124, Germany, Tel +49 1718 4502 47, Email [email protected]
Purpose: In Germany, there are several microcurrent medical devices that are certified for the treatment of patients suffering from one of the indications chronic back pain, skeletal system pain, fibromyalgia, migraine or depression. While certification is based on controlled, randomized clinical trials, evidence of efficacy and safety under real-world conditions is limited to very few observational studies. To fill this gap, this study was conducted.
Patients and Methods: Fifty patients per indication already using the investigational device before study entry were included and followed for a total 6 months. Each participant used the Healy in an individualized schedule to optimize the treatment of his/her special indication. This means that each participant performed on average 1– 2 microcurrent applications per day for 20 to 30 minutes each. In all indications, the improvement of health-related quality of life was assessed by the SF-36 questionnaire and other validated indication specific surveys.
Results: In all indications, the improvement of health-related quality of life as assessed by the SF-36 questionnaire was statistically highly significant and clinically relevant. These findings were supported by more specific outcome measures applied in each indication. Only four adverse events related to the application of microcurrent occurred during the trial.
Conclusion: Microcurrent therapy has been demonstrated to be efficient and safe under real-world conditions for the treatment of each of the conditions for which the device is certified.
Keywords: microcurrent therapy, pain, anxiety, sleep disturbances
Introduction
Microcurrent therapy, also known as microcurrent electrical neuromuscular stimulation (MENS), involves the application of low-level electrical currents to specific areas of the body for therapeutic purposes. It has a history that spans several decades.
In the 19th century, scientists such as Luigi Galvani and Alessandro Volta made significant discoveries in the field of bioelectricity.1 They found that low-level electric currents can trigger muscle contractions in animals and proved that electric currents exist in living organisms.
In the late 19th and early 20th centuries, electrotherapy became a popular medical treatment for various conditions.2 Devices such as the faradic battery, which produced electrical currents for therapeutic use, were developed and used by physicians.
Based on animal experiments and clinical investigations in the second half of the 20th century, the neurophysiological mechanisms of microcurrent therapy were elucidated in more detail.2
In the 1970s, Dr. Thomas Wing, an American physician, developed the concept of microcurrent therapy.3 He discovered that extremely low-level electrical currents, in the range of millionths of an ampere (microamperes), could effectively treat pain and promote healing in tissues.
Over the years, researchers and practitioners made significant advancements in microcurrent therapy. They developed more sophisticated devices that can deliver precise and controlled microcurrents to target specific tissues and conditions.
In the 1990s, the United States Food and Drug Administration (FDA) approved microcurrent devices for the treatment of various medical conditions, including pain management, wound healing and muscle rehabilitation.
Carolyn McMakin4 provided an in-depth understanding of the FSM (Frequency Specific Microcurrent) technique and its applications. Her work has also significantly contributed to the education and training of healthcare professionals in the field of microcurrent therapy.
In recent years, there has been an increase in the availability of microcurrent devices for home use. These portable devices allow individuals to self-administer microcurrent therapy for pain relief and other therapeutic purposes.
The exact mode of action of microcurrent therapy is not yet fully understood, and research in this area is still ongoing. Several theories have been proposed to explain how microcurrent therapy may exert its therapeutic effects on the organism:
ATP Production: Microcurrent therapy may enhance the production of adenosine triphosphate (ATP) within the cells.5,6 ATP is the energy currency of the body, and increased ATP levels can promote cellular metabolism and facilitate tissue repair processes.
Cellular Communication and Signaling: Microcurrents applied to the body may stimulate cell-to-cell communication and enhance intercellular signaling.7,8 This can influence various physiological processes, including pain modulation, inflammation reduction and tissue regeneration.
Electrochemical Effects: The electrical currents delivered during microcurrent therapy can alter the electrochemical environment in the tissues.9,10 This can affect ion exchange, pH levels and cell membrane potential, leading to changes in cellular activity and function.
Blood Flow and Circulation: Microcurrent therapy has been suggested to enhance blood flow and microcirculation in the treated area.11,12 Improved circulation can promote the delivery of oxygen, nutrients and immune cells to the tissues, supporting healing and reducing inflammation.
Neurological Effects: Microcurrents may have direct effects on the nervous system, influencing nerve conduction, reducing pain signals and promoting neuromuscular function.13,14 This can be beneficial in pain management and muscle rehabilitation.
Modulation of Cellular Processes: Microcurrent therapy may modulate various cellular processes, such as protein synthesis,15,16 gene expression17 and enzymatic activity.18 These effects can contribute to tissue repair, regeneration and modulation of inflammatory responses.
It is important to note that the exact mode of action may vary depending on the specific condition being treated and the parameters of the microcurrent therapy applied, such as frequency, waveform and intensity. Additionally, different mechanisms may work synergistically to produce the therapeutic effects observed in microcurrent therapy.
Several microcurrent devices similar to the Healy device have successfully been used in clinical trials to demonstrate the efficacy and safety of microcurrent therapies for different indications:
Pain Management: Numerous studies have investigated the use of microcurrent therapy for pain management.18–57 Research has shown positive results in various conditions, such as fibromyalgia, osteoarthritis, skeletal system pain and postoperative pain.
Anxiety and Depression: Microcurrent therapy has been studied as a non-invasive treatment option for anxiety and depression.58–78 Clinical trials have demonstrated benefits in reducing symptoms and improving overall mood.
Insomnia and Sleep Disorders: Some research has explored the use of microcurrent therapy for sleep disorders and insomnia.79,80 Studies have shown improvements in sleep quality and duration.
The Healy device has the same clinical, technical and biological properties as the devices used in these studies and is CE marked since 2017.
In this study, the Healy device was used solely within its approved intended use, including the following indications: chronic back pain, skeletal system pain, fibromyalgia, migraine and depression, anxiety and associated sleep disorders.
An observational period of 6 months and a total study population of 250 participants (50 participants per indication) were assumed to be sufficient to achieve reliable results concerning the treatment’s efficacy and safety. Because this was an observational trial designed to collect data on normal use of the Healy device, participants were asked to use the device according to their needs and indications.
Materials and Methods
Design
The study was a monocentric observational post market follow-up study with a treatment duration of six months, with measurement points at the beginning (V1), after 28 days (V2), after 3 months (V3) and at the end of the study after 6 months (V4). A study protocol was established before recruitment began. Participants were recruited via an existing network of persons interested in this type of treatment and suffering from one of the approved indications (chronic back pain, skeletal system pain, fibromyalgia, migraine and depression, anxiety and associated sleep disorders). The confirmation of the diagnosis (indication) was diligently carried out by the therapist through a pre-screening, following the rules of the professional code of conduct for non-medical practitioners (BoH). Because of the corona virus situation at baseline, all study visits were conducted remotely.
