Back to Journals » Research and Reports in Neonatology » Volume 14
Obstacles to the Early Diagnosis and Management of Patent Ductus Arteriosus
Authors Gowda SH, Philip R, Weems MF
Received 1 October 2023
Accepted for publication 15 February 2024
Published 29 February 2024 Volume 2024:14 Pages 43—57
DOI https://doi.org/10.2147/RRN.S409744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
Sharada H Gowda,1 Ranjit Philip,2 Mark F Weems3
1Department of Pediatrics and Division of Neonatology, Baylor College of Medicine, Houston, TX, USA; 2Department of Pediatrics, Division of Cardiology, Le Bonheur Children’s Hospital, University of Tennessee Health Science Center, Memphis, TN, USA; 3Department of Pediatrics, Division of Neonatology, Le Bonheur Children’s Hospital, University of Tennessee Health Science Center, Memphis, TN, USA
Correspondence: Mark F Weems, University of Tennessee Health Science Center, Department of Pediatrics, Division of Neonatology, 853 Jefferson Ave, Rout Bldg E201, Memphis, TN, 38163, USA, Tel +1 901-448-5950, Fax +1 901-448-1691, Email [email protected]
Abstract: “What we know is little, and what we are ignorant of is immense”; the last words of Laplace still apply to the diagnosis and management of the patent ductus arteriosus (PDA). Despite decades of research, we are searching for the right approach to care for patients with PDA. Nuances of myocardial structural changes and cardiopulmonary interactions with prolonged exposure to excess pulmonary blood flow have played an important role in decision-making. The availability of medical treatments with poor efficacy and, historically, surgical ligation as the only available definitive therapy further widened the gap between observation and definitive closure. As more extremely low birth weight neonates born at earlier gestational ages survive, we are faced with a population whose physiological immaturity and structural alignment of the myocardium predisposes them to myocardial dysfunction and dysregulated vascular tone. Therefore, it may be time to replace historical approaches with a more precise patient-centric therapeutic model. A comprehensive serial echocardiography assessment of the heart function, hemodynamic significance, and clinical context with respect to pulmonary insufficiency and gut perfusion aids the neonatologist in making PDA management decisions. A targeted approach balances risks and benefits of therapy, avoids treatment for infants likely to have early spontaneous closure, and limits prolonged exposure to the pathologic PDA shunt in high-risk infants. There is significant variability in the diagnosis and treatment of the PDA, both within and across centers. This review highlights the clinical obstacles contributing to the variability and illustrates the need for a standardized approach to PDA diagnosis and management.
Keywords: preterm, neonate, echocardiogram, neonatology, cardiology, shunt
Introduction
“What we know is little, and what we are ignorant of is immense”; it is said that these were Laplace’s last words before his death.1 Laplace’s law and his alleged last words still hold true in PDA physiology and management a few centuries later. The ductus arteriosus (DA) was discovered as early as the second century, AD. Galen first described a fetal shunt from the pulmonary artery to the aorta, circumventing the lungs.2 A remnant of the sixth embryonic aortic arch, this connection between the left pulmonary artery and aorta allows the majority of right-sided cardiac output to bypass the lungs, sending blood flow to the descending aorta, feeding the systemic circulation and returning blood to the placenta via umbilical arteries (Figure 1A). The ductal tissue maintains patency in utero by multiple mechanisms, most prominently prostaglandin E2 (PGE2). Over time, the ductal tissue changes in morphology and response to chemical mediators such that the ductus at term gestation is significantly different from the preterm ductus.3
By the 16th century, spontaneous closure of the DA had been described, but understanding the mechanism of closure remains incomplete.4 At delivery, simultaneous decreases in placenta-derived PGE2 and pulmonary vascular resistance with an increase in blood oxygen content contribute to ductal constriction.5 In the term infant, this typically leads to functional closure within hours of birth. Compression of vaso vasorum supplying the ductal smooth muscle leads to tissue necrosis and permanent closure. The preterm infant, however, is at risk for prolonged patent ductus arteriosus (PDA) due to underdeveloped smooth muscle, immature vaso vasorum, and decreased responsiveness to changes in PGE2 compared to the term infant.3 In this case, the fetal shunt reverses, sending left ventricular output across the PDA and back to the lungs (Figure 1B). While it is well known that nearly all preterm infants are born with a PDA and spontaneous closure may be delayed, the diagnosis of pathologic PDA and the benefit of interventions aimed at reducing the negative effects of the PDA remain controversial. Decision-making for PDA treatment has been historically centered around clinical presentation in the absence of objective echocardiographic findings. There is a growing body of literature supporting the use of targeted hemodynamic assessment by echocardiography to guide the bedside management of the PDA.6–8
Negative Outcomes Associated with the Ductus Arteriosus
After birth, prolonged ductal patency has been associated with multiple negative outcomes. The systemic to pulmonary shunt reduces blood flow to the systemic circulation, including the brain, gastrointestinal tract, and kidneys, a phenomenon called “ductal steal”. Additionally, ductal shunting results in lower diastolic pressure which impairs coronary perfusion pressure and organ perfusion pressure.9 In the early neonatal period, decreased cerebral perfusion has been associated with intraventricular hemorrhage.10 A prolonged shunt is associated with poor brain growth, a risk factor for poor neurodevelopmental outcomes.11 Similarly, in the gastrointestinal tract, decreased perfusion is a risk factor for necrotizing enterocolitis.12
In contrast to most other developing organ systems, the lungs are faced with increased blood flow. Shunting from the aorta across the PDA to the pulmonary artery leads to excess pulmonary blood flow.13,14 Increased pulmonary arterial flow leads to an increase in interstitial lung fluid. This is generally tolerated in the first few days after birth because there is a responsive increase in lymph flow allowing flow to return to the right atrium via the lymphatic system. However, when coupled with inflammation induced by mechanical ventilation, lymph drainage compensation cannot maintain homeostasis.15 At this point, additional flow may contribute to dilation of the left atrium, left ventricle, and aortic root (Figure 1C). Persistent excess flow leads to pulmonary edema. In severe cases, patients suffer from pulmonary hemorrhage which is more common when there is a restrictive atrial shunt or when there is a rapid decrease in mean airway pressure as in the case of extubation.16,17 If the shunt remains significant beyond the transitional period, progressive pulmonary edema and inflammation lead to hypoxemia. The bedside clinician may respond by increasing oxygen delivery or by increasing ventilator pressure, both risk factors for developing bronchopulmonary dysplasia.15 It is described that prolonged ducal patency beyond eight to ten days increases the risk of bronchopulmonary dysplasia (BPD) or death, and patency beyond eight weeks increases the risk of developing pulmonary hypertension.18–20 In addition to the associations prolonged ductal patency has with negative outcomes in specific neonatal organ systems, a prolonged shunt is associated with an 8-fold increase in mortality in the neonatal intensive care unit (NICU).21
Treatment Trends
Differing Views by Specialty
While the patent ductus arteriosus is clearly associated with morbidities and mortality in the NICU, there remains disagreement about the value of intervention. A recent survey of two hundred neonatologists and pediatric cardiologists asked the question, “In your opinion and experience, does closing a hemodynamically significant PDA make a difference in morbidity and mortality for children born <28 weeks’ gestation?”22 In this study, the cardiology group overwhelmingly responded that closing the PDA does make a difference (88 vs 12%), while the neonatologists were split (53 vs 46%).22 A similar survey study among European neonatologists shows wide variability in PDA assessment, diagnosis, and treatment. Furthermore, there is little clinical equipoise, suggesting that PDA practices are more strongly related to personal biases than clinical evidence.23
Decreasing Intervention
Despite a long-standing recognition that the patent ductus is associated with negative outcomes, it is less clear that treatment of the ductus improves outcomes. Multiple analyses of all trials of medical therapy for PDA show that pharmacotherapy decreases ductal patency but does not reduce the incidence of any negative outcome associated with the PDA.24 It has been suggested that PDA may not be a cause of negative outcomes, but simply a marker of a patient who is at risk for the negative outcomes associated with prematurity.25 Alternatively, it may be that closing the PDA does reduce the risk of negative outcomes, but the therapies studied to date, including NSAIDs and surgical ligation, carry a side-effect profile that is harmful enough to counteract the benefits of PDA closure.
