Back to Journals » Clinical Interventions in Aging » Volume 19
Obstacles to the Early Diagnosis and Management of Sarcopenia: Current Perspectives
Received 6 November 2023
Accepted for publication 14 February 2024
Published 20 February 2024 Volume 2024:19 Pages 323—332
DOI https://doi.org/10.2147/CIA.S438144
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Hoyli Ooi,1 Carly Welch1,2
1Department of Ageing and Health, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, London, UK; 2Department of Twin Research and Genetic Epidemiology, School of Life Course and Population Sciences, St Thomas’ Campus, King’s College London, London, UK
Correspondence: Carly Welch, Department of Twin Research & Genetic Epidemiology King’s College London, St Thomas’ Campus 3rd & 4th Floor South Wing Block D Westminster Bridge Road, London, SE1 7EH, UK, Tel +44 (0) 20 7188 6765, Email [email protected]
Abstract: Research in sarcopenia has grown exponentially over the last 15 years in geriatrics and gerontology, as well as other specialties, including oncology and hepatology. There is now strong evidence for the role of resistance exercise to prevent declines in muscle strength and function, especially when combined with nutritional optimization with protein supplementation. However, there remains a disparity between research evidence and clinical practice. There are multiple factors for this, which relate to the current diagnostic criteria for sarcopenia, practical and logistical aspects of diagnosis of sarcopenia, clinician knowledge of both diagnosis and management, and the availability of pathways for interventions. Sarcopenia is currently defined based on the identification of muscle strength, in combination with muscle size or quality, below cut-off thresholds at a single timepoint. This defines sarcopenia as a binary process of either present or not present, thus early diagnosis can be challenging. In this article, we summarize current obstacles to early diagnosis and management of sarcopenia in clinical practice, and make recommendations to how these might be overcome. This includes our recommendation of incorporation of handgrip strength measurement into standard care, to enable dynamic assessment and identification of early declines in handgrip strength, so that interventions can be implemented to prevent disability.
Keywords: EWGSOP2, dynamic change, handgrip strength, implementation, exercise
Introduction
Sarcopenia has gained increasing recognition among researchers and clinicians, as it has been shown to be associated with increased likelihood of adverse outcomes including falls,1 fractures,2 physical disability,3 and mortality.1 Multiple operational working groups including the European Working Group on Sarcopenia in Older People [EWGSOP (2010)],4 revised EWGSOP2 (2019),5 Asian Working Group for Sarcopenia (AWGS),6 International Working Group on Sarcopenia (IWGS),7 and Foundation for the National Institute of Health (FNIH),8 have proposed sarcopenia definitions. The prevalence of sarcopenia ranges between 10% and 27% dependent on definition used.9 In 2010, The European Working Group on Sarcopenia in Older People (EWGSOP) produced a landmark paper that classifies sarcopenia as a geriatric syndrome characterized by loss of muscle mass and function.4 Ten years following this, with advances in clinical research, EWGSOP revised its original definition in 2019 (EWGSOP2). EWGSOP2 focuses more on low muscle strength as the main criterion for sarcopenia, and the diagnosis is confirmed by low muscle quality or quantity, with poor physical performance as an indicator for severe sarcopenia.5 The shift towards focus on muscle strength was driven by evidence demonstrating that grip strength was a greater predictor of adverse outcomes than muscle mass.10 The Asian Working Group for Sarcopenia (AWGS) 2019 echoed the general EWGSOP2 definition, with its differences mainly on the diagnostic cut-off values reflective of the regional population and having two separate algorithms for community vs hospital settings.6 Evidence has shown that progressive resistance exercise training can improve muscle strength and muscle mass in older adults.11 The “Sarcopenia and Physical Frailty in Older People: Multicomponent Treatment Strategies” (SPRINTT) randomized controlled trial (RCT) showed positive effects of physical activity and nutrition intervention to prevent mobility disability and improve physical performance.12 Despite a myriad of clinical research findings and recommendations in place to manage sarcopenia, it remains challenging to achieve early diagnosis and management of sarcopenia in clinical practice. This article summarizes the multiple obstacles at different stages (Figure 1), and recommendations about how these might be overcome.
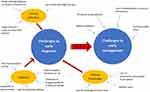 |
Figure 1 Challenges to early diagnosis and management, including inter-relation between diagnosis and management. |
Challenges to Early Diagnosis
Challenges Related to Current Diagnostic Criteria for Sarcopenia
Diagnosis Based on Set Cut-off Values
Muscle strength is now considered the primary determinant of sarcopenia. EWGSOP2 recommends using assessment of grip strength or chair stand test to identify probable sarcopenia. The sarcopenia diagnosis is confirmed by demonstration of reduced muscle mass or muscle quality. A wide variety of tools have been proposed for measurement, depending on local availability.13 Diagnostic cut-off values for both muscle strength and quantity are generally accepted as two standard deviations below the mean of a young healthy reference population.14 Considering sarcopenia in this way considers sarcopenia as a binary concept of either present or not present. EWGSOP2 have proposed that additional reductions in physical performance should be defined as severe sarcopenia.4 However, there will always be a tipping point at which people cross the boundary of meeting each of these criteria. In fact, by the stage patients meet criteria for sarcopenia, it is already by definition impacting them significantly and impairing function. Loss of skeletal muscle mass occurs gradually and progressively from middle age at the rate of 0.5% to 1% per year. This process accelerates over the age of 60.15 Therefore, “early diagnosis” of sarcopenia can only be made in the case of patients who have just crossed the threshold of set cut-offs, this is comparable to diagnosing the “end stage” of muscle dysfunction based on current definition.16 This is considered akin to only diagnosing chronic kidney disease in patients who have end stage kidney disease. In addition, it should be noted that different cut-off points are proposed for different populations. EWGSOP2 proposed cut-offs of <16 kg for women and <27 kg in men5 for diagnosis of sarcopenia based on handgrip strength. However, AWGS proposed cut-offs of <18 kg for women and <28 kg for men.6 These differences may lead to earlier diagnosis of sarcopenia if utilizing AWGS compared to EWGSOP2.
