Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Occurrence of Methicillin-Resistant Staphylococcus aureus (MRSA) in Bovine Bulk Milk and Farm Workers in Smallholder Dairy Farming Systems in Northwestern Ethiopia
Authors Kassa HY, Belete MA , Yihunie FB , Bayu A , Demlie TB, Tassew H
Received 9 December 2023
Accepted for publication 4 March 2024
Published 11 March 2024 Volume 2024:15 Pages 71—80
DOI https://doi.org/10.2147/VMRR.S454193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Haregua Yesigat Kassa,1 Mequanint Addisu Belete,1 Fanuel Bizuayehu Yihunie,2 Azeb Bayu,3 Tiliksew Bialfew Demlie,4 Habtamu Tassew4
1Department of Veterinary Laboratory Technology, College of Agriculture and Natural Resource, Debre Markos University, Debre Markos, Ethiopia; 2College of Veterinary Medicine and Animal Science, Samara University, Samara, Ethiopia; 3Department of Veterinary Science, College of Agriculture and Environmental Science, Debre Tabor University, Debre Tabor, Ethiopia; 4Department of Veterinary Science, College of Agriculture and Environmental Sciences, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Mequanint Addisu Belete, Email [email protected]; [email protected]
Background and Purpose: Staphylococcus aureus is a common pathogen responsible for causing various human and animal infections and is well known for its ability to develop resistance to multiple antibiotics. This study aimed to evaluate the occurrence of methicillin-resistant Staphylococcus aureus (MRSA) in bulk milk and dairy farms in northwestern Ethiopia and to determine their phenotypic and genotypic antimicrobial susceptibility patterns.
Methods: We collected 50 bulk milk samples from 50 dairy farms and 50 hand swabs from dairy milkers. The cefoxitin disk diffusion test and PCR-based assays were used to identify MRSA isolates. In addition, cefoxitin-resistant isolates were tested for susceptibility to other antibiotics using the Kirby-Bauer disk diffusion method.
Results: The results showed that MRSA was detected in 8 samples: 6 from bulk milk samples (12%) and 2 from hand swabs (4%). All MRSA isolates exhibited a high resistance rate to penicillin (100%), followed by tetracycline (75%), ciprofloxacin (25%), chloramphenicol (25%), erythromycin (25%), gentamycin (12.5%), and trimethoprim-sulfamethoxazole (12.5%). Moreover, 72% of the isolates showed resistance to three or more antibiotic classes and were classified as multidrug-resistant.
Conclusion: This study identified methicillin-resistant Staphylococcus aureus and multidrug-resistant MRSA in bulk milk and dairy farms in northwestern Ethiopia. These findings highlight the potential risk of transmission of these antibiotic-resistant bacteria to humans and the need for improved antibiotic stewardship in the dairy sector using the One Health approach.
Keywords: dairy farms, Ethiopia, humans, MRSA, bulk milk, resistance
Introduction
Milk is a rich source of fats, proteins, carbohydrates, minerals, and vitamins essential for the development of mammals. Nevertheless, it is also an excellent medium for growing and sourcing several foodborne pathogens.1,2 In many low- and middle-income countries (LMICs), milk and dairy products are produced by smallholder farmers under unsanitary conditions, making them vulnerable to contamination by pathogenic microorganisms.3–6 At the farm level, milk can be contaminated with pathogenic microorganisms through the exterior of the udder and adjusting areas, dairy utensils, the hands of the dairy worker, and the environment.7,8
Staphylococcus aureus (S. aureus) is a significant pathogen affecting humans and animals. It has long been recognized as a worthy cause of mastitis in cattle, with infected udder of animals serving as a vital reservoir and having the ability to shed through their milk.9–11 Staphylococcal mastitis compromises the health of animals and harms the economy of nations by reducing production and decreasing milk quality.10 In humans, S. aureus is one of the primary causes of food-borne diseases. It results from the contamination of food by highly heat-stable staphylococcal enterotoxins.12,13 It is commonly reported as a bacterial hazard in animal-derived foods in Ethiopia and other East African countries.14
Antimicrobials are commonly and often inappropriately used on dairy farms, leading to the development of drug resistance that has progressed from single-drug to multi-drug-resistant strains.15,16 Methicillin-resistant Staphylococcus aureus (MRSA) strains are resistant to methicillin and related anti-staphylococcal drugs.17 This resistance is due to the acquisition of the mecA gene, which encodes a modified penicillin-binding protein called PBP2a with a low affinity for beta-lactam antibiotics. The mecA gene indicates potential resistance and serves as a marker for identifying MRSA.18
MRSA infections are becoming a growing global health concern in human and veterinary medicine.19 Livestock-associated MRSA (LA-MRSA) has recently emerged and has been detected in different livestock species, foods of animal origin, and humans.20–25 LA-MRSA demonstrates a greater capacity for establishing and transmitting within livestock herds, posing a higher potential for spreading and infecting humans compared to other human MRSA strains.19,26
In disk diffusion tests, cefoxitin is considered the best indicator of MRSA due to its increased sensitivity, specificity, and strength as an inducer of mecA. However, PCR-based detection of the mecA gene is still the gold standard for identifying MRSA isolates.27,28 Several studies have been carried out in Ethiopia to investigate MRSA infections in different regions of the country.29–36 However, there is a lack of information regarding MRSA in raw bulk milk and among farm workers. Thus, the aim of this study was to detect MRSA strains from bulk milk and dairy milkers and determine their phenotypic and genotypic antimicrobial profiles.