Uncertainty about the benefits of PDA closure has led to a reduction in PDA therapy over time. A study of PDA management trends in the United States from 2006 to 2015 show a reduction in PDA ligation across all gestational age groups 23 to 30 weeks. Similarly, there was a reduction in the use of indomethacin or ibuprofen beginning in 2006 for patients born 27 to 30 weeks’ gestation and beginning in 2010 for patients born 23 to 26 weeks’ gestation.26 This downward trend is also seen in the diagnosis of PDA, but there is no evidence that the number of preterm infants who are born with a patent ductus has changed over time. Therefore, a decreasing trend in diagnosis suggests that physicians who are uncertain about PDA management prefer to ignore the PDA rather than try to understand how it may be affecting the patient.
Surgical Ligation and Catheter-Based Closure
Surgical ligation of the PDA was first proposed in 1907, and the technique was first developed in the 1930s.27 Ligation in neonates was increasing in frequency until the mid-2000’s at which point it began to decline.28 The decrease in surgical ligation of the PDA has been more significant than the decrease in medical therapy. Bixler, et al, show that from 2006 to 2015, PDA ligations decreased from nearly 25% in infants born at 23 to 24 weeks’ gestation to approximately 10%. For infants born at 25 to 26 weeks, PDA ligation decreased from approximately 17% to 8%.26 The decrease in surgical ligation is likely due to the recognition of unacceptable side effects of ligation via thoracotomy. Data from the Trial of Indomethacin Prophylaxis in Preterms (TIPP) show that surgical ligation did not affect mortality but was associated with increased BPD, neurosensory impairment, and severe retinopathy of prematurity (ROP).29 However, these data have been challenged with a suggestion that confounding variables in the ligation group are responsible for the poor outcomes. A more recent study shows that neonates who go on to surgical ligation require a significantly higher mean airway pressure than those who do not go on to ligation. This increased need for respiratory support prior to ligation could be the underlying cause of increased BPD, neurodevelopmental delay, and ROP among ligation patients. In fact, Weisz, et al, found improved survival and no differences in neurodevelopmental impairment, chronic lung disease, or severe ROP.30
Although the long-term effects of ligation remain unclear, there are clear negative short-term effects associated with ligation. Multiple studies have shown that patients frequently require an increase in cardiorespiratory support after surgical ligation, a phenomenon called “post-ligation syndrome”.31,32 Recurrent laryngeal nerve injury and vocal cord paralysis are found in up to 40% of patients after ligation.33 Pneumothorax and pulmonary hemorrhage have also been reported.34,35
A relatively new therapy is transcatheter PDA closure (TCPC). This is a well-established intervention for infants greater than 5 kg, but new devices and approaches have allowed this intervention to be offered to infants as small as approximately 600 g.36 While there are no randomized studies comparing these procedures, TCPC is currently increasing in use as it becomes available in more centers. TCPC has been shown to have a low rate of adverse events and may be associated with faster respiratory improvement compared to surgical ligation.36–39
Flawed Studies
There are more than five hundred clinical trials addressing the PDA published from 1976 to 2022.40 The most common findings among these trials are that medical therapy for PDA has an efficacy rate of only 67% and there is no consistent benefit to outcomes at NICU discharge. However, all randomized trials to date have been trials of low-efficacy medical therapy or high-risk surgical ligation. Furthermore, recurrent flaws in study design make it difficult to apply study findings to the present-day clinical practice. The mean date of publication for all PDA clinical trials is 2005 (Figure 2);40 therefore, the majority of PDA trials were conducted on more mature neonatal populations than is commonly seen in the NICU today.
 |
Figure 2 Number of PDA clinical trials published each year, 1976–2022. Mean publication date is 2005.40 |
Among these published trials, there is inconsistency in the definition of PDA. Some such reports have used clinical definitions only (ie, murmur or bounding pulses) without ensuring the clinical signs are actually due to the PDA. Other reports have used the presence of a PDA on echocardiogram as a binary variable without assessment of shunt flow or clinical effects of the shunt.41,42 Still, other reports assessed the PDA prior to therapy with no post-intervention echocardiogram to determine efficacy. Recent studies have used more sophisticated PDA assessment tools, but the most consistent echocardiography variable used to define a PDA is diameter which has very poor correlation with shunt volume and hemodynamic significance.43–46
In addition to poorly representing current preterm populations and poorly defining the PDA, studies are plagued with excessive crossover. Hundscheid, et al, show that among 32 randomized clinical trials published from 1980 to 2014, greater than 50% of the conservative management groups still received medical therapy for the PDA, and in some studies, as many as 85% of patients randomized to conservative management still received medical therapy.47
Timing of assessment and therapy has a significant impact on study results. Most PDA trials have used treatment in the first few days after birth.5,24 These studies likely enrolled a significant number of patients during a period when the spontaneous closure rate is highest. On the other hand, the few studies with primary interventions beyond one week of age likely enrolled patients who were exposed to a pathologic shunt for a long enough duration that they were already high-risk for negative outcomes even if the PDA closure therapy is successful. As shown by Semberova, et al, very low birthweight infants born greater than 27 weeks’ gestation have an approximately 50% PDA closure rate in the first two weeks and greater than 80% closure by three weeks of age. Therefore, routine PDA therapy in this group would not likely impact the development of negative outcomes as it would shift PDA closure forward in time by only one to two weeks. Infants born before 26 weeks’ gestation, however, are expected to have a much longer duration of PDA. Half of these ducts remain significant at ten weeks of age, and 30% never close spontaneously.48
There is also variability in spontaneous PDA closure across centers. The PDA-Tolerate Trial showed that the spontaneous closure rate in the first week for infants born less than 26 weeks’ gestation varied from 8 to 50%.49 This difference highlights that there are many other interventions in neonatal medicine that affect the PDA. It is reasonable to presume that centers with a different spontaneous closure rates would see different impacts from early medical therapy. However, the PDA-Tolerate trial also shows that neonatologist biases can interfere with study recruitment. Two hundred and two patients were recruited, but 157 were not eligible because the clinical neonatologist treated the infant before enrollment. This means there was a population of infants, nearly as large as the enrolled population that was deemed too sick to be recruited. The pre-treated infants had worse respiratory disease at birth, 8 days of age, and 15 days of age; and this group had a median duration of PDA exposure of only 11 days compared to 22 days in the trial group. Despite being a higher-risk population, the pre-treated group had significantly lower mortality (3 vs 14%, p<0.001) compared to the randomized population.50 This suggests it may be beneficial to treat the PDA early in higher risk patients.