Muscle Loss as a Spectrum
Sarcopenia is a musculoskeletal disease, with primary sarcopenia defined as muscle loss related to primary age-related processes (eg, senescence), and secondary sarcopenia defined by disease-related muscle loss.17 In fact, it is now accepted that sarcopenia as a process can occur with any chronic disease regardless of age (eg sarcopenia in the context of liver disease).18 However, diagnostic strategies are not disease-specific and it is not known if different diagnostic strategies may be superior in different populations. Additionally, the more broadly sarcopenia is considered, the more it can be considered to overlap with other conditions within the muscle loss spectrum eg, cachexia, Intensive Care Unit-Acquired Weakness, neurodegenerative disorders, or inflammatory disorders.16,19 The underlying mechanisms will of course significantly differ, but the current definition of sarcopenia is based on clinical criteria, and in fact the underlying mechanisms of sarcopenia are still not fully understood. There are numerous proposed causes of age-related sarcopenia, including loss of motor units innervating muscle, systemic inflammation, oxidative stress, decline in anabolic hormones, “anorexia of ageing”, reduced protein absorption and disturbances in the gastrointestinal microbiome,20 and reduced physical activity.17
Dynamic Changes and Timeframe of Measurement
Age-related decline in grip strength can start as early as the fifth decade of life, and not all muscle loss means sarcopenia. Considering normative data on grip strength across the life course in the UK, there are three overall periods: 1) An increase to peak in early adult life, 2) Maintenance through to midlife, and 3) Decline from midlife onwards.17 During this time, some people may experience declines in muscle mass and function that are significant to them, but that do not lead them to technically meet the criteria of sarcopenia when they are assessed objectively. This is a significant limitation when considering comparators against the population, rather than the individual. People generally have different levels of early or midlife muscle mass along with muscle function.21 Muscle losses also progress at different levels with age, being affected by multiple factors including genetic susceptibility, lifestyle factors, and chronic diseases,22 and may also be accelerated in the context of acute illness and bedrest.23 Diagnosing sarcopenia from single timepoints, rather than as a dynamic process, risks failure to identify patients who have experienced significant relative declines for them as an individual.24 Where serial measurements are performed, the timing of these will depend upon the clinical situation. Repeat measurements within as little as one week may be necessary in the context of acute sarcopenia (defined as incident sarcopenia within six months),23,24 whereas annual assessment alongside other health checks (eg, blood pressure) may be appropriate to detect change in stable health conditions. A change of 5–6.5 kg in handgrip strength has been shown to be clinically significant.25
Overlap with Other Conditions and Syndromes
Whilst sarcopenia has been increasingly recognized as a unique entity with its own diagnostic criteria, it should be appreciated that there is significant overlap between sarcopenia and other conditions. Frailty is a syndrome of increased vulnerability to poor resolution of homeostasis following a stressor event, which can be defined using either a Frailty Index26 or a phenotypic definition. The Fried phenotype is defined as the presence of three out of five of the characteristics of weight loss, low muscle strength, self-reported exhaustion, low physical performance, or low physical activity.27 There has been shown to be significant overlap between frailty and sarcopenia with multiple diagnostic criteria for frailty. Cachexia is a wasting syndrome caused by cancer and other inflammatory processes. The most recent consensus definition on cachexia defines cachexia as body weight loss of at least 5% in 12 months or less (or body mass index (BMI) <20 kg/m2) and at least three of the five conditions: decreased muscle strength, fatigue, anorexia, low fat-free mass index, or abnormal biochemistry.28 Deconditioning is another term that is commonly used within clinical medicine. Deconditioning is a non-specific term that can be considered to refer to a multi-system process of reduced function following a physical stressor event.29 Whilst these terms can be considered distinct and complementary, the overlap between definitions may be confusing and distracting for clinicians in offering an early sarcopenia diagnosis when diagnostic criteria for other condition are also met.