Written informed consent was obtained from participants before undertaking the screening and baseline assessments. The trial received research ethics committee approval from the International Medical & Dental Ethics Commission (IMDEC) prior to including the first participant into the study (Ethics committee approval reference No. 2021/117).
This study was conducted according to the principles outlined in the World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects.81
Participants
All participants were already using the investigational microcurrent device for at least three months at the time of study entry for the treatment of one of the five indications mentioned above. They gave written informed consent to participate prior to any study-related procedure. Volunteers were excluded if they were younger than 18, older than 70 years, pregnant, had a pacemaker or any other electronic or metallic device at or near the place of application on the body, open wounds, scar tissue or insensitivity or radiation therapy near the place of application, or a history of epilepsy.
Treatment Device
For application of individualized frequency modulated microcurrent applications, the investigational device (Healy device) was to be attached to the body via adhesive electrodes, ear clips or bracelet electrodes and cables on various places depending on the program and objective (see Figure 1, Healy application for chronic back pain).
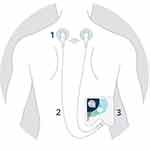 |
Figure 1 Example of the application of the investigational medical product (Healy device); 1: Adhesive electrodes, 2: Cables, 3: Healy device. |
Healy uses frequencies from 0.1 Hz to 1 MHz. It applies an electrical current between 0 μA and 4000 μA. The maximum applied voltage is 10 V.
Treatments Administered
Each participant used the Healy in an individualized schedule to optimize the treatment of his/her special indication. This means that each participant performed on average 1–2 microcurrent applications per day for 20 to 60 minutes each. The frequency pattern of these applications was designed by experienced therapists, not involved in any study-specific procedures.
Outcome Measurements
SF 36
Scoring the SF 36-Item Health Survey is a two-step process. First, pre-coded numeric values are recoded according to the scoring key.82–84 All items are scored so that a high score defines a more favorable health state. In addition, each item is scored on a scale from 0 to 100, so that the lowest and highest possible scores are 0 and 100, respectively. The score indicates the percentage of the total possible score. In step 2, the items in the same scale are averaged to create the 8 scale scores. Items in which one or more answers are left blank (missing data) are not considered when calculating the scale scores. Thus, the scale scores represent the average for all items in the scale that the respondent answered.
After the eight scale scores are calculated, a z-score is determined for each by subtracting the scale mean of a sample of the US general population from an individual’s scale score and then dividing by the standard deviation from the US general population. Each of the eight z-scores is then multiplied by the corresponding factor scoring coefficient for the scale.85,86 There are two different sets of factor-scoring coefficients, one for the physical component summary (PCS) and another for the mental component summary (MCS). The products of the z-scores and factor scoring coefficients for the PCS are then summed together and a similar calculation is performed for the MCS. Each resulting sum is multiplied by 10 and added to 50 to linearly transform the PCS or MCS to the T-score metric, which has a mean of 50 and a standard deviation of 10 for the US general population.
Pain Assessment Score (Valid for Chronic Back Pain and Skeletal System Pain)
Pain Scores were assessed by three 10-point Likert scales (average pain, maximal pain, current pain).87,88 The assessment is carried out by the patients themselves. Pain scores were evaluated at each study visit starting with the baseline visit according to the following verbal descriptive endpoints of the scale:
0 = no pain at all; 10 = unbearable pain
Severity of Migraine Assessment (MiDAS; Only Valid for the Indication “Migraine”)
The severity of migraine was assessed by use of the Migraine Disability Assessment (MiDAS) questionnaire. The MiDAS Questionnaire was developed to assess headache-related disability with the aim of improving migraine care.89–91 Headache sufferers answer five questions, scoring the number of days, in the past 3 months of activity limitations due to migraine. All assessments were carried out by the patient using various questionnaires. Scores were evaluated at each study visit starting with the baseline visit.
Mental Illness Assessment (Valid for Indications “Fibromyalgia” and “Depression” Only)
In terms of mental illness, several rating scales were used. All assessments were carried out by the patient using various questionnaires. Scores were evaluated at each study visit starting with the baseline visit.
Based on a recent study performed by the RKI in Germany,92 the Patient Health Questionnaire (PHQ 9)93 is a suitable instrument to assess the severity of depression symptoms.
It consists of 9 questions measuring the frequencies of depression-related complaints during the previous 14 days by use of a 4-point Likert scale:
Not at all, On single days, On more than half of the days, On almost every day.
Anxiety Assessment (Valid Only for the Indications Fibromyalgia and Depression)
Anxiety is measured according to GAD-7 anxiety severity.94,95 Scores are dependent on frequency of symptoms (0: not at all / 1: several days / 2: more than half the days / 3: nearly every day).
Sleep Quality (Valid for Indications “Fibromyalgia” and “Depression” Only)
Insomnia severity was measured according to Insomnia Severity Index (ISI), based on 7-point questionnaire.96,97 Total score categories are defined as follows.
- 0–7 no clinically significant insomnia
- 8–14 subthreshold insomnia
- 15–21 clinical insomnia (moderate severity)
- 22–28 clinical insomnia (severe)
Statistics
Efficacy Analysis
All efficacy variables were listed by subject. Data was summarized by treatment group. N, Mean, Standard Deviation, Minimum and Maximum were used to summarize continuous efficacy variables, whereas number and percent were used to summarize categorical efficacy variables.
All analyses of the continuous efficacy variables (eg, pain score) were performed as analysis of variance for repeated measurements. In case Mauchly tests for sphericity yielded departure from sphericity, Greenhouse-Geisser and Huynh-Feldt corrections were performed. All indications were tested at the two-sided 5% significance level.
To confirm test results in case of severe deviations from sphericity, additional non-parametric tests (Friedman test) were performed.
For group comparison of total effect sizes (differences between final and baseline values), covariance analysis was performed with baseline adjustments.
Safety Analysis
All newly occurring diseases and deterioration of existing diseases were recorded as adverse events. All adverse events were listed by study participants as recorded and as coded according to the ICD-10 classification. Incidences of Adverse Events (AEs) per organ class and group and incidences of AEs assessed as potentially induced by the study procedure were listed and compared with incidence rates in comparable populations.