Materials and Methods
Study Area and Farm Characteristics
The study was conducted on dairy farms located in the town of Debre Markos, the administrative center for East Gojjam in the Amhara regional state. Debre Markos is located 300 kilometers northwest of the capital, Addis Ababa. The geographical location of the study area is situated between 10°17′00″ and 10°21′30″ N latitude and 37°42′00″ to 37°45′30″ E longitude, and its elevation ranges from 2350 to 2500 m above sea level (Figure 1).
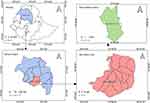 |
Figure 1 Geographic location of the study area. |
In recent years, small-scale urban and peri-urban dairy farms have rapidly grown throughout the city, serving as an income generator by selling milk and milk products and live animals. The dairy herd comprises an average of 50 lactating cows milked twice daily. Owners of cows or farm workers usually milk their animals by hand and store the milk in cans at room temperature for sale and daily consumption. As a result, this practice leads to an increase in milk with a high level of microbial contamination, which adversely affects the safety and quality of milk.
Study Design and Sampling
The present cross-sectional study was conducted from November 2020 to June 2021 on 50 dairy farms. The study included all available, personally funded, and microenterprise dairy farms. Four of the 54 dairy farms contacted had no lactating cows and ceased milk production during the study period. Therefore, 50 dairy farms were included in the study. The data collection methods used were 1) a structured questionnaire to assess the overall general characteristics of the farm and 2) direct observations of the hygiene practices conducted on farms.
Approximately 100 mL of bulk milk samples per type of farm were aseptically collected using sterile falcon tubes. Hand swabs were collected from 50 dairy milkers involved in milking cows using cotton-tipped swabs kept in transport media (1 farm worker per farm). The samples were immediately transported in an icebox to the microbiology laboratory of the Debre Markos referral hospital for bacteriological analysis.
S. aureus Isolation and Identification
One milliliter of each collected sample was enriched in 10 mL of Tryptone soya broth (Himedia, India) at 37 °C for 18–24 hrs. Next, bacterial cultures were individually inoculated on the selective medium, mannitol salt agar (MSA) (Himedia, India). The plates were incubated aerobically at 37 OC for 18–24 hrs. Golden-yellow colonies on the MSA plate were considered presumptive S. aureus. Afterward, the oxidation fermentation (OF) test was performed to distinguish the Staphylococcus species from the Micrococcus species. Subsequently, potential Staphylococcal colonies grew on mannitol salt agar and fermented on OF media, were subcultured on blood agar with 5% sheep blood (Oxoid, UK). The colonies with beta-hemolytic patterns on blood agar were kept, and further identifications were done by conventional methods (oxidase test, DNase test, catalase test, and coagulase tube test) according to the procedure outlined by37,38.