Practical/Logistical Aspects in Diagnosis of Sarcopenia
Case Finding: How to Identify
Sarcopenia is closely related to frailty,30 which is a syndrome of increased vulnerability to poor resolution of homeostasis following a stressor event. Clinicians have become more greatly aware of frailty in clinical practice over the last five years, with simple screening tools often embedded into clinical pathways. However, this has not been the case for sarcopenia. Where screening is performed, this is normally undertaken at a single timepoint by asking binary questions for signs or symptoms of sarcopenia, (eg, falls, weakness, slowness in walking, difficulty rising from a chair, weight loss, or muscle wasting). Sarcopenia cases can be easily missed if healthcare professionals do not ask these questions. EWGSOP2 has recommended the use of SARC-F questionnaire as a validated tool to elicit self-reported possible sarcopenia cases,1 but it is not incorporated as a routine screening component in Comprehensive Geriatric Assessment in clinical practice.31,32
Case Finding: Where to Identify
Despite important progress in the screening process, the crux of the matter remains: clinicians do not know who to target for screening. Few centers have formal pathways to identify sarcopenia cases. Currently, operational clinical services that are most likely to identify sarcopenia cases include frailty units, falls clinics, bone health clinics, and specialist services such as Geriatric-Oncology liaison. In the community setting, General Practitioners, in the UK, are now widely using the electronic Frailty Index to identify older people who are likely to be living with frailty.33 Sarcopenia screening could be embedded as part of routine review to aid in early identification of probable sarcopenia cases. This could be performed through questionnaire utilizing the SARC-F, which could be recorded via telephone, electronic, or postal responses. Alternatively, handgrip strength measurement could be incorporated into yearly anthropometric measurements, alongside weight and blood pressure in General Practice for all older adults. If sarcopenia could be set as a Quality and Outcome Framework (QOF) indicator, or equivalent national standard, this would significantly propel sarcopenia awareness forwards.
Measurement of Muscle Strength
Handgrip Strength and Chair Stand Test are proposed by EWGSOP2 as objective diagnostic measures for probable sarcopenia. EWGSOP2 revised probable sarcopenia as low muscle strength in 2019. This supersedes the original term of presarcopenia, which is characterized as low muscle mass only by EWGSOP. Challenges to implementation of these diagnostic strategies were identified as lack of awareness among healthcare professionals, acquisition of equipment, and time constraints in clinical settings.34 Handgrip strength should be measured using a dynamometer. This can be performed with ease by any trained professional; measurement has been shown to be robust to type of dynamometer and position of measurement.21 However, the availability of dynamometers remains limited outside of research-active centers,35 with equipment shortages in clinical practice,34 especially in lower income countries.36 EWGSOP2 proposed Chair Stand Test as an alternative if dynamometers are unavailable in the clinical setting; however, it is evident that this is not being measured either. However, the CST has high floor effects in people with lower limb pathology.37 In principle, if a patient cannot do the CST, they can be assumed to be sarcopenic, but there might be other reasons such as lack of motivation, low energy levels, or bone injury or instability behind this.36
Measurement of Muscle Quantity or Quality
EWGSOP2 recommends assessment of muscle quantity and quality for technical confirmation only, and reinforces implementation of intervention strategies based on low muscle strength alone. However, there is some recognition that measurement of muscle quantity or quality may be of relevance in identifying early-stage muscle dysfunction, before patients develop significant loss of muscle function (presarcopenia). Muscle quality has been used as a term to define both what the muscle looks like (eg, using surrogate markers for adipose infiltration), and what the muscle is able to do (strength/unit mass). The Global Leadership in Sarcopenia (GLIS) consortium has produced a glossary of terms towards an international standardized approach, and now recommends avoiding the term muscle quality but referring more specifically to the characteristics measured.38 Dual-energy X-ray Absorptiometry, Computed Tomography (CT), and Magnetic Resonance Imaging (MRI) are recommended as gold standard for muscle quantity measurement in sarcopenia diagnosis.4 However, each of these tools are costly, time-consuming, and need to be performed in dedicated hospital environments. Bioelectrical impedance analysis (BIA) and Ultrasound are alternatives that can be performed serially in any environment, and can be used to measure other muscle metrics.39–41 Ultrasound offers a potentially promising method of detecting early loss of muscle quantity at the quadriceps. Total fat mass, and inter- and intra-muscular fat infiltration to not form part of the criteria for sarcopenia diagnosis, but there is evidence that these metrics can affect how the muscle functions. However, there are less widely standardized protocols for assessment of these metrics eg, phase angle with BIA, or echogenicity with ultrasound.42 Appendicular muscle mass measurements can be affected by factors such as exercise, position,43 and fluid status.44 Moreover, changes in muscle quantity and quality are heterogenous within hospitalized populations.36 Importantly, even more so than dynamometers, devices for measurement like BIA are infrequently available in clinical environments. An alternative approach might be to estimate muscle quantity and/or quality from imaging performed as part of standard care (eg, estimation of abdominal muscle cross-sectional area and/or quantification of fat infiltration on CT or MRI scans performed for cancer staging).45 The assessment of calf circumference alone has been proposed as a pragmatic tool to estimate muscle quantity alongside muscle strength to offer increased specificity. Calf circumference is a very crude marker that will certainly be affected by adiposity and fluid balance.46
Measurement of Physical Performance
Objective measurement of physical performance is emerging as part of routine clinical assessment.47 These include measurements such as gait speed, 400 m timed walk, Short Physical Performance Battery, and Timed Up and Go (TUG).48 The TUG has been embedded into the clinical pathway for Comprehensive Geriatric Assessment in some specialist units but is rarely measured by clinicians outside of this setting.49–52 A barrier to early assessment in clinical practice can be the concern of healthcare professionals of increased falls risk when mobilizing patients.53 However, this risk is low if properly supervised, and further education and support to staff may help to overcome this barrier.