Results
Demographics
The first screening was performed in June 2021, the first subject was included (date of IC signature) on 30th June 2021. Screening was completed in August 2022; the last subject was included on 11th August 2022. The last patient completed the clinical part of the study (last patient out) on Jan 13, 2023.
Of the 317 potential participants screened for study participation, 256 persons signed the informed consent form and were included into one of the five indication groups. Seven of these included participants terminated the study prior to performing any study-related procedure (no survey completed, no study visit performed), resulting in 249 participants with study-related data.
Five participants terminated the study prematurely between visit 1 and visit 4, resulting in 244 study completers. Forty-eight participants were diagnosed with chronic back pain or migraine, respectively, 49 with skeletal system pain and fibromyalgia and 50 with depression, anxiety and associated sleep disorder.
Two hundred and twenty female patients and 29 male patients were included in the study. Two hundred and forty-seven patients were Caucasians, 2 Asians. The average age of the complete full analysis set was 51.3 years, average height was 1694 cm, average weight 73.3 kg (Table 1).
 |
Table 1 Summary of Demographic Data, All Study Participants and Participants Suffering from the Different Indications, Mean Values and Standard Deviation in Brackets. Second Line Minimum and Maximum |
Differences concerning the demographic data between the indication groups are small, with participants in the skeletal system pain group being slightly older (on average 55.62 years) and participants in the Migraine group being slightly younger (on average 46.27 years) than the total participants’ average age.
Previous and Concomitant Diseases and Previous and Concomitant Therapies
Three hundred and eighty previous relevant diseases were reported in 165 patients.
Most prominent are diseases of the musculoskeletal system and connective tissue (116), endocrine, nutritional and metabolic diseases (59), diseases of the nervous system (38) and diseases of the circulatory system (36).
None of the recorded previous diseases fulfilled any of the exclusion criteria.
Three hundred and eighty-one previous and concomitant treatments were reported in 125 patients. All pharmacological treatments were coded according to the WHO drug dictionary, all non-pharmacological treatment were classified as “non-pharmacological”.
The most prominent medications were for diseases of the musculoskeletal system (64), alimentary tract and metabolism (53), respiratory system (41), treatments for diseases of the nervous system (41) and various others (51).
Beside these, 21 non-pharmacological treatments were reported, these are physiotherapy (8), orthopedic surgeries (3), rehabilitation facility stays (3), psychotherapy (2), hearing aids (1), acupuncture (1), ultrasound therapy (1), dental treatment (1), laser eye treatment (1).
None of the recorded previous treatments fulfilled any of the exclusion criteria.
Primary Endpoint: Quality of Life Assessment by SF-36 Questionnaire (All Indication Groups)
In all indication groups, the SF-36 total score steadily increased (Figure 2a). Repeated-measures ANOVA yielded highly significant results for both the overall time effect and the time effects within each individual indication group after Greenhouse-Geisser or Huynh-Feldt corrections for deviation from sphericity (Table 2). These results could be confirmed by non-parametric Friedman rank sum tests which were performed additionally because of departure from sphericity.
 |
Table 2 Results of Repeated Measurement ANOVA and Friedman Test of SF-36 Scores and Physical and Mental Components. Clinically Significant Results (P < 0.05) are Indicated in Bold |
The highest daily increase (slope of the curve) could be found in all groups except the migraine group between the initial and the second study visit, whereas for migraine the highest improvement rate occurred between study days 28 and 84.
Group differences are due to the low baseline scores of the fibromyalgia (50) and depression (59) groups, in the other groups, the baseline value is about 65 scoring points.
The highest overall increases in SF-36 score occurred in the fibromyalgia and depression group (20 scoring points), whereas in the other groups the scores increased by about 12 points.
Group differences are caused due to differences in baseline values; after adjustment for baseline score, no significant differences could be detected (ANCOVA for group differences in final score with baseline adjustment, Df = 4, F = 1.342, p = 0.2548).
When separating the SF-36 score into a physical and a mental component, both scores increased steadily during the study (Figure 2b and c). For all but the mental component in the chronic back pain group, these increases are statistically significant (Table 2).
For the physical component, significant group differences (group effect of repeated-measure ANOVA with Greenhouse-Geisser correction for departure from sphericity; DF = 4;241, F = 10.78, p <2e-16) were mainly due to the comparable lower increase in the depression group (about 4 scoring points) in comparison to the other groups (7–8 scoring points).
For the mental component, the situation is reversed; here significant group effects (group effect of repeated measure ANOVA with Greenhouse-Geisser correction for departure from sphericity; DF = 4;241, F = 16.87, p <2e-16) are mainly due to the much higher increase in the depression group (about 16 scoring points), whereas the increases in the other groups were between about 3 (chronic back pain) and 9 scoring points (fibromyalgia).
The relative partitioning of both components mirrors its importance in the different indications: For the indication group with pain-related issues (back and skeletal system pain), the improvement of the physical component was dominant, whereas in the depression group the effect of the mental component is prevailing. In fibromyalgia and migraine both components improved to almost the same extent.
The increase in total score (12–20 scoring points) is at least twice as high as the minimal clinically important differences as limit to indicate clinical relevance; these are 5 scoring points.98 For the physical component scale, in all indication groups but the depression group, the increase of the score (about 7 points) is higher than the mentioned limit for clinical relevance (4); for the mentioned component the fibromyalgia group (about 9) and the depression group (15), the increase was above the limit for clinical relevance (3.2).
Secondary Endpoints
In each of the five indications additionally to the generic SF-36 instrument at least one more specific assessment instrument was used for efficacy assessment. For chronic back pain (Figure 3a) and skeletal system pain (Figure 3b) a standardized pain assessment instrument was used, for fibromyalgia (Figure 3c) and depression (Figure 3d) anxiety severity was measured by use of GAD7, insomnia severity by use of ISI and for severity of depression the PHQ9 instrument was used. Migraine severity was assessed by the MiDAS questionnaire in the respective indication group (Figure 3e).
In both pain groups, the chronic back pain and the skeletal system pain group, pain intensity dropped steadily from moderate intensity (>4) at baseline by almost 2 scoring points. Final values are within the range of mild intensity. This effect is statistically highly significant (Table 3) and clinically relevant.99,100
For anxiety severity (GAD-7),101,102 severity of depression symptoms (PHQ-9)101,103 and insomnia severity (ISI)96,104 the decline in scores during the study is both highly significant (Table 3) and clinically relevant in the fibromyalgia and the depression group.