Determining the timing of intervention to improve outcomes remains elusive, however, because, while the expected benefits of earlier PDA closure increase with lower gestational age, the efficacy of medical therapy decreases. Multiple courses of unsuccessful medical therapy followed by late definitive closure prolong exposure to pathologic shunting, and the interventions may have side effects which negatively impact outcomes. El-Kaffash, et al, show that in patients born less than 28 weeks’ gestation, exposure to a hemodynamically significant PDA beyond eight days is associated with BPD or death with an adjusted odds ratio of 6.5 [1.7–25.5]. His group developed a four-component PDA score that was able to determine a high likelihood of spontaneous closure within the first two weeks after birth. Those with low likelihood of spontaneous closure were randomized to treatment or conservative management. The spontaneous closure group had the lowest rate of BPD or death followed by the group that had successful medical therapy. The highest rate of BPD or death was found in the group of PDAs that were treated but did not close with therapy.18,51 There are currently no validated data to predict response to medical therapy, and studies show the efficacy of this population is no more than 50–55%.18,41,42,52
Support for Closing the PDA
The neonatal heart, especially the premature myocardium, is less compliant due to decreased number of sarcomeres and actin/myosin complexes leading to baseline diastolic dysfunction compared to the mature myocardium. A study comparing myocardial performance index (MPI) and cardiac output (CO) in term and preterm neonates on 28th day of life demonstrated lower MPI and CO in preterm neonates along with lower ventricular mass and myocardial thickness.53 Frank Starling’s Law states that the stroke volume of the left ventricle correlates with myocyte stretch and ventricular volume. With intrinsic impairment in both these parameters, a neonatal heart with increased left-to-right shunting and an increase in pulmonary venous return stretches the limits of ventricular compliance and further impedes contractility, ultimately leading to an enlarged chamber size (Figure 1C). Takahashi, et al, describe this phenomenon: “premature infants had less capacity to generate adequate left ventricular stroke volume in proportion to the quantity of the left-to-right ductal shunting than the mature infants in presence of PDA”.54 Su, et al, observe the preterm left ventricle has limited ability to adapt to increased afterload and preload, resulting in myocardial dysfunction and hemodynamic deterioration.55 This may lead to post-capillary pulmonary hypertension (PH) in neonates with prolonged left-to-right shunting leading to increased pulmonary blood flow and pulmonary edema. Zhang, et al, describe their single center experience of cardiopulmonary ultrasound in the treatment of PDA and hypoxic respiratory failure (HRF) in preterm neonates. In their cohort of 76 neonates weighing ≤1500 grams, they utilized ultrasound to guide management in 39 patients, while 37 patients were treated traditionally. They noted that ultrasound cohort had earlier treatment for ductal closure, decreased need for invasive respiratory support, and lower incidence of moderate to severe BPD.56
Several recent studies provide more evidence supporting ductal closure. Relangi, et al, studied outcomes in a single center before and after a clinical change to a more conservative approach to PDA therapy. They conclude, “the change in approach to diagnosis and management of PDA, from a more proactive and aggressive approach during the earlier epoch one to a more expectant approach during the subsequent epoch two, was associated with worse respiratory outcomes, including increase in BPD and in BPD or Death”.57 Similarly, Altit, et al, show outcome differences before and after a change from PDA treatment by clinical judgement to a strict conservative approach. In patients born prior to 26 weeks, the rate of BPD or death increased significantly following a change to strict conservative management.58 El-Khuffash and his colleagues attempt to reconcile the contradiction between studies that show no benefit from PDA therapy and studies that show worse outcomes from conservative management by analyzing their randomized study by treatment effect rather than by intention to treat. They show that the rate of BPD or death is lowest among infants who have spontaneous ductal closure in the first two weeks after birth, and successful PDA treatment is associated with similarly good outcomes. The worst outcomes were associated with prolonged PDA with our without PDA treatment.18,51 Together, these studies suggest that optimal patient selection, optimal timing, and high treatment efficacy are needed to show benefit from PDA therapy.
Barriers to Treatment
PDA is a condition with significant diagnostic and physiological heterogeneity. Patients with ductal-dependent cardiac lesions require the PDA to be patent for a longer period in order to maintain either pulmonary or systemic circulation. Among preterm neonates without congenital cardiac disease, there are currently no standardized prediction models to understand which PDAs will quickly close without intervention and which PDAs might benefit from interventions aimed to drive closure. Furthermore, there are anatomic and hemodynamic variations among patients and dynamic variations over time which make it difficult to classify PDAs into clear categories that can be correlated with clear outcomes.
A major barrier to any therapy or intervention is the absence of large, well-designed randomized trials to prove benefit from the proposed intervention. To that extent, an important limitation in PDA therapy is that there are no randomized trials to clearly demonstrate the benefit of PDA therapy on long-term outcomes such as bronchopulmonary dysplasia (BPD), neurodevelopmental outcomes, or duration of mechanical ventilation.48
Even today, neonatologists and cardiologists encounter similar dilemmas faced by Gross’s team when he operated on a child with PDA and successfully closed in 1939: lack of physician equipoise.23,27,50 The neonatal community is (1) divided on whether or not to treat PDA, (2) inconsistent on timing of initiation, duration of therapy, and the number of courses of acetaminophen, indomethacin, or Ibuprofen, and (3) hesitant to commit to definitive closure methods such as TCPC or surgical ligation after medical therapy failure. This variability in management may be explained by institutional data and experience to some extent. Adoption of TCPC is limited by the lack of established interventional catheterization program in some centers, adverse outcomes after surgical closure such as post PDA ligation syndrome, and unjustified fears limb-threatening arterial thrombus formation.59,60
Surgical ligation and its associated mortality and morbidity may be the greatest barrier to closing the ductus. Occurrence of low cardiac output, also called as post-PDA ligation syndrome, after surgical ligation may be a result of systemic inflammatory response, sudden increase in afterload to left ventricle and strain on the myocardium in the immediate post-operative period.31 Increased rates of ROP and BPD are noted after surgical ligation in secondary analysis of infants treated for DA from the Trial of Indomethacin Prophylaxis in Preterms (TIPP).29 Similarly, Mirea, et al, noted in their cohort of preterm babies <32 weeks that those with surgical ligation had lower mortality with higher odds of comorbidities such as ROP and BPD.61 This was re-demonstrated in meta-analysis comparing medical management with surgical ligation by Weisz et al.62 These studies highlight the negative consequences of surgical ligation after a prolonged period left-to-right shunting which causes reduced afterload on the left ventricle and concomitant lung injury due to the inflammatory cascade caused by ventilator-induced trauma and oxygen toxicity.