Burden of Time Within Clinical Service
Despite the simple algorithm introduced by EWGSOP2 as a pragmatic approach to improve the uptake of sarcopenia cases, it is still perceived as a time-consuming process, and thus remains unpopular among many clinicians. However, diagnostic measurements such as handgrip strength, physical performance, BIA, and ultrasound scan can be performed within minutes, with ultrasound being the longest test as it is operator-dependent.31 Importantly, patients themselves do not perceive these tests as being time-consuming.39 Therefore, concerns about tests being time-consuming relate more greatly to clinicians’ own perceptions of the value of their own time, rather than concerns over burden to patients.
Clinician Knowledge
Based on a UK survey in 2019, it has been demonstrated that physicians generally have a low level of awareness of sarcopenia. There was a low response rate to this survey, with only 28% (49/177) of NHS trusts responding. Of those who responded, 73% (36/49) reported use of any diagnostic tools, and only 6% (3/49) applied diagnostic algorithms for sarcopenia.54 True rates are likely to be even lower if accounting for responder bias. Particularly outside of geriatric medicine, but even amongst some experienced geriatricians, therapeutic nihilism is often a barrier to sarcopenia diagnosis.55 Therapeutic nihilism is highly prevalent in age-related conditions in general. However, it should be acknowledged that patients are deserving of all diagnoses related to them as individuals, and the potential outcomes of treatment should not prevent diagnosis in and of itself.
Challenges to Early Management
Challenges Related to Diagnosis
It is inherently problematic that if sarcopenia is not being diagnosed early, then it cannot be managed at an early stage. Therefore, all the aforementioned challenges in early diagnosis of sarcopenia, are relevant in preventing early management of sarcopenia. From a treatment perspective, research trials that focus on sarcopenia as a binary outcome, may prevent opportunities to identify interventions that could have positive outcomes if delivered prior to declines in muscle mass and function below the current consensus cut-offs points.
Additionally, as described, diagnosis is mainly based on phenotype rather than the underlying mechanistic processes. This means that early management of sarcopenia focuses only on early clinical trajectories, rather than targeting biological pathways before sarcopenia becomes clinically evident. The metabolic syndrome is characterized by excessive accumulation of visceral fat, hypertension, raised fasting blood glucose, and lipid levels. These lead to persistent oxidative stress, inflammatory cytokine release, mitochondrial dysfunction, and insulin resistance, which all contribute to loss of muscle mass.56 It is unsurprising, therefore, that there is a higher prevalence of sarcopenia amongst people with metabolic syndromes (eg, Diabetes Mellitus,57 following androgen deprivation therapy).58 A related problem of renin–angiotensin–aldosterone system dysfunction has also been implicated in muscle dysfunction.59 In other people, pathways involving primary cellular senescence, Growth Hormone depletion, myostatin upregulation, or denervation may be involved. By targeting treatment based on phenotype alone, it also means that treatment may not be appropriately stratified to target the underlying mechanistic pathways, which are likely to significantly differ for individual patients dependent on the underlying pathophysiology. For example, the use of Angiotensin-Converting Enzyme inhibitors to improve endothelial function, angiogenesis, and reduce inflammation,59 which could be beneficial for some but harmful for others. The lack of standardization in guidance for sarcopenia case finding for clinical trials has also historically hindered the identification of new pharmacological treatment strategies towards those at risk.60
Availability of Pathways for Interventions
As mentioned earlier, a common barrier to diagnosis is therapeutic nihilism, as many clinicians perceive that there is little they can do differently if they do identify sarcopenia. However, resistance-based exercise has shown compelling evidence in improvement of muscle quantity and function. It is acknowledged that a lot of evidence for efficacy has been extrapolated from trials of people without sarcopenia or pre-sarcopenia, and few studies have specifically measured sarcopenia as the target outcome.61 Nevertheless, there are wide variations of exercise prescriptions in clinical practice, and there is no standardized guidance in individualized resistance exercise prescription, especially in terms of frequency of exercise sessions, duration of the program, exercise intensity, and repetitions.61
Sarcopenia is a grey entity in public healthcare systems, thus there is scarcity of financial support and limited availability of resources to develop and invest in sarcopenia services. Early management is further impeded by long waiting lists within many public healthcare systems.62 After identifying sarcopenia from the case finding process, no clear pathway has been established on how and when to refer older adults to other services. There is often a lack of infrastructure for appropriate treatment, as well as diagnosis.34 Geriatricians may be reluctant to pursue onward referrals to physiotherapy or dieticians if they are aware of long waiting lists for these services, or if these services do not offer specialist consultation for sarcopenia as a condition.