In the migraine group, no relevant changes in total MiDAS score, consisting of 5 categories asking for days of impairment during the previous three months, could be detected during the study.105,106 The indicated number of days of impairments declined marginally from visit 1 to visit 2, remains unchanged up to visit 3 and then slightly increased again. These changes were statistically not significant (p = 0.5777 for repeated measure ANOVA after Greenhouse-Geisser correction; 14.2.4).
Numbers of days with headache declined slightly during the first study period and thereafter stayed almost constant until study completion. The decline in the first study period was statistically significant (p = 0.004).
For pain intensity, a slight, but significant decline by only 0.7 assessment points could be observed (p = 0.04), mainly in the first 3 study months (up to visit 3).
Safety Evaluation
In total, 246 adverse events occurred in 161 participants of the safety data set (256 participants who signed the informed consent form).
Most frequent AEs were related to COVID-19 (88), the respiratory system (54), the musculoskeletal system and connective tissue (23) and diseases of the digestive system.
A listing of the frequency of AEs per ICD10 class is given in Table 4.
 |
Table 4 Adverse Events According to ICD-10 Class Assignment in Descending Order According to the Frequencies |
Seventeen adverse events occurred after the person signed the informed consent form but prior to Visit 1.
All these pre-study AEs were assessed as being not related to the Healy device electrostimulation.
In total 230 adverse events occurred during the study, after visit 1.
In total 12 serious adverse events (SAEs) occurred in 12 different participants during the study (Table 5). All were resolved during the study. The severity of these SAEs was assessed as being “mild” in 2 cases, “moderate” in 2 cases and “severe” in 8 cases. For all events, at least one treatment was applied. None of the SAEs was related to the application of electrostimulation by the Healy device. The intensity of most AEs was assessed as mild (199), 22 were assessed as being of moderate intensity, 9 AEs were severe.
 |
Table 5 Serious Adverse Events per Patient |
Two hundred and twenty-six AEs were assessed as being not related to the Healy electrostimulation; 4 AEs as related to the electrostimulation with the Healy device.
The average incidence of AEs is slightly higher than the average incidence of diseases within a comparable population of persons not suffering from chronic diseases in Germany during the COVID-19 pandemic.107
Four adverse events in total, all non-serious, were assessed to be related to the Healy device. In 2 cases, the electrostimulation induced additional pain of mild intensity, the other 2 events were adverse skin reactions. The respective conditions are mentioned in the current Healy Instructions for Use as potential side effects of Healy electrostimulation. In 2 of the 4 cases, the respective AEs are the cause of premature study termination.
Discussion
For all indications, most commonly fibromyalgia, the baseline values of the SF-36 scores are below the average values of a comparable population collected in a large survey in Germany.106 This, along with the fact that values increased most during the first phase of the study, makes it clear that participants were using microcurrent therapy rather sub optimally before the study began. The consultation with the investigator at the beginning of the study, together with increased motivation, obviously leads to a better and possibly more frequent use of the investigated microcurrent therapy.
During the first month of study, SF-36 scores as measure for the health-related quality of life initially increased sharply. After the first 2 visits, this increase flattened out, but continued until the end of the study. At this time, for all subclasses except fibromyalgia, the level of a healthy comparison population was reached,108 whereas for fibromyalgia, the final values were slightly lower.
The same pattern as described for SF-36 was also detected for pain assessment in the chronic back pain and skeletal system pain group, for Generalized Anxiety Disorder (GAD), Patient Health Questionnaire (PHQ) and Insomnia Severity Index (ISI) in the fibromyalgia and depression group. After a large improvement, scores in these cases continued to decline to a lesser extent during the initial phase of the study (up to the second visit) until the conclusion of the study.
This is clear evidence of the effectiveness of the therapy under investigation. The low baseline values and the gradual improvements in the first 4 study weeks may indicate that optimization of the Instructions for Use and better support channels are required to safeguard the optimal use of the device, also under non-study conditions.
Except for the MiDAS score for all parameters measured during the study, almost the same pattern of score improvement could be detected: Starting from baseline values within a range indicating at least moderate impairment,109 the levels improved markedly until study completion. At the end of the study, the respective levels were within the range of a comparable healthy population (for SF-36) or improved by at least one degree in severity, eg, from moderate impairments to mild impairments for GAD in the fibromyalgia and depression group. These effects are clinically significant, and the changes are evidently above the levels for Minimum Clinically Important Differences (MCID, for values and sources see Table 6).
 |
Table 6 Minimum Clinically Important Differences as Mentioned in Scientific Publications |
The Minimum Clinically Important Difference (MCID) represents the smallest improvement considered worthwhile by a patient. This concept is offered as the new standard for determining effectiveness of a given treatment and describing patient satisfaction in reference to that treatment.111 The patient-centered approach of MCID has some shortcomings in terms of methods of determination, but is still a standardized measure of therapy success.
For the MiDAS total score, no improvements could be detected within the complete study. This may be caused by the fact that this instrument refers to changes within a 3-months period, resulting in some overlapping and in some difficulties in reporting reliable values.
Despite this methodical issue, for the migraine group a positive effect could be detected as well, as assessed by the SF-36 instruments and the additional MiDAS questions asking for days with headache (improvement by 4 days per period) and intensity of headache (improvement by 0.7 scoring points).
Cohen’s approach112,113 to determine the effect size calculates the ratio of the difference between the group means and the value dispersion. Values less than 0.5 are considered small (unhedged) effects. Values between 0.5 and 0.8 are considered medium and values greater than 0.8 are considered strong effects. According to this classification, there are strong effects for the changes in all parameters in this observational study, except for MiDAS, as further evidence of the effectiveness of Healy electrostimulation. For MiDAS, the values are in the range of unhedged effects. It is possible that for this instrument neither the number of participants nor the length of the observational period was sufficient to detect any positive effects. The other potential explanation is that microcurrent may have a strong positive effect on the general health status in patients suffering from migraine, but the direct effect on the symptoms of the disease itself is weak.
In randomized blinded clinical trials, microcurrent therapy was only considered effective when its effect was better than the placebo response triggered by a sham treatment. Nevertheless, in patient care, effective treatment is unavoidably delivered with additional placebo response. This is also applicable in this observational study. Given that the beneficial effects of the placebo effect are often clinically significant, especially in chronic pain,14,114 assessing the overall treatment effect that includes placebo response is important in optimizing patient care.
To assess the effect of concomitant therapies on study outcome parameters, analyses of the complete populations were compared with results excluding all participants applying any therapy potentially having a positive effect on study outcome. Twenty participants in total were excluded in the secondary analysis set due to this assessment.
The comparisons of the two analyses were performed for all parameters and yielded only marginal differences for the two populations (<3%).