There has been a dictum in neonatology that a “less is more” approach or “benign neglect” may be the optimal approach. Advocates against ductal closure argue that the ductus is a marker of neonatal illness rather than a cause of neonatal illness.63 There are also advocates for closing ductus early in the transitional period, as early as 72 hours after birth, when most argue that the normal neonatal transitional circulatory adaptations have not completed.25,64 Perhaps, the more realistic statement is somewhere in between: not all babies need the ductus closed, not all of them need it closed early, and in some, waiting longer might be deleterious. This idea supported by Isayama, et al, who show that NICUs at both high and low extremes for PDA treatment have higher rates of death or severe neurologic injury; there is likely an optimal treatment strategy that sits somewhere between routine therapy and strict conservative management.65
Role of Transthoracic Echocardiography
Transthoracic echocardiography (TTE) is a vital armament to the clinician for guiding decision-making in the diagnosis and management of a PDA. The assessment of hemodynamic significance of a PDA is no longer limited to merely measuring the size of the PDA by TTE. The contemporary definition for assessment of hemodynamic significance is more so a combined evaluation of clinical and echocardiographic parameters. Furthermore, there must be consistency in the reporting of TTEs to provide the neonatologist with the necessary information to make clinical decisions based on “echocardiographic significance of a PDA”.
Echocardiographic Assessment of Hemodynamic Significance
A diagnostic cardiac catheterization in conjunction with a cardiac MRI is the gold standard for assessment of cardiac hemodynamics.19 Unfortunately, the feasibility of these tests in premature infants can be challenging. When evaluating hemodynamic significance of a PDA by TTE, the quintessential question is how to estimate the magnitude of shunt volume and its impact on both pulmonary and systemic circulations. In addition, it is often underappreciated how the immature myocardium handles increased preload from the PDA in the setting of potential impaired coronary artery perfusion.9 The three key elements of PDA evaluation by TTE include assessment of the PDA’s physical characteristics, signs of pulmonary over-circulation, and evidence of systemic hypo-perfusion. There are various Doppler parameters to evaluate each of these elements which may be used in various scoring systems and institutional protocols to determine if the PDA is of hemodynamic significance. These scoring systems, albeit useful in predicting outcomes, can sometimes delay care or complicate decision-making if not performed with precision and accuracy. The pragmatic approach is to assess each of the three key PDA elements using available Doppler measures.
PDA Physical Characteristics
Size and Morphology
The morphology of the PDA in preterm infants is usually long and tortuous with a “hockey stick” shaped curvature at the pulmonary end (Figure 3A and B).66 This is referred to as the “Type F” PDA. The size of the PDA is usually insinuated by the measurement at the pulmonary end which typically is the narrowest dimension of the PDA. Grading a PDA as small, moderate, or large varies between studies, but most agree that the PDA size at the pulmonary end of less than 1.5 mm is considered small. From a size perspective, most PDAs measuring 2.5 mm or more correlate with other TTE evidence of hemodynamic significance. The size of the PDA does matter based on weight. A 2 mm PDA in a 700 gram neonate is more significant than a similar sized PDA in a 1.7 kg neonate. Hence, many centers adopt indexing the PDA size to body weight and grade the PDA as small, moderate, or large based on a size of 1.5 mm/kg, 1.5 to 3 mm/kg, and greater than 3 mm/kg, respectively. Regardless of the grading system used, PDA measurements may be variable and can change with time and the clinical status. It is reassuring, however, that there is fair correlation between PDA size as measured by TTE when compared to angiograms in the cardiac catheterization laboratory.67 However, PDA size has very poor correlation with the magnitude of shunting.46 If TCPC is being considered, TTE also provides information (length of the PDA and the size at the aortic end) to the interventional cardiologist to aid in sizing of the potential device (Figure 3C).
 |
Figure 3 (A) Ductal morphology in premature low birth weight infants referred to as “Type F” PDA.66 (B) It is long and tortuous, similar to the fetal ductus, giving the appearance of a hockey stick. (C) Length and width at each end can be measured. Abbreviations: PA, pulmonary artery, PDA, patent ductus arteriosus, Ao, aorta, Dist, distance. |
Direction and Velocity of Shunt Across the PDA
The direction of shunt flow is assessed using pulsed- and continuous-wave Doppler as well as color Doppler and is dependent on the difference in systemic and pulmonary pressures. The direction of flow in a PDA follows the pressure gradient, generally from the high-pressure aorta to the low-pressure pulmonary artery, ie, left to right. However, it can be right to left or bidirectional when there is high pulmonary vascular resistance. Right-to-left shunting across a PDA is a contra-indication to ductal closure as it serves as a pop-off valve for the right ventricle and a source of systemic cardiac output, albeit at the cost of oxygen saturation, in the case of high pulmonary vascular resistance.
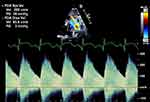
A low peak velocity in a PDA indicates a non-restrictive shunt (usually <2.5–3 m/sec). If the peak systolic velocity is high, pulsatility can be assessed by the ratio of the peak systolic and diastolic velocity. If the peak systolic velocity is at least 2 times the peak diastolic velocity, it indicates a non-restrictive shunt. A restrictive shunt has a high peak systolic velocity with a low ratio of peak systolic to diastolic velocity (Figure 4).
Pulmonary Over-Circulation
A PDA is a post-tricuspid shunt. The increased pulmonary blood flow from left-to-right shunting across the PDA leads to increased pulmonary venous return or preload to the left side of the heart with consequent left atrial dilation. If the shunting persists, the immature left ventricle eventually dilates (Figure 1C). A significant atrial level shunt can alter this process and mask left ventricular dilation. There are numerous markers for pulmonary over-circulation, all of which have a small margin of error in measurement, and hence multiple measures are incorporated into the published scoring systems. The markers for pulmonary over-circulation can be sub-categorized into the following three groups:
Volume Overloading of the Left Heart
Left Atrial Dilation
In the absence of significant diastolic dysfunction of the immature left ventricle, left atrial dilation can be used as an indicator of volume overload from a significant PDA shunt. Invariably, these preterm infants have an atrial level communication which may offload the left side. When there is a large left-to-right atrial shunt, the left atrium may not be dilated despite a hemodynamically significant PDA shunt.
Measurement
- Left atrium to aortic annulus (LA:Ao) ratio: The LA:Ao ratio by M-mode is used as a marker for left atrial dilation indicating increased pulmonary venous return from the PDA. The aortic annulus (Ao) measurement is reproducible and does not change with loading conditions. An LA:Ao ratio of greater than 1.4 is considered significant. A ratio of greater than 2 is considered severe left atrial dilation (Figure 5A).
Pitfall: The left atrium may be foreshortened in some instances unless anatomic correction of the cursor is done.
- Left atrial volume: Normal values for left atrial volume in preterm infants are not well established possibly due to errors in measurement with the atrial septal defect in this cohort. Anecdotal experience suggests left atrial volumes are in the range of 26 to 30 mL/m2.
Left Ventricular Dilation
This is usually a later finding with a persistent large PDA. In the presence of a large atrial septal defect which offloads the left side, left ventricular dilation may not be seen. There is usually qualitative left ventricular dilation with a globular appearance of the heart on an apical 4-chamber view (Figure 5B).