Clinical Knowledge of Current Evidence Base
As discussed, clinician knowledge of sarcopenia diagnosis is often limited. However, even where clinicians have awareness of how to diagnose sarcopenia, many clinicians may lack awareness of the current evidence base for interventions. New intervention strategies are constantly being trialed, and new research is emerging all the time. Therefore, many clinicians are likely to be unaware when evidence is generated for sarcopenia. Clinicians are often unaware of management strategies, with over two-thirds of patients identified with sarcopenia not being referred to physiotherapy (ie, for individualized resistance exercise approaches) or to dieticians (ie, for implementation of PROT-AGE study group guidance).34 The management of sarcopenia requires a multiprofessional approach and this is associated with additional inherent challenges, as processes of interdisciplinary working and referral differ between centers and countries.63 Although physiotherapists and dieticians may have enhanced knowledge of management, accessing such treatment may be difficult if physicians do not have the required knowledge to refer.
Despite sarcopenia being identified as a skeletal muscle disease, it does not fit into the medical model of treatment, as research to date does not support any pharmacological treatment for sarcopenia.64 Pathogenesis of sarcopenia is not fully understood. It is hypothesized that the imbalance between muscle protein synthesis and degradation may cause the onset of sarcopenia. There are both intrinsic factors within skeletal muscles and extrinsic factors in systemic environment that can contribute to this process. Extensive research aiming on a wide array of molecular targets led to development of drugs like myostatin inhibitor, activin receptor, exercise mimetics, anabolic hormones and natural compound with anti-ageing effects, but none of them has proven efficacy. This may lead to doctors being disengaged from sarcopenia interest, as they may view it as something that other professionals are better placed to treat, and that there is little that they can offer as a doctor.65 This is opposed to osteoporosis, where the evidence-base for pharmacological agents for osteoporosis has promoted the development of bone health clinics. Studies have shown that osteoporosis strongly increases the risk of sarcopenia, and vice versa. Despite its bidirectional relationship, sarcopenia remains well under-detected and under-related as it is not an integral assessment component in bone health clinic.66 This is despite the best evidence-based treatments for osteoporosis being nutrition and exercise, in line with sarcopenia. It is likely that the identification of drug treatments for sarcopenia will help to promote infrastructure for delivery of early interventions.
Patient Perception and Compliance with Interventions
Resistance exercise and even optimal nutrition takes time and requires self-discipline. Patients can be easily demotivated due to slow progress with results that are not clearly obvious to them.55,67 This may also limit clinicians from implementing treatment strategies due to uncertainty about treatment adherence.
Current Evidence Base for Interventions
Although sarcopenia is an area of increasing research interest, few trials have specifically targeted interventions to patients with sarcopenia alone. It is increasingly recognized that recruiting patients with sarcopenia to clinical trials in the real world is challenging. As an example, in the UK, the Leucine and ACE inhibitors as therapies for sarcopenia (LACE) trial struggled to recruit to the original target.59 Maintaining strict criteria for sarcopenia in clinical studies will limit participation, but will also limit the applicability of results to patients at the early stages of their muscle loss trajectory.
It is also important to consider that current research has targeted sarcopenia based on its phenotypic presentation, rather than targeting underlying mechanisms. Thus, it is difficult for clinicians to be certain which treatments will benefit individual patients. Whilst there is strong evidence for resistance exercise in improving muscle mass and strength, as described, it remains unclear to what extent this can help to reverse sarcopenia once patients meet criteria for this. The evidence for nutritional interventions is somewhat weaker.68 The latest evidence shows Whey Protein, Leucine, and Vitamin D Supplementation can improve muscle strength and function but Vitamin D as a monotherapy is ineffective.69,70 Exercise and food supplementation can be considered to have synergistic effects against inflammation in older adults. Apart from that, many research trials for sarcopenia are currently ongoing, many of which have been shown to be promising in early phase trials (eg, neuromuscular electrical stimulation,71 low energy light therapy, pharmaceutical agents such as myostatin blockers).72 Of course, evidence-based interventions cannot be translated into clinical practice until they have shown to have efficacy and safety in late phase trials.
Implementation Science: Bridging the Gap
In all fields of medicine, it is known that there has repeatedly been a significant delay for research findings to reach clinical practice. Historically, this has been quoted as 17 years on average,73 although this has improved with enhanced rapidity of communication through electronic means and global collaboration. One of the challenges that limits translation of research into clinical practice is that historically professionals have worked either within academia (ie evidence generation), or clinical practice, with fewer professional workers at the interface between these. Clinicians working in implementation science offer the potential to bridge this gap, and there is clearly an urgent need for this in the field of sarcopenia to enable evidence-based resistance exercise and nutritional interventions to be integrated into clinical practice pathways.
Recommendations and Future Directions
Recommendations for Current Clinical Practice
- Clinicians should be familiar with the current sarcopenia definition and diagnose sarcopenia in a pragmatic approach according to EWGSOP2, AWGS, IWGS, or FNIH criteria. The Global Leadership Initiative in Sarcopenia (GLIS) aims to offer a unified definition of sarcopenia to be used worldwide.38
- Proactive screening for self-reported sarcopenia patients based on SARC-F questionnaire in clinical practice, particularly in community settings, will assist with case finding and stratification.
- Annual measurement of handgrip strength will enable the identification of dynamic changes over time, and a shift towards a life course approach to management of sarcopenia.
- Geriatricians should lead and collaborate with dieticians and physiotherapists in developing local clinical pathways for sarcopenia, to aid in diagnosis and treatment plans, and bidirectional knowledge sharing.