This study was performed in participants already using the Healy device and having a positive attitude concerning its effectiveness. This has to be taken into account, when generalizing the study results. The effectiveness and effect sizes within this group may be higher than in a comparable group, having no experiences in microcurrent applications.
In total, 230 adverse events occurred during the study. This could be interpreted as an average incidence of about 1.8 events per participant per year. In comparison to the average sick days per year in the German adult population (1.1 sick days per year in 2021 and 2022),107,108 this seems to be quite high. But the difference could be easily explained by the fact that the average age of the study population is higher than the mean of the employed population. Additionally, all participants in the study were suffering from at least one chronic condition. Both factors (higher age and chronic diseases) are increasing the incidence of diseases in comparison with younger healthy persons.
Four adverse events of mild intensity were related with the application of electrostimulation. The corresponding complaints (pain twice, skin reactions twice) are already listed in the Healy Instructions for Use as possible side effects. Considering that the 250 study participants have used the investigational microcurrent device at least once per day for 6 months, the incidence rate of side effects in this study is very low (1 case per 10.000 applications). This underpins that microcurrent electrostimulation is safe and well tolerated by its users.
Conclusion
For all parameters except one, the improvements between study entry (V1) and study completion (V4) are both significant and clinically relevant. Being aware and taking into consideration that the mode of action of microcurrent electrostimulation therapy is not fully understood, this observational study clearly demonstrated its effectiveness and safety.
Acknowledgments
Mrs. Annemarie Blam contributed as investigator in the study; she performed the remote study visits with the participants and verified the inclusion/exclusion criteria. Mrs. Stephanie Reddig drafted some essential study documents, performed the submission to the ethics committee and contributed to data management activities. Mr. Georg Salcher reviewed the first draft version of this report.
Disclosure
PM is an employee of Healy GmbH, the sponsor of the study. WW received consulting fees from Healy GmbH. The authors report no other conflicts of interest in this work.
References
1. Piccolino M. Luigi Galvani and animal electricity: two centuries after the foundation of electrophysiology. Trend Neurosci. 1997;20(10):443–448. doi:10.1016/S0166-2236(97)01101-6
2. Heidland A, Fazeli G, Klassen A, et al. Neuromuscular electrostimulation techniques: historical aspects and current possibilities in treatment of pain and muscle waisting. Clin Nephrol. 2013;79(Suppl 1):S12–S23.
3. Wing TW. Microcurrent Therapy, Reprints 1. Pioneering Microcurrent Research and Applied Electro-Acupoint & Therapy Articles. Available from: https://earthen.com/products/author/dr-thomas-w-wing.
4. Chaitow L, McMakin C. McMakin Frequency Specific Microcurrent in Pain Management, 2010, Churchill Livingstone (Verlag), 978-0-443-06976-5 (ISBN).
5. Cheng N, Van Hoof H, Bockx E, et al. The effects of electric currents on ATP generation, protein synthesis and membrane transport of rat skin. Clin Orthop Relat Res. 1982;171:264–272.
6. Mannheimer JS. The effect of microcurrent stimulation on ATP synthesis in the human masseter as evidenced by 31P magnetic resonance spectroscopy. Seton Hall University Dissertations and Theses (ETDs); 2005. Available from: https://scholarship.shu.edu/dissertations/1544.
7. Konstantinou E, Zagoriti Z, Pyriochou A, Poulas K. Microcurrent stimulation triggers MAPK signaling and TGF-β1 release in fibroblast and osteoblast-like cell lines. Cells. 2020;9(9):1924. doi:10.3390/cells9091924
8. Scholkmann F, Fels D, Cifra M. Non-chemical and non-contact cell-to-cell communication: a short review. Am J Transl Res. 2013;5(6):586–593.
9. Kolimechkov S, Seijo M, Swaine I, et al. Physiological effects of microcurrent and its application for maximising acute responses and chronic adaptations to exercise. Eur J Appl Physiol. 2023;123(3):451–465. doi:10.1007/s00421-022-05097-w
10. Mercola J, Kirsch D. The basis for micro current electrical therapy in conventional medical. J Adv Med. 1995;8:2.
11. Kadrya B. Efficacy of high frequency versus low frequency micro current electrical stimulation on resistivity index and blood flow volume in normal subjects. J Circ. 2018;2(1):3.
12. Park R, Son H, Kim K, Kim S, Taeyoung O. The effect of microcurrent electrical stimulation on the foot blood circulation and pain of diabetic neuropathy. J Physi Ther Sci. 2011;23:515–518. doi:10.1589/jpts.23.515
13. Sabel BA, Zhou W, Huber F, et al. Non-invasive brain microcurrent stimulation therapy of long-COVID-19 reduces vascular dysregulation and improves visual and cognitive impairment. Restor Neurol Neurosci. 2021;39(6):393–408. doi:10.3233/RNN-211249
14. Iijima H, Takahashi M. Microcurrent therapy as a therapeutic modality for musculoskeletal system pain: a systematic review accelerating the translation from clinical trials to patient care. Arch Rehabil Res Clin Transl. 2021;3(3):100145. doi:10.1016/j.arrct.2021.100145
15. Moon YS, Kwon DR, Lee YJ. Therapeutic effect of microcurrent on calf muscle atrophy in immobilized rabbit. Muscle Nerve. 2018;58(2):270–276. doi:10.1002/mus.26110
16. Ohno Y, Fujiya H, Goto A, et al. Microcurrent electrical nerve stimulation facilitates regrowth of mouse soleus muscle. Int J Med Sci. 2013;10(10):1286–1294. doi:10.7150/ijms.5985
17. Lerman I, Hauger R, Sorkin L, et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation. 2016;19(3):283. doi:10.1111/ner.12398
18. Yu C, Hu ZQ, Peng RY. Effects and mechanisms of a microcurrent dressing on skin wound healing: a review. Military Med Res. 2014;1(24). doi:10.1186/2054-9369-1-24
19. Heffernan M. The effect of variable microcurrents on EEG spectrum and pain control. Can J Clin Med. 1997;4:4–11.
20. Holubec J. Cumulative response from cranial electrotherapy stimulation, (CES) for chronic pain. Pract Pain Manag. 2009;9:1.