Cefoxitin Disk Diffusion Method
Cefoxitin susceptibility testing was employed with 30 µg of cefoxitin disks to identify methicillin resistance, following the Clinical Laboratory Standards Institute (CLSI) criteria.39 The identified isolate inoculum was prepared by adjusting the turbidity to 0.5 McFarland standards and was inoculated on a Mueller Hinton agar plate (Himedia, India). The disks were applied to MHA, and the plates were incubated at 37 °C for 24 h. Isolates that showed an inhibition zone ≤ 21 mm were considered to be MRSA, whereas isolates that showed an inhibition zone ≥ 22 mm were identified as MSSA (Methicillin-sensitive S. aureus), respectively.39 A standard strain of MRSA (ATTC 33591) and MSSA (ATTC 25923) were used as positive and negative controls in all assays, respectively. All phenotypically confirmed and purified MRSA isolates were preserved in Brain Heart Infusion (BHI) broth (Himedia, India) with 20% glycerol at −20 °C for further molecular conformation.
DNA Extraction and Polymerase Chain Reaction
Overnight cultures in BHI broth (Himedia, India) were used for DNA extraction. The extraction process was done using a Bio Basic Genomic DNA Extraction Kit (Bio Basic Group, Canada) according to the instructions of the manufacturer. A Nanodrop 2000 Spectrophotometer (Thermo Scientific TM, USA) was used to check the concentration and quality of isolated DNA.
Cefoxitin-resistant isolates were further subjected to PCR (polymerase chain reaction) to detect the presence of the mecA gene using primers and protocols as described previously.40,41 Amplification was done using the Prima 96 Plus Thermal Cycler (Himedia, India). Initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 1 min, annealing at 60°C for the subsequent 40s, extension at 72°C for 1 min, and final extension at 72°C for 10 min. PCR products were submitted to electrophoresis in a 1.5% (w/v) agarose gel (Bio Basic Inc. Canada) stained with 10 mg/mL ethidium bromide. The electrophoresis was carried out at 120 V for 40 min in 1X TAE buffer (40 mM Tris, 1 mM EDTA, and 20 mM glacial acetic acid, pH 8.0). A 100-bp DNA molecular weight marker (Solis BioDyne, Estonia) was used to estimate the size of the product. The resulting band pattern was visualized and recorded using a gel documentation system (BioRAD, USA).
Antimicrobial Susceptibility Testing
The Kirby-Bauer disc diffusion method was used to determine the antimicrobial susceptibility profile of MRSA isolates according to the CLSI guidelines.39 The antimicrobials tested included those of relevance for the treatment of MRSA infections in humans and animals, namely, penicillin (10 μg), tetracycline (30 μg), gentamycin (10 μg), chloramphenicol (30 μg), erythromycin (15 μg), ciprofloxacin (10 μg), and trimethoprim-sulfamethoxazole (25 μg). The antimicrobials used in this study were obtained from Thermo Scientific™ Oxoid™, United Kingdom.
Data Analyses
The data collected was coded and entered into the Microsoft Excel spreadsheet (Microsoft ® Office Excel 2016). The statistical software SPSS (Statistical Package for Social Sciences), version 23 (IBM, USA) was used for the analysis. Descriptive statistics were carried out to describe the results of the occurrence and hygiene practices at the farm level.
Results
A total of fifty dairy farms of three different types were enrolled: family 10 (20%), enterprise 22 (44%), and private 18 (36%) (Table 1). In this study, 50, 32, and 18% of farms had concrete, soil, and stone slabs, respectively. About 60% of dairy farms possess lactating cows 20–50, while 28% have 1–20 and are managed under semi-intensive production systems. The assessment revealed that only 5 (10%) practiced pre- and post-teat dipping, and 10 (20%) employed hand washing before and between the milking process (Table 1).
 |
Table 1 Background Information on Farm Characteristics and Isolation Frequency of S. aureus and MRSA from Bulk Milk and Hand Swab Samples in 50 Dairy Farms in Debre Markos, Ethiopia |
In total, 100 samples were sampled, comprising 50 bulk milk samples and 50 hand swab samples. The collected samples were tested for potential staphylococci by broth enrichment and culture on MSA selective plates. Out of 100 samples, 75 revealed bacterial growth. Based on morphological traits and biochemical assays, S. aureus was found in 14 of the 100 samples. The positivity constituted 11 isolates in bulk milk samples and 3 in workers’ hand swab samples (Figure 2). S. aureus was detected at the farm level in 11 of 50 (22%) dairy farms.
 |
Figure 2 The overall distributions of S. aureus, MRSA, and MSSA in bulk milk and hand swabs of farm workers among 50 investigated dairy farms