Measurement
- Left ventricular end diastolic dimension (LVeDd): LVeDd can be measured in both parasternal long and short axis images. In the case of a PDA, LVeDd usually measures near the upper limit of normal for age and size. There are published Z-scores for LVeDd.68
Pitfall: A large ASD may offload the volume on the left side resulting in a normal LVeDd. In general, LVeDd is a late sign and may not be seen early even with a hemodynamically significant PDA.
Mitral Valve Regurgitation
In the presence of a persistent large PDA, the volume overload with left atrial and ventricular dilation leads to stretching of the mitral valve annulus with consequent mitral valve insufficiency. It is an indication of a significant long-standing left-to-right shunt.
Pitfall: Mitral valve regurgitation is a late sign and is not seen in all infants with a large hemodynamically significant PDA (hsPDA). Though not common, congenital mitral valve dysplasia can also cause mitral valve regurgitation.
Forward Pulmonary Flow in Diastole in the Left Pulmonary Artery
The PDA connects the aorta to the roof of the main pulmonary artery near the origin of the left pulmonary artery (LPA). The presence of antegrade blood flow in the LPA indicates diastolic shunting from the PDA (Figure 5C).
Measurement
- Pulsed-wave Doppler interrogation of the LPA shows diastolic flow. The end diastolic velocity can be measured. An end diastolic velocity measurement of greater than 0.2 m/s is a marker of a significant PDA shunt.69
Pulmonary Vein Flow
Increased pulmonary venous return from a large PDA is reflected in dilated pulmonary veins by 2D and color Doppler.
Measurement
- Qualitative assessment of the pulmonary veins by 2D and Color Doppler on the “crab view”.
Pitfall: This is subjective and fraught with variability in assessment
- Pulmonary vein D-wave velocity: The “D” wave refers to the diastolic wave which is due to opening of the mitral valve and reduction in the left atrial pressure. Doppler measurement is obtained from an apical 4-chamber view with the cursor parallel to the direction of blood flow. Neonates with an hsPDA have a high pulmonary vein D-wave velocity. A D-wave velocity of greater than 0.5 m/s is indicative of a significant PDA shunt.
Pitfall: This metric may vary based on the hydration/volume status of the neonate.
Left Side Pressure Loading
Mitral Valve E/A Ratio
This is an easily reproducible measure in TTE and is usually an indicator of myocardial compliance. The E-velocity refers to the early passive filling of the left ventricle which occurs when the left atrial pressure supersedes the left ventricular pressure and the mitral valve opens. The A-velocity refers to the active filling of the left ventricle that occurs during atrial systole. Normally, the mitral E/A ratio is <1 in preterm neonates due to poor compliance with immature myocardium leading to low early diastolic filling (ie, low E-velocity). In the presence of an hsPDA, due to increased pulmonary venous return and consequent increased left atrial pressure, the E-velocity (early diastolic filling) increases leading to reversal in E/A ratio >1. A mitral E-velocity of more than 80cm/sec is indicative of a significant left-to-right shunt.
Pitfall: With time, even in preterm infants with no PDA, as the myocardial compliance improves, the E/A ratio gradually becomes >1. The E-velocity is also dependent on the volume status.
Decreased Isovolumic Relaxation Time (IVRT)
This is the time between closure of the aortic valve and opening of the mitral valve. It is a timeframe in diastole when blood is neither entering nor exiting the left ventricle. With a large PDA with a significant shunt, the volume overload leads to rapid atrial filling which shortens the IVRT, ie, time to mitral valve opening. An IVRT less than 30 milliseconds is considered significant.
Pitfall: This measurement is fraught with error if not done routinely and consistently. Reliably obtaining an accurate angle of insonation of the cursor to the blood flow can be challenging. There is also a very small margin of error when measuring the time on the Doppler profile.
Left Ventricular Outflow (LVO) Estimation
Left Ventricular Outflow to Right Ventricular Outflow (LVO:RVO) Ratio
In the presence of a large PDA, left ventricular outflow is increased due to the significant left-to-right shunting beyond the semilunar valves. Similarly, the right ventricular outflow (RVO) can also be measured. In theory, estimation of the ratio of LVO: RVO provides a surrogate for estimation of Qp: Qs (pulmonary blood flow to systemic blood flow ratio by cardiac catheterization). A Qp: Qs ratio greater than 1.5:1 is considered a significant shunt. Unfortunately, there are many levels of error with this measure as described below unless done routinely by a core group of sonographers.
Pitfalls: A small error in measurement of the aortic or pulmonary valve annulus makes a big difference in estimation of cardiac output. In addition, it can be challenging to obtain a reproducible and accurate LVO/left ventricular velocity time integral as the angle of insonation of the cursor in the left ventricular outflow must be parallel to the blood flow (Figure 6).
Assessment of Systemic Hypo-Perfusion
Reversal of flow in the abdominal aorta indicates systemic steal from a significant PDA shunt. This echocardiographic measure has the strongest correlation with PDA shunt volume when assessed with cardiac MRI.70 This measure is easily obtained and has good reproducibility and purely from a hemodynamic perspective implies a significant PDA shunt regardless of any of the other aforementioned TTE metrics in the absence of severe aortic valve insufficiency or an arteriovenous shunt (Figure 7). Absent diastolic blood flow in the descending aorta below the ductal ampulla or in the celiac or superior mesenteric artery have also been described as markers of large shunt volume.69
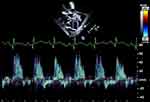 |
Figure 7 Pulsed-wave Doppler of the abdominal aorta demonstrates reversal of flow during diastole. |
Pitfall: There is a level of apprehension in evaluation of the celiac and superior mesenteric artery as it is thought to be beyond the scope of a congenital cardiology sonographer.
Other Considerations
The inherent diastolic dysfunction of the premature left ventricular myocardium is often forgotten during assessment of the hemodynamic assessment of a PDA. With increased pulmonary blood flow in a setting of left ventricular diastolic dysfunction, the pulmonary venous hypertension worsens which can eventually affect right ventricular performance.71
Early Echocardiography
There is currently no consensus for timing or indication for screening of the PDA in extremely preterm infants. Recent randomized controlled trials have suggested that the persistence of a moderate to large PDA beyond seven days of life in an extremely preterm infant increases the risk of BPD and death.20,72 The intent for early screening comes from the assumption that early screening would result in earlier treatment which may impact outcomes. The argument against early screening has been that though the presence of a hsPDA is associated with poor outcomes, closing the PDA has not changed these outcomes. Large national population-based prospective cohort studies have tried to answer the question on the utility of early screening echocardiography for PDA. The EPIPAGE 2 study showed that screening TTE before day 3 of life was associated with lower in-hospital mortality and the likelihood of pulmonary hemorrhage.73 However, there was no difference in the incidence of necrotizing enterocolitis, severe BPD or severe cerebral lesions. Geisinger, et al, propose early echocardiography directed therapy in preterm infants less than 24 weeks’ gestation, showing an increase in survival, free of severe morbidity.74 Although safety data on early TTE screening in extremely preterm infants are lacking the presumed safety profile of this non-invasive assessment, it should not limit the use of this modality for early screening. There may be a disagreement on whether closing the PDA improves clinical outcomes. However, if available routinely, early TTE screening may help clinicians better understand the hemodynamics and physiology of extremely preterm infants and improve precision when making clinical decisions.