- Whilst the field is developing, individual hospital sites should develop their own audit standards for monitoring purposes, to ensure that improvement strategies relevant to the local practice are effective in their implementation. We encourage the sharing of summary data between sites to promote development of national standards for sarcopenia diagnosis and treatment.
Future Directions
There are currently no drugs approved for treatment of sarcopenia.53 Pharmacological intervention remains imperative as a future direction to reduce sarcopenia burden, alongside non-pharmacological strategies. Any new development in pharmacological intervention will be encouraging, and be able to spur on early diagnosis of sarcopenia.
Conclusion
From sarcopenia diagnosis to management, there are multilevel obstacles in translating sarcopenia research advancements into clinical practice. Challenges in early diagnosis relate to the application of diagnostic criteria towards established disease, and practical/logistical aspects of diagnosis such as the availability of equipment, time pressures, and knowledge of staff. Challenges in early management related directly to the challenges related to early diagnosis, as well as the availability of established pathways for interventions. With significant adverse outcomes associated with sarcopenia, we must commit ourselves to overcome the challenges, and treat sarcopenia just like any other major geriatric syndrome. Future research should aim to enhance understanding of fundamental mechanisms with the development of targeted interventions towards a stratified medicine approach, whilst simultaneously striving to further implement evidence-based interventions within clinical practice.
Disclosure
The authors report no conflict of interest in this work.
References
1. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population‐based sample of community‐dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7(3):290–298. doi:10.1002/jcsm.12073
2. Zhang Y, Hao Q, Ge M, Dong B. Association of sarcopenia and fractures in community-dwelling older adults: a systematic review and meta-analysis of cohort studies. Osteoporos Int. 2018;29(6):1253–1262. doi:10.1007/s00198-018-4429-5
3. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O, Wright JM. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One. 2017;12(1):e0169548. doi:10.1371/journal.pone.0169548
4. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
5. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
6. Chen LK, Woo J, Assantachai P, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi:10.1016/j.jamda.2019.12.012
7. Rolland Y, Czerwinski S, van Kan GA, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12(7):433–450. doi:10.1007/bf02982704
8. McLean RR, Shardell MD, Alley DE, et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the national institutes of health (FNIH) sarcopenia project. J Gerontol a Biol Sci Med Sci. 2014;69(5):576–583. doi:10.1093/gerona/glu012
9. Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. doi:10.1002/jcsm.12783
10. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures: the longitudinal aging study Amsterdam. J Gerontol a Biol Sci Med Sci. 2018;73(9):1199–1204. doi:10.1093/gerona/glx245
11. Bao W, Sun Y, Zhang T, et al. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: a systematic review and meta-analysis. Aging Dis. 2020;11(4):863. doi:10.14336/ad.2019.1012
12. Landi F, Cesari M, Calvani R, et al.; on behalf of the SPRINTT Consortium. The “Sarcopenia and Physical fRailty IN older people: multi-componenT Treatment strategies” (SPRINTT) randomized controlled trial: design and methods. Aging Clin Exp Res. 2017;29(1):89–100. doi:10.1007/s40520-016-0715-2
13. Talar K, Hernández-Belmonte A, Vetrovsky T, Steffl M, Kałamacka E, Courel-Ibáñez J. Benefits of resistance training in early and late stages of frailty and sarcopenia: a systematic review and meta-analysis of randomized controlled studies. J Clin Med. 2021;10(8):1630. doi:10.3390/jcm10081630
14. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1(2):129–133. doi:10.1007/s13539-010-0014-2
15. Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; A quantitative review. Front Physiol. 2012;3. doi:10.3389/fphys.2012.00260
16. Welch C, Hassan-Smith ZK, Greig CA, Lord JM, Jackson TA. Acute sarcopenia secondary to hospitalisation - an emerging condition affecting older adults. Aging Dis. 2018;9(1):151. doi:10.14336/ad.2017.0315
17. Bauer J, Morley JE, Schols AMWJ, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. 2019;10(5):956–961. doi:10.1002/jcsm.12483
18. Sayer AA, Cruz-Jentoft A. Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing. 2022;51(10). doi:10.1093/ageing/afac220
19. Welch C. Acute sarcopenia: definition and actual issues. In: Practical Issues in Geriatrics. Springer International Publishing; 2021:133–143.