21. Tan G, Rintala DH, Thornby JI, et al. Using cranial electrotherapy stimulation to treat pain associated with spinal cord injury. J Rehabil Res Dev. 2006;43(4):461–473. doi:10.1682/JRRD.2005.04.0066
22. Facci L, Nowotny JP, Tormem F, et al. Effects of transcutaneous electrical nerve stimulation (TENS) and interferential currents (IFC) in patients with nonspecific chronic low back pain: randomized clinical trial. São Paulo Med J. 2011;129(4):206–216. doi:10.1590/S1516-31802011000400003
23. Rajfur J, Pasternok M, Rajfur K, et al. Efficacy of selected electrical therapies on chronic low back pain: a comparative clinical pilot study. Med Sci Monit. 2017;23:85–100. doi:10.12659/MSM.899461
24. Boldt I. Non-pharmacological interventions for chronic pain in people with spinal cord injury. Cochrane Database Syst Rev. 2014;11:CD009177.
25. O’Connell N, Wand BM, Marston L, et al. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014. doi:10.1002/14651858.CD008208.pub3
26. Tan G, Alvarez JA, Jensen MP, et al. Complementary and alternative medicine approaches to pain management. J Clin Psychol. 2006;62(11):1419–1431. doi:10.1002/jclp.20321
27. Moreno-Duarte I, Morse LR, Alam M, et al. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage. 2014;85:1003–1013. doi:10.1016/j.neuroimage.2013.05.097
28. Lichtbroun A, Raicer M-MC, Smith RB, et al. The treatment of fibromyalgia with cranial electrotherapy stimulation. J Clin Rheumatol. 2001;7(2):72–78. doi:10.1097/00124743-200104000-00003
29. Cork R. The effect of cranial electrotherapy stimulation (CES) on pain associated with fibromyalgia. Internet J Anesthesiol. 2004;8:1092–1406.
30. Taylor A, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327–335. doi:10.1016/j.pmn.2011.07.002
31. Taylor A, Anderson JG, Riedel SL, et al. A randomized, controlled, double-blind pilot study of the effects of cranial electrical stimulation on activity in brain pain processing regions in individuals with fibromyalgia. Explor J Sci Heal Heal. 2013;9:32–40.
32. Löfgren M, Norrbrink C. Pain relief in women with fibromyalgia: a cross-over study of superficial warmth stimulation and transcutaneous electrical nerve stimulation. J Rehabil Med. 2009;41(7):557–562. doi:10.2340/16501977-0371
33. Carbonario F, Matsutani LA, Yuan SLK, et al. Effectiveness of high-frequency transcutaneous electrical nerve stimulation at tender points as adjuvant therapy for patients with fibromyalgia. Eur J Phys Rehabil Med. 2013;49(2):197–204.
34. Gur A. Physical therapy modalities in management of fibromyalgia. Curr Pharm Des. 2006;12(1):29–35. doi:10.2174/138161206775193280
35. Gilula M. Cranial electrotherapy stimulation and fibromyalgia. Expert Rev Med Devices. 2007;4(4):489–495. doi:10.1586/17434440.4.4.489
36. de Silva Salazar A, Stein C, Marchese RR, et al. Electric stimulation for pain relief in patients with fibromyalgia: a systematic review and meta-analysis of randomized controlled trials. Pain Physician. 2017;20(20;2):15–25. doi:10.36076/ppj/2017/25
37. Johnson M, Claydon LS, Herbison GP, et al. Transcutaneous electrical nerve stimulation for fibromyalgia in adults. Cochrane Database Syst Rev. 2017;2017(10). doi:10.1002/14651858.CD012172.pub2
38. Benlidayi I. The effectiveness and safety of electrotherapy in the management of fibromyalgia. Rheumatol Int. 2020;40(10):1571–1580. doi:10.1007/s00296-020-04618-0
39. Rockstroh G, Schleicher W, Krummenauer F, et al. Effectiveness of microcurrent therapy as a constituent of post-hospital rehabilitative treatment in patients after total knee alloarthroplasty - a randomized clinical trial. Rehabilitation. 2010;49(03):173–179. doi:10.1055/s-0029-1246152
40. Tedesco D, Gori D, Desai KR, et al. Drug-free interventions to reduce pain OR opioid consumption after total knee arthroplasty a systematic review and meta-analysis. JAMA Surg. 2017;152(10):1–13. doi:10.1001/jamasurg.2017.2872
41. Kaya Mutlu E, Ercin E, Razak Ozdıncler A, et al. A comparison of two manual physical therapy approaches and electrotherapy modalities for patients with knee osteoarthritis: a randomized three arm clinical trial. Physiother Theory Pract. 2018;34(8):600–612. doi:10.1080/09593985.2018.1423591
42. Ranker A, Husemeyer O, Cabeza-Boeddinghaus N, et al. Microcurrent therapy in the treatment of knee osteoarthritis: could it be more than a placebo effect? A randomized controlled trial. Eur J Phys Rehabil Med. 2020;56(4):459–468. doi:10.23736/S1973-9087.20.05921-3
43. Zuim P, Garcia AR, Turcio KHL, et al. Evaluation of microcurrent electrical nerve stimulation (MENS) effectiveness on muscle pain in temporomandibular disorders patients. J Appl Oral Sci. 2006;14(1):61–66. doi:10.1590/S1678-77572006000100012
44. Rajpurohit B, Khatri S, Metgud D, et al. Effectiveness of transcutaneous electrical nerve stimulation and microcurrent electrical nerve stimulation in bruxism associated with masticatory muscle pain - a comparative study. Indian J Dent Res. 2010;21(1):104. doi:10.4103/0970-9290.62816
45. Saranya B, Ahmed J, Shenoy N, et al. Comparison of transcutaneous electric nerve stimulation (TENS) and microcurrent nerve stimulation (MENS) in the management of masticatory muscle pain: a comparative study. Pain Res Manag Nov. 2019;2019:1–5. doi:10.1155/2019/8291624
46. Kang D, Jeon J-K, Lee J-H, et al. Effects of low-frequency electrical stimulation on cumulative fatigue and muscle tone of the erector spinae. J Phys Ther Sci. 2015;27(1):105–108. doi:10.1589/jpts.27.105
47. Poltawski L, Johnson M, Watson T, et al. Microcurrent therapy in the management of chronic tennis elbow: pilot studies to optimize parameters. Physiother Res Int. 2012;17(3):157–166. doi:10.1002/pri.526
48. Brotman P. Low-intensity transcranial electrostimulation improves the efficacy of thermal biofeedback and quieting reflex training in the treatment of classical migraine headache. Am J Electromed. 1989;6:120–123.