Horizon
The Percutaneous Intervention Versus Observational Trial of Arterial Ductus in Low-weight Infants (PIVOTAL) is a step in the right direction, addressing many of the limitations of previous randomized control trials in terms of patient selection by strict TTE and clinical criteria and the comparison with definitive therapy in the form of percutaneous intervention which bypasses many of the limitations of surgical ligation. As this trial unfolds, secondary anecdotal benefits are already being seen in many centers in terms of more standardized TTE acquisition and reporting which has aided in protocol driven clinical management of preterm neonates with a large PDA. As the questions continue as to who (<26 weeks’ gestation?), when (10–21 days of age), and how (if failed medical therapy, then TCPC) does the PDA need to be closed, trials of this nature, through precision-based medicine help bridge the gap between the academic thought groups who do not believe in PDA closure and those who believe in closing every PDA in preterm neonates.
Conclusion
As an essential fetal shunt supplying oxygen to the systemic circulation, ductus arteriosus plays an important role in fetal growth and development. However, patency beyond fetal life is fraught with hemodynamic changes. The PDA in preterm neonates is a pathologic left-to-right shunt that is associated with pulmonary over circulation which may play a role in evolution of chronic lung disease, altered myocardial function with loading condition changes on left ventricle, and decreased systemic perfusion affecting end organs such as gastrointestinal tract and renal parenchyma with added comorbidities of necrotizing enterocolitis and acute kidney injury. Additionally, medical management with diuretics and fluid restriction may lead to electrolyte disturbances and further worsening of kidney injury. The dynamic nature of the PDA especially with the relationship to mean airway pressure on mechanical ventilation makes assessment more nuanced and thus contributes to confusion.
A major drawback with multiple attempts at randomized control trials is an inability to show better outcomes with closure despite such advantages shown on institutional case series. Perhaps, limitations in study design or variability at participating institutions make it challenging to apply evidence to an individual patient. As neonatologists and cardiologists gain better understanding of PDA pathology and its effects on micro and macro circulation, it is important to apply rigorous clinical and echocardiographic criteria to defining an hsPDA.
With many unanswered questions ranging from whether a PDA is contributing to any clinical co-morbidities to risks of medical, transcatheter closure, and surgical ligations, the medical community is yet to agree upon optimal strategies for PDA management. Individualizing therapeutic approaches based on the clinical phenotype and risk stratification of therapies may mitigate adverse effects and prove beneficial.
Disclosure
Dr. Gowda is a consultant for the American Academy of Pediatrics, serving on the editorial board of NeoReviews Plus and is Chair of the American Academy of Pediatrics Early Career Leadership Alliance (ECLA) program curriculum. Dr. Philip is a consultant for Abbott Congenital Heart. Dr. Weems is a consultant for the American Academy of Pediatrics, serving as Associate Editor of Pediatrics in Review, and is a consultant and speaker for Abbott Congenital Heart.
References
1. Carrillo SA. “What we know is little, and what we are ignorant of is immense”. J Thorac Cardiovasc Surg. 2017;154(6):2052–2053. doi:10.1016/j.jtcvs.2017.08.111
2. Siegel RE. Galen’s experiments and observations on pulmonary blood flow and respiration. Am J Cardiol. 1962;10(5):738–745. doi:10.1016/0002-9149(62)90250-3
3. Hundscheid T, van den Broek M, van der Lee R, de Boode WP. Understanding the pathobiology in patent ductus arteriosus in prematurity-beyond prostaglandins and oxygen. Pediatr Res. 2019;86(1):28–38. doi:10.1038/s41390-019-0387-7
4. Reese J. Towards a greater understanding of the ductus arteriosus. Semin Perinatol. 2018;42(4):199–202. doi:10.1053/j.semperi.2018.05.001
5. Hamrick SEG, Sallmon H, Rose AT, et al. Patent Ductus Arteriosus of the Preterm Infant. Pediatrics. 2020;146(5). doi:10.1542/peds.2020-1209
6. Singh Y, Katheria A, Tissot C. Functional Echocardiography in the Neonatal Intensive Care Unit. Indian Pediatr. 2018;55(5):417–424. doi:10.1007/s13312-018-1286-4
7. Singh Y, Gupta S, Groves AM, et al. Expert consensus statement ‘Neonatologist-performed Echocardiography (NoPE)’-training and accreditation in UK. Eur J Pediatr. 2016;175(2):281–287. doi:10.1007/s00431-015-2633-2
8. Paudel G, Joshi V. Echocardiography of the patent ductus arteriosus in premature infant. Congenit Heart Dis. 2019;14(1):42–45. doi:10.1111/chd.12703
9. Vaisbourd Y, Sharif D, Riskin A, et al. The effect of patent ductus arteriosus on coronary artery blood flow in premature infants: a prospective observational pilot study. J Perinatol. 2020;40(9):1366–1374. doi:10.1038/s41372-020-0622-4
10. Noori S, Seri I. Hemodynamic antecedents of peri/intraventricular hemorrhage in very preterm neonates. Semin Fetal Neonatal Med. 2015;20(4):232–237. doi:10.1016/j.siny.2015.02.004
11. Lemmers PM, Benders MJ, D’Ascenzo R, et al. Patent Ductus Arteriosus and Brain Volume. Pediatrics. 2016;137(4). doi:10.1542/peds.2015-3090
12. Dollberg S, Lusky A, Reichman B. Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr. 2005;40(2):184–188. doi:10.1097/00005176-200502000-00019
13. Homedi A, De La Hoz A, Miller MR, Lalitha R, McClean M, Bhattacharya S. Impact of Targeted Neonatal Echocardiography on Patent Ductus Arteriosus Management in a Canadian Tertiary Care Neonatal Unit: a Retrospective Cohort Study. Am J Perinatol. 2023. doi:10.1055/s-0043-1774313