20. Ni Lochlainn M, Bowyer R, Steves C. Dietary protein and muscle in aging people: the potential role of the gut microbiome. Nutrients. 2018;10(7):929. doi:10.3390/nu10070929
21. Dodds RM, Syddall HE, Cooper R, et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12):e113637. doi:10.1371/journal.pone.0113637
22. Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13(1):1–7. doi:10.1097/mco.0b013e328333c1c1
23. Welch C, Greig C, Lewis D, et al. Trajectories of muscle quantity, quality and function measurements in hospitalized older adults. Geriatr Gerontol Int. 2022;22(4):311–318. doi:10.1111/ggi.14366
24. Welch C, Greig C, Majid Z, et al. Induced frailty and acute sarcopenia are overlapping consequences of hospitalisation in older adults. J Frailty Sarcopenia Falls. 2022;07(03):103–116. doi:10.22540/jfsf-07-103
25. Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci. 2019;31(1):75–78. doi:10.1589/jpts.31.75
26. Rockwood K. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi:10.1503/cmaj.050051
27. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–M157. doi:10.1093/gerona/56.3.m146
28. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:10.1016/j.clnu.2008.06.013
29. Chen Y, Almirall-Sánchez A, Mockler D, Adrion E, Domínguez-Vivero C, Romero-Ortuño R. Hospital‐associated deconditioning: not only physical, but also cognitive. Int J Geriatr Psychiatry. 2022;37(3). doi:10.1002/gps.5687
30. Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6. doi:10.3389/fnagi.2014.00192
31. Chong E, Bao M, Goh EF, Lim WS. SARC-F at the emergency department: diagnostic performance for frailty and predictive performance for reattendances and acute hospitalizations. J Nutr Health Aging. 2021;25(9):1084–1089. doi:10.1007/s12603-021-1676-5
32. Pachołek K, Sobieszczańska M. Sarcopenia identification during comprehensive geriatric assessment. Int J Environ Res Public Health. 2021;19(1):32. doi:10.3390/ijerph19010032
33. Lansbury LN, Roberts HC, Clift E, Herklots A, Robinson N, Sayer AA. Use of the electronic Frailty Index to identify vulnerable patients: a pilot study in primary care. Br J Gen Pract. 2017;67(664):e751–e756. doi:10.3399/bjgp17x693089
34. Reijnierse EM, de van der Schueren MAE, Trappenburg MC, Doves M, Meskers CGM, Maier AB. Lack of knowledge and availability of diagnostic equipment could hinder the diagnosis of sarcopenia and its management. PLoS One. 2017;12(10):e0185837. doi:10.1371/journal.pone.0185837
35. Sayer AA. Sarcopenia. BMJ. 2010;341(2):c4097. doi:10.1136/bmj.c4097
36. Eckman M, Gigliotti C, Sutermaster S, Mehta K. Get a grip! Handgrip strength as a health screening tool. In:
37. Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. 1999;70(2):113–119. doi:10.1080/02701367.1999.10608028
38. Cawthon PM, Visser M, Arai H, et al. Defining terms commonly used in sarcopenia research: a glossary proposed by the Global Leadership in Sarcopenia (GLIS) Steering Committee. Eur Geriatr Med. 2022;13(6):1239–1244. doi:10.1007/s41999-022-00706-5
39. Welch C, Greig C, Masud T, Jackson TA. Muscle quantity and function measurements are acceptable to older adults during and post- hospitalisation: results of a questionnaire-based study. BMC Geriatr. 2021;21(1). doi:10.1186/s12877-021-02091-3
40. Perkisas S, Brockhattingen K, Welch C, Bahat G. Using ultrasound in the assessment of muscle. ejgg. 2021;3(1):1–3. doi:10.4274/ejgg.galenos.2021.2-1
41. Welch C, Greig C, Majid Z, et al. The feasibility of conducting acute sarcopenia research in hospitalised older patients: a prospective cohort study. Eur Geriatr Med. 2022;13(2):463–473. doi:10.1007/s41999-021-00565-6
42. Akamatsu Y, Kusakabe T, Arai H, et al. Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. J Cachexia Sarcopenia Muscle. 2022;13(1):180–189. doi:10.1002/jcsm.12860
43. Welch C, Majid Z, Andrews I, et al. Effect of position and exercise on measurement of muscle quantity and quality: towards a standardised pragmatic protocol for clinical practice. BMC Sports Sci Med Rehabil. 2021;13(1). doi:10.1186/s13102-020-00227-3
44. Stanley B, Greig C, Jackson T, et al. Investigating the impact of fluid status on the ultrasound assessment of muscle quantity and quality in the diagnosis of sarcopenia – a multidimensional cross-sectional study. BMC Geriatr. 2023;23(1). doi:10.1186/s12877-023-04177-6
45. Gomindes AR, Appleton JP, Chugh R, Welch C. Muscle quantity at C3 and/or L3 on routine trauma series computed tomography correlate with brain frailty and clinical frailty scale: a cross-sectional study. Cureus. 2021. doi:10.7759/cureus.15912
46. Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16(1). doi:10.1186/s12877-016-0349-4
47. Cawthon PM. Assessment of lean mass and physical performance in sarcopenia. J Clin Densitom. 2015;18(4):467–471. doi:10.1016/j.jocd.2015.05.063
48. Bijlsma AY, Meskers CGM, van den Eshof N, et al. Diagnostic criteria for sarcopenia and physical performance. Age. 2014;36(1):275–285. doi:10.1007/s11357-013-9556-5