49. Solomon S, Elkind A, Freitag F, et al. Safety and effectiveness of cranial electrotherapy in the treatment of tension headache. Headache J Head Face Pain. 1989;29(7):445–450. doi:10.1111/j.1526-4610.1989.hed2907445.x
50. Vernon H, Hagino C, Vernon H, et al. Systematic review of randomized clinical trials of complementary/alternative therapies in the treatment of tension-type and cervicogenic headache. Complement Ther Med. 1999;7(3):142–155. doi:10.1016/S0965-2299(99)80122-8
51. Perini F, De Boni A. Peripheral neuromodulation in chronic migraine. Neurol Sci. 2012;33(S1):31–33. doi:10.1007/s10072-012-1039-4
52. Tong K, Lo SK, Cheing GL, et al. Alternating frequencies of transcutaneous electric nerve stimulation: does it produce greater analgesic effects on mechanical and thermal pain thresholds? Arch Phys Med Rehabil. 2007;88(10):1344–1349. doi:10.1016/j.apmr.2007.07.017
53. Tan G, Rintala DH, Jensen MP, et al. Efficacy of cranial electrotherapy stimulation for neuropathic pain following spinal cord injury: a multi-site randomized controlled trial with a secondary 6-month open-label phase. J Spinal Cord Med. 2011;34(3):285–296. doi:10.1179/2045772311Y.0000000008
54. Nizard J, Lefaucheur J-P, Helbert M, et al. Non-invasive stimulation therapies for the treatment of refractory pain. Discov Med. 2012;14(74):21–31.
55. Gabriel A, Sobota R, Gialich S, et al. The use of targeted microcurrent therapy in postoperative pain management. Plast Surg Nurs. 2013;33(1):6–8; quiz 9–10. doi:10.1097/PSN.0b013e3182844219
56. Nardone R, Höller Y, Leis S, et al. Invasive and non-invasive brain stimulation for treatment of neuropathic pain in patients with spinal cord injury: a review. J Spinal Cord Med. 2014;37(1):19–31. doi:10.1179/2045772313Y.0000000140
57. Pal U, Kumar L, Mehta G, et al. Trends in management of myofacial pain. Natl J Maxillofac Surg. 2014;5(2):109. doi:10.4103/0975-5950.154810
58. Schmitt R, Capo T, Boyd E, et al. Cranial electrotherapy stimulation as a treatment for anxiety in chemically dependent persons. Alcohol Clin Exp Res. 1986;10(2):158–160. doi:10.1111/j.1530-0277.1986.tb05064.x
59. Gibson T, O’Hair D. Cranial application of low level transcranial electrotherapy vs. relaxation instruction in anxious patients. Am J Electromed. 1987;4:18–21.
60. Winick RL. Cranial electrotherapy stimulation (CES): a safe and effective low cost means of anxiety control in a dental practice. Gen Dent. 1999;47(1):50–55.
61. Overcash S. Cranial electrotherapy stimulation in patients suffering from acute anxiety disorders. Am J Electromed. 1999;16:49–51.
62. Bystritsky A, Kerwin L, Feusner J, et al. A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. J Clin Psychiatry. 2008;69(3):412–417. doi:10.4088/JCP.v69n0311
63. Kim H, Kim WY, Lee YS, et al. The effect of cranial electrotherapy stimulation on preoperative anxiety and hemodynamic responses. Korean J Anesthesiol. 2008;55(6):657–661. doi:10.4097/kjae.2008.55.6.657
64. Lee S, Kim W-Y, Lee C-H, et al. Effects of cranial electrotherapy stimulation on preoperative anxiety, pain and endocrine response. J Int Med Res. 2013;41(6):1788–1795. doi:10.1177/0300060513500749
65. Koleoso O, Osinowo HO, Akhigbe KO. The role of relaxation therapy and cranial electrotherapy stimulation in the management of dental anxiety in Nigeria. J Dent Med Sci. 2013;10:51–57.
66. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm. 2012;120(5):821–827. doi:10.1007/s00702-012-0908-6
67. McClure D, Greenman SC, Koppolu SS, et al. A pilot study of safety and efficacy of cranial electrotherapy stimulation in treatment of bipolar II depression. J Nerv Ment Dis. 2015;203(11):827–835. doi:10.1097/NMD.0000000000000378
68. Yixin C, Lin Y, Jiuping Z, et al. Results of cranial electrotherapy stimulation to children with mixed anxiety and depressive disorder. Shanghai Arch Psych. 2007;19:203–205.
69. Barclay T, Barclay R. A clinical trial of cranial electrotherapy stimulation for anxiety and comorbid depression. J Affect Disord. 2014;164:171–177. doi:10.1016/j.jad.2014.04.029
70. Roh H, So W. Cranial electrotherapy stimulation affects mood state but not levels of peripheral neurotrophic factors or hypothalamic-pituitary-adrenal axis regulation. Technol Heal Care. 2017;25(3):403–412. doi:10.3233/THC-161275
71. Barabasz A. Treatment of insomnia in depressed patients by hypnosis and cerebral electrotherapy. Am J Clin Hypn. 1976;19(2):120–122. doi:10.1080/00029157.1976.10403850
72. Wagenseil B, Garcia C, Suvorov AV, et al. The effect of cranial electrotherapy stimulation on sleep in healthy women. Physiol Meas. 2018;39(11):114007. doi:10.1088/1361-6579/aaeafa
73. Kavirajan H, Lueck K, Chuang K. Alternating current cranial electrotherapy stimulation (CES) for depression. Cochrane Database Syst Rev. 2013;2013:1
74. Philip N, Nelson BG, Frohlich F, et al. Low-intensity transcranial current stimulation in psychiatry. Am j Psychiatry. 2017;174(7):628–639. doi:10.1176/appi.ajp.2017.16090996
75. Gilula M, Kirsch D. Cranial electrotherapy stimulation review: a safer alternative to psychopharmaceuticals in the treatment of depression. J Neurother. 2005;9(2):37–41. doi:10.1300/J184v09n02_02
76. Novakovic V, Sher L, Lapidus KB, et al. Brain stimulation in posttraumatic stress disorder. Eur J Psychotraumatol. 2011;2(1):5609. doi:10.3402/ejpt.v2i0.5609
77. Kirsch D, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169–176. doi:10.1016/j.psc.2013.01.006
78. Huang Y, Lane H-Y, Lin C-H, et al. New treatment strategies of depression: based on mechanisms related to neuroplasticity. Neural Plast. 2017;2017:1–11. doi:10.1155/2017/4605971
79. O’Caoimh R, Mannion H, Sezgin D, et al. Non-pharmacological treatments for sleep disturbance in mild cognitive impairment and dementia: a systematic review and meta-analysis. Maturitas. 2019;127:82–94. doi:10.1016/j.maturitas.2019.06.007
80. Lande R, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8–13. doi:10.1016/j.ctim.2012.11.007
81. Carlson RV, Boyd KM, Webb DJ. The revision of the declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 2004;57(6):695–713. doi:10.1111/j.1365-2125.2004.02103.x
82. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): i. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-00002
83. Hays RD, Shapiro MF. An overview of generic health-related quality of life measures for HIV research. Qual Life Res. 1992;1(2):91–97. doi:10.1007/BF00439716
84. Steward AL, Sherbourne C, Hayes RD, et al. Summary and discussion of MOS measures. In: Stewart AL, Ware JE, editorwebs. Measuring Functioning and Well-Being: The Medical Outcome Study Approach. Durham, NC: Duke University Press; 1992:345–371.
85. Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395–404. doi:10.1023/A:1012552211996
86. Ware JE, Kosinski M, Bayliss MS, McHorney C, Rogers WH, Raczek A. Comparison of methods for scoring and statistical analysis of the SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med Care. 1995;33:AS264–79.
87. Harris V, Hughes M, Roberts R, Dolan G, Williams EM. The development and testing of a Chemotherapy-Induced Phlebitis Severity (CIPS) scale for patients receiving anthracycline chemotherapy for breast cancer. J Clin Med. 2020;9(3):701. doi:10.3390/jcm9030701
88. Bolognese JA, Schnitzer TJ, Ehrich EW. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarth Cartil. 2003;11(7):499–507. doi:10.1016/S1063-4584(03)00082-7
89. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MiDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–8. doi:10.1212/wnl.56.suppl_1.s20
90. Stewart WF, Lipton RB, Whyte J. An international study to assess reliability of the Migraine Disability Assessment (MiDAS) score. Neurology. 1999;53(5):S. 988–994. doi:10.1212/WNL.53.5.988
91. Agosti R, Chrubaski JE, Kohlmann T. Der MiDAS-Fragebogen. Sprachliche Validierung der deutschen Version, Veröffentlichungen des Kopfwehzentrums Hirslanden.
92. Busch M, Maske U, Ryl L, Schlack R, Hapke U. Prävalenz von depressiver Symptomatik und diagnostizierter Depression bei Erwachsenen in Deutschland, Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Publikationsserver des RKI; 2013.
93. Kroenke RL, Spitzer RL, Williams JBW. The PHQ-9. Validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):S. 606–613. doi:10.1046/j.1525-1497.2001.016009606.x
94. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–1097. doi:10.1001/archinte.166.10.1092
95. Mossman SA, Luft MJ, Schroeder HK, et al. The Generalized Anxiety Disorder 7-item scale in adolescents with generalized anxiety disorder: signal detection and validation. Ann Clin Psychiatry. 2017;29(4):227–234A.
96. Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601
97. Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi:10.1016/S1389-9457(00)00065-4
98. Badhiwala JH, Witiw CD, Nassiri F, et al. Minimum clinically important difference in SF-36 Scores for use in degenerative cervical myelopathy. Spine (Phila Pa 1976). 2018;43(21):E1260–E1266. doi:10.1097/BRS.0000000000002684
99. Olsen MF, Bjerre E, Hansen MD, et al. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(35). doi:10.1186/s12916-016-0775-3
100. Laigaard J, Pedersen C, Rønsbo TN, Mathiesen O, Karlsen APH. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: a systematic review. Br J Anaesth. 2021;126(5):1029–1037. doi:10.1016/j.bja.2021.01.021
101. Bauer-Staeb C, Kounali D-Z, Welton NJ, et al. Effective dose 50 method as the minimal clinically important difference: evidence from depression trials. J Clin Epidemiol. 2021;137:200–208. doi:10.1016/j.jclinepi.2021.04.002
102. Toussaint A, Hüsing P, Gumz A, et al. Sensitivity to change and minimal clinically important difference of the 7-item Generalized Anxiety Disorder Questionnaire (GAD-7). J Affect Disord. 2020;265:395–401. doi:10.1016/j.jad.2020.01.032
103. Lynch CP, Cha EDK, Jenkins NW, et al. The minimum clinically important difference for patient health questionnaire-9 in minimally invasive transforaminal interbody fusion. Spine. 2021;46(9):603–609. doi:10.1097/BRS.0000000000003853
104. Yang M, Morin CM, Schaefer K, Wallenstein GV. Interpreting score differences in the insomnia severity index: using health-related outcomes to define the minimally important difference. Curr Med Res Opin. 2009;25(10):2487–2494. doi:10.1185/03007990903167415
105. Carvalho GF, Luedtke K, Braun T. Minimal important change and responsiveness of the Migraine Disability Assessment Score (MIDAS) questionnaire. J Headache Pain. 2021;22(1):126. doi:10.1186/s10194-021-01339-y
106. Buse DC, Lipton RB, Hallström Y, et al. Migraine-related disability, impact, and health-related quality of life among patients with episodic migraine receiving preventive treatment with erenumab. Cephalalgia. 2018;38(10):1622–1631. doi:10.1177/0333102418789072
107. Informationsdienst des Instituts der deutsche Wirtschaft; Der Krankenstand in Deutschland; 2023. Available from: https://www.iwd.de/artikel/krankenstand-in-deutschland-498654.
108. Ellert U. Gesundheitsbezogene Lebensqualität bei Erwachsenen in Deutschland Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1) Bundesgesundheitsbl. Robert Koch-Institut; 2013:56:643–649. doi:10.1007/s00103-013-1700-y
109. Agosti RETO, Julia C, Kohlmann E. Der MIDAS-Fragebogen. Ars Medici. 2008;16:700–701.
110. Brigden A, Parslow RM, Gaunt D, et al. Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: triangulation using three different methods. Health Qual Life Outcomes. 2018;16(1):202. doi:10.1186/s12955-018-1028-2
111. Copay AG, Subach BR, Glassman SD, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi:10.1016/j.spinee.2007.01.008
112. Cohen J. Jacob Cohen: a power primer. Psychol Bull Band. 1992;112(1):155–159. doi:10.1037/0033-2909.112.1.155
113. Cohen J. Statistical Power Analysis for the Behavioral Sciences.
114. Zhang W, Robertson J, Jones AC, Dieppe PA, Doherty M. The placebo effect and its determinants in osteoarthritis: meta-analysis of randomized controlled trials. Ann Rheum Dis. 2008;67(12):1716–1723. doi:10.1136/ard.2008.092015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