14. Rios DR, Bhattacharya S, Levy PT, McNamara PJ. Circulatory Insufficiency and Hypotension Related to the Ductus Arteriosus in Neonates. Front Pediatr. 2018;6:62. doi:10.3389/fped.2018.00062
15. Willis KA, Weems MF. Hemodynamically significant patent ductus arteriosus and the development of bronchopulmonary dysplasia. Congenit Heart Dis. 2019;14(1):27–32. doi:10.1111/chd.12691
16. Kappico JM, Cayabyab R, Ebrahimi M, et al. Pulmonary hemorrhage in extremely low birth weight infants: significance of the size of left to right shunting through a valve incompetent patent foramen ovale. J Perinatol. 2022;42(9):1233–1237. doi:10.1038/s41372-022-01464-9
17. Scholl JE, Yanowitz TD. Pulmonary hemorrhage in very low birth weight infants: a case-control analysis. J Pediatr. 2015;166(4):1083–1084. doi:10.1016/j.jpeds.2014.12.032
18. Bussmann N, Smith A, Breatnach CR, et al. Patent ductus arteriosus shunt elimination results in a reduction in adverse outcomes: a post hoc analysis of the PDA RCT cohort. J Perinatol. 2021;41(5):1134–1141. doi:10.1038/s41372-021-01002-z
19. Philip R, Waller BR, Chilakala S, et al. Hemodynamic and clinical consequences of early versus delayed closure of patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2021;41(1):100–108. doi:10.1038/s41372-020-00772-2
20. Clyman RI, Kaempf J, Liebowitz M, et al. Prolonged Tracheal Intubation and the Association Between Patent Ductus Arteriosus and Bronchopulmonary Dysplasia: a Secondary Analysis of the PDA-TOLERATE trial. J Pediatr. 2021;229:283–288 e282. doi:10.1016/j.jpeds.2020.09.047
21. Noori S, McCoy M, Friedlich P, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–144. doi:10.1542/peds.2008-2418
22. Sathanandam S, Whiting S, Cunningham J, et al. Practice variation in the management of patent ductus arteriosus in extremely low birth weight infants in the United States: survey results among cardiologists and neonatologists. Congenit Heart Dis. 2019;14(1):6–14. doi:10.1111/chd.12729
23. Hundscheid T, El-Khuffash A, McNamara PJ, de Boode WP. Survey highlighting the lack of consensus on diagnosis and treatment of patent ductus arteriosus in prematurity. Eur J Pediatr. 2022;181(6):2459–2468. doi:10.1007/s00431-022-04441-8
24. Benitz WE, Bhombal S. The use of non-steroidal anti-inflammatory drugs for patent ductus arteriosus closure in preterm infants. Semin Fetal Neonatal Med. 2017;22(5):302–307. doi:10.1016/j.siny.2017.07.004
25. Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30(4):241–252. doi:10.1038/jp.2010.3
26. Bixler GM, Powers GC, Clark RH, Walker MW, Tolia VN. Changes in the Diagnosis and Management of Patent Ductus Arteriosus from 2006 to 2015 in United States Neonatal Intensive Care Units. J Pediatr. 2017;189:105–112. doi:10.1016/j.jpeds.2017.05.024
27. Gross RE. Surgical Management of the Patent Ductus Arteriosus: with Summary of Four Surgically Treated Cases. Ann Surg. 1939;110(3):321–356. doi:10.1097/00000658-193909000-00001
28. Reese J, Scott TA, Patrick SW. Changing patterns of patent ductus arteriosus surgical ligation in the United States. Semin Perinatol. 2018;42(4):253–261. doi:10.1053/j.semperi.2018.05.008
29. Kabra NS, Schmidt B, Roberts RS, et al. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150(3):229–234, 234 e221. doi:10.1016/j.jpeds.2006.11.039
30. Weisz DE, Mirea L, Rosenberg E, et al. Association of Patent Ductus Arteriosus Ligation With Death or Neurodevelopmental Impairment Among Extremely Preterm Infants. JAMA Pediatr. 2017;171(5):443–449. doi:10.1001/jamapediatrics.2016.5143
31. Serrano RM, Madison M, Lorant D, Hoyer M, Alexy R. Comparison of ‘post-patent ductus arteriosus ligation syndrome’ in premature infants after surgical ligation vs. percutaneous closure. J Perinatol. 2020;40(2):324–329. doi:10.1038/s41372-019-0513-8
32. El-Khuffash AF, Jain A, Weisz D, Mertens L, McNamara PJ. Assessment and treatment of post patent ductus arteriosus ligation syndrome. J Pediatr. 2014;165(1):46–52 e41. doi:10.1016/j.jpeds.2014.03.048
33. Benjamin JR, Smith PB, Cotten CM, Jaggers J, Goldstein RF, Malcolm WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2010;30(6):408–413. doi:10.1038/jp.2009.124
34. Ashfaq A, Rettig RL, Chong A, Sydorak R. Outcomes of patent ductus arteriosus ligation in very low birth weight premature infants: a retrospective cohort analysis. J Pediatr Surg. 2022;57(7):1201–1204. doi:10.1016/j.jpedsurg.2022.02.037
35. Ishida S, Yamaguchi A, Ooka M, Kenmochi M, Nakanishi H. Evaluation of postoperative complications for patent ductus arteriosus in extremely-low-birthweight infants. Pediatr Int. 2022;64(1):e14759. doi:10.1111/ped.14759
36. Sathanandam S, Balduf K, Chilakala S, et al. Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv. 2019;93(1):89–96. doi:10.1002/ccd.27808
37. Rodriguez Ogando A, Planelles Asensio I, de la Blanca ARS, et al. Surgical Ligation Versus Percutaneous Closure of Patent Ductus Arteriosus in Very Low-Weight Preterm Infants: which are the Real Benefits of the Percutaneous Approach? Pediatr Cardiol. 2018;39(2):398–410. doi:10.1007/s00246-017-1768-5
38. Bischoff AR, Kennedy KF, Backes CH, Sathanandam S, McNamara PJ. Percutaneous Closure of the Patent Ductus Arteriosus in Infants </=2 kg: IMPACT Registry insights. Pediatrics. 2023.
39. Bischoff AR, Jasani B, Sathanandam SK, Backes C, Weisz DE, McNamara PJ. Percutaneous Closure of Patent Ductus Arteriosus in Infants 1.5 kg or Less: a Meta-Analysis. J Pediatr. 2021;230:84–92 e14. doi:10.1016/j.jpeds.2020.10.035
40. PubMed, National Library of Medicine. Search term: “patent ductus arteriosus”. Filters: clinical Trial + 1976-2023. https://pubmed.ncbi.nlm.nih.gov/?term=patent%20ductus%20arteriosus&filter=pubt.clinicaltrial&filter=years.1976-2023.