49. Chen YM, Chuang YW, Liao SC, et al. Predictors of functional recovery (FR) for elderly hospitalized patients in a geriatric evaluation and management unit (GEMU) in Taiwan. Arch Gerontol Geriatr. 2010;50:S1–S5. doi:10.1016/s0167-4943(10)00041-5
50. Anita K, Biswas TK. A study of timed get up and go scores as falls risk assessment in elderly in a tertiary care centre. Int J Res Med Sci. 2020;8(3):841–846. doi:10.18203/2320-6012.ijrms20200519
51. Freter SH, Fruchter N. Relationship between timed “up and go” and gait time in an elderly orthopaedic rehabilitation population. Clin Rehabil. 2000;14(1):96–101. doi:10.1191/026921500675545616
52. Riis J, Byrgesen SM, Kragholm KH, Mørch MM, Melgaard D. Validity of the GAITRite walkway compared to functional balance tests for fall risk assessment in geriatric outpatients. Geriatrics. 2020;5(4):77. doi:10.3390/geriatrics5040077
53. Geelen SJG, van Dijk - Huisman HC, de Bie RA, et al. Barriers and enablers to physical activity in patients during hospital stay: a scoping review. Syst Rev. 2021;10(1). doi:10.1186/s13643-021-01843-x
54. Offord NJ, Clegg A, Turner G, Dodds RM, Sayer AA, Witham MD. Current practice in the diagnosis and management of sarcopenia and frailty – results from a UK-wide survey. J Frailty Sarcopenia Falls. 2019;71–77. doi:10.22540/jfsf-04-071
55. Dismore L, Hurst C, Sayer AA, Stevenson E, Aspray T, Granic A. Study of the older adults’ motivators and barriers engaging in a nutrition and resistance exercise intervention for sarcopenia: an embedded qualitative project in the MIlkMAN pilot study. Gerontol Geriatr Med. 2020;6:2333721420920398. doi:10.1177/2333721420920398
56. Nichikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic Syndrome and Sarcopenia. Nutrients. 2021;13(10):3519. doi:10.3390/nu13103519
57. Izzo A, Massimino E, Riccardi G, Della Pepa G. A narrative review on sarcopenia in type 2 diabetes mellitus: prevalence and associated factors. Nutrients. 2021;13(1):183. doi:10.3390/nu13010183
58. Couderc AL, Villani P, Berbis J, et al. HoSAGE: sarcopenia in older patient with intermediate / high-risk prostate cancer, prevalence and incidence after androgen deprivation therapy: study protocol for a cohort trial. BMC Cancer. 2022;22(1). doi:10.1186/s12885-021-09105-8
59. Witham MD, Achison M, Aspray TJ, et al. Recruitment strategies for sarcopenia trials: lessons from the LACE randomized controlled trial. JCSM Rapid Commun. 2021;4(2):93–102. doi:10.1002/rco2.38
60. Reginster JY, Cooper C, Rizzoli R, et al. Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res. 2016;28(1):47–58. doi:10.1007/s40520-015-0517-y
61. Hurst C, Robinson SM, Witham MD, et al. Resistance exercise as a treatment for sarcopenia: prescription and delivery. Age Ageing. 2022;51(2). doi:10.1093/ageing/afac003
62. O’Dowd A. NHS waiting list hits 14 year record high of 4.7 million people. BMJ. 2021;n995. doi:10.1136/bmj.n995
63. Bury TJ, Stokes EK. A global view of direct access and patient self-referral to Physical Therapy: implications for the profession. Phys Ther. 2013;93(4):449–459. doi:10.2522/ptj.20120060
64. Haase CB, Brodersen JB, Bülow J. Sarcopenia: early prevention or overdiagnosis? BMJ. 2022;e052592. doi:10.1136/bmj-2019-052592
65. Kwak JY, Kwon KS. Pharmacological interventions for treatment of sarcopenia: current status of drug development for sarcopenia. Ann Geriatr Med Res. 2019;23(3):98–104. doi:10.4235/agmr.19.0028
66. Yu X, Sun S, Zhang S, et al. A pooled analysis of the association between sarcopenia and osteoporosis. Medicine (Baltimore). 2022;101(46):e31692. doi:10.1097/md.0000000000031692
67. Vasudevan A, Ford E. Motivational factors and barriers towards initiating and maintaining strength training in women: a systematic review and meta-synthesis. Prev Sci. 2022;23(4):674–695. doi:10.1007/s11121-021-01328-2
68. Gielen E, Beckwée D, Delaere A, et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev. 2021;79(2):121–147. doi:10.1093/nutrit/nuaa011
69. Chang MC, Choo YJ. Effects of whey protein, leucine, and vitamin D supplementation in patients with sarcopenia: a systematic review and meta-analysis. Nutrients. 2023;15(3):521. doi:10.3390/nu15030521
70. Prokopidis K, Giannos P, Katsikas Triantafyllidis K, et al. Effect of vitamin D monotherapy on indices of sarcopenia in community‐dwelling older adults: a systematic review and meta‐analysis. J Cachexia Sarcopenia Muscle. 2022;13(3):1642–1652. doi:10.1002/jcsm.12976
71. Ikeda T, Katayama S, Kitagawa T. The combined intervention of neuromuscular electrical stimulation and nutrition therapy: a scoping review. Clin Nutr ESPEN. 2023;54:239–250. doi:10.1016/j.clnesp.2023.01.027
72. Mellen RH, Girotto OS, Marques EB, et al. Insights into pathogenesis, nutritional and drug approach in sarcopenia: a systematic review. Biomedicines. 2023;11(1):136. doi:10.3390/biomedicines11010136
73. Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–520. doi:10.1258/jrsm.2011.110180
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