41. Gupta S, Subhedar NV, Bell JL, et al. Trial of Selective Early Treatment of Patent Ductus Arteriosus with Ibuprofen. N Engl J Med. 2024;390(4):314–325. doi:10.1056/NEJMoa2305582
42. Hundscheid T, Onland W, Kooi EMW, et al. Expectant Management or Early Ibuprofen for Patent Ductus Arteriosus. N Engl J Med. 2023;388(11):980–990. doi:10.1056/NEJMoa2207418
43. El-Khuffash A, James AT, Corcoran JD, et al. A Patent Ductus Arteriosus Severity Score Predicts Chronic Lung Disease or Death before Discharge. J Pediatr. 2015;167(6):1354–1361 e1352. doi:10.1016/j.jpeds.2015.09.028
44. Kluckow M, Jeffery M, Gill A, Evans N. A randomised placebo-controlled trial of early treatment of the patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 2014;99(2):F99–F104. doi:10.1136/archdischild-2013-304695
45. Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. 2012;160(6):929–935 e921. doi:10.1016/j.jpeds.2011.12.031
46. Smith A, Mullaly R, Franklin O, El-Khuffash A. Reproducibility of the EL-Khuffash PDA severity score and PDA diameter measurements in extremely preterm infants. Early Hum Dev. 2023;184:105832. doi:10.1016/j.earlhumdev.2023.105832
47. Hundscheid T, Onland W, van Overmeire B, et al. Early treatment versus expectative management of patent ductus arteriosus in preterm infants: a multicentre, randomised, non-inferiority trial in Europe (BeNeDuctus trial). BMC Pediatr. 2018;18(1):262. doi:10.1186/s12887-018-1215-7
48. Semberova J, Sirc J, Miletin J, et al. Spontaneous Closure of Patent Ductus Arteriosus in Infants </=1500 g. Pediatrics. 2017;140(2). doi:10.1542/peds.2016-4258
49. Clyman RI, Liebowitz M, Kaempf J, et al. PDA-TOLERATE Trial: an Exploratory Randomized Controlled Trial of Treatment of Moderate-to-Large Patent Ductus Arteriosus at 1 Week of Age. J Pediatr. 2019;205:41–48 e46. doi:10.1016/j.jpeds.2018.09.012
50. Liebowitz M, Katheria A, Sauberan J, et al. Lack of Equipoise in the PDA-TOLERATE Trial: a Comparison of Eligible Infants Enrolled in the Trial and Those Treated Outside the Trial. J Pediatr. 2019;213:222–226 e222. doi:10.1016/j.jpeds.2019.05.049
51. El-Khuffash A, Bussmann N, Breatnach CR, et al. A Pilot Randomized Controlled Trial of Early Targeted Patent Ductus Arteriosus Treatment Using a Risk Based Severity Score (The PDA RCT). J Pediatr. 2021;229:127–133. doi:10.1016/j.jpeds.2020.10.024
52. Davidson JM, Ferguson J, Ivey E, Philip R, Weems MF, Talati AJ. A randomized trial of intravenous Acetaminophen versus indomethacin for treatment of hemodynamically significant PDAs in VLBW infants. J Perinatol. 2021;41(1):93–99. doi:10.1038/s41372-020-0694-1
53. Bokiniec R, Wlasienko P, Borszewska-Kornacka MK, Madajczak D, Szymkiewicz-Dangel J. Myocardial performance index (Tei index) in term and preterm neonates during the neonatal period. Kardiol Pol. 2016;74(9):1002–1009. doi:10.5603/KP.a2016.0056
54. Takahashi Y, Harada K, Ishida A, Tamura M, Takada G. Left ventricular preload reserve in preterm infants with patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. 1994;71(2):F118–121. doi:10.1136/fn.71.2.F118
55. Su BH, Lin HY, Huang FK, Tsai ML, Huang YT. Circulatory Management Focusing on Preventing Intraventricular Hemorrhage and Pulmonary Hemorrhage in Preterm Infants. Pediatr Neonatol. 2016;57(6):453–462. doi:10.1016/j.pedneo.2016.01.001
56. Zhang Z, Lou X, Hua L, Jia X, Xu L, Zhao M. Cardiopulmonary Ultrasound-Guided Treatment of Premature Infants with Respiratory Failure and Patent Ductus Arteriosus: a Randomized, Controlled Trial. Indian J Pediatr. 2023;90(11):1103–1109. doi:10.1007/s12098-023-04489-w
57. Relangi D, Somashekar S, Jain D, et al. Changes in Patent Ductus Arteriosus Treatment Strategy and Respiratory Outcomes in Premature Infants. J Pediatr. 2021;235:58–62. doi:10.1016/j.jpeds.2021.04.030
58. Altit G, Saeed S, Beltempo M, Claveau M, Lapointe A, Basso O. Outcomes of Extremely Premature Infants Comparing Patent Ductus Arteriosus Management Approaches. J Pediatr. 2021;235:49–57 e42. doi:10.1016/j.jpeds.2021.04.014
59. Weisz DE, McNamara PJ. Patent ductus arteriosus ligation and adverse outcomes: causality or bias? J Clin Neonatol. 2014;3(2):67–75. doi:10.4103/2249-4847.134670
60. Backes CH, Cheatham SL, Deyo GM, et al. Percutaneous Patent Ductus Arteriosus (PDA) Closure in Very Preterm Infants: feasibility and Complications. J Am Heart Assoc. 2016;5(2). doi:10.1161/JAHA.115.002923
61. Mirea L, Sankaran K, Seshia M, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012;161(4):689–694 e681. doi:10.1016/j.jpeds.2012.05.007
62. Weisz DE, More K, McNamara PJ, Shah PS. PDA ligation and health outcomes: a meta-analysis. Pediatrics. 2014;133(4):e1024–1046. doi:10.1542/peds.2013-3431
63. Benitz WE. Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97(2):F80–82. doi:10.1136/archdischild-2011-300381
64. Sallmon H, Koehne P, Hansmann G. Recent Advances in the Treatment of Preterm Newborn Infants with Patent Ductus Arteriosus. Clin Perinatol. 2016;43(1):113–129. doi:10.1016/j.clp.2015.11.008
65. Isayama T, Kusuda S, Reichman B, et al. Neonatal Intensive Care Unit-Level Patent Ductus Arteriosus Treatment Rates and Outcomes in Infants Born Extremely Preterm. J Pediatr. 2020;220:34–39 e35. doi:10.1016/j.jpeds.2020.01.069
66. Philip R, Waller BR, Agrawal V, et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. 2016;87(2):310–317. doi:10.1002/ccd.26287
67. Paudel G, Johnson JN, Philip R, et al. Echocardiographic versus Angiographic Measurement of the Patent Ductus Arteriosus in Extremely Low Birth Weight Infants and the Utility of Echo Guidance for Transcatheter Closure. J Am Soc Echocardiogr. 2021;34(10):1086–1094. doi:10.1016/j.echo.2021.06.005
68. Abushaban L, Vel MT, Rathinasamy J, Sharma PN. Normal reference ranges for left ventricular dimensions in preterm infants. Ann Pediatr Cardiol. 2014;7(3):180–186. doi:10.4103/0974-2069.140832
69. El Hajjar M, Vaksmann G, Rakza T, Kongolo G, Storme L. Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed. 2005;90(5):F419–422. doi:10.1136/adc.2003.027698
70. Broadhouse KM, Price AN, Durighel G, et al. Assessment of PDA shunt and systemic blood flow in newborns using cardiac MRI. NMR Biomed. 2013;26(9):1135–1141. doi:10.1002/nbm.2927
71. Smith A, El-Khuffash AF. Defining “Haemodynamic Significance” of the Patent Ductus Arteriosus: do We Have All the Answers? Neonatology. 2020;117(2):225–232. doi:10.1159/000506988
72. Clyman RI, Hills NK, Cambonie G, et al. Patent ductus arteriosus, tracheal ventilation, and the risk of bronchopulmonary dysplasia. Pediatr Res. 2022;91(3):652–658. doi:10.1038/s41390-021-01475-w
73. Roze JC, Cambonie G, Marchand-Martin L, et al. Association Between Early Screening for Patent Ductus Arteriosus and In-Hospital Mortality Among Extremely Preterm Infants. JAMA. 2015;313(24):2441–2448. doi:10.1001/jama.2015.6734
74. Giesinger RE, Hobson AA, Bischoff AR, Klein JM, McNamara PJ. Impact of early screening echocardiography and targeted PDA treatment on neonatal outcomes in “22-23” week and “24-26” infants. Semin Perinatol. 2023;47(2):151721. doi:10.1016/j.semperi.2023.151721
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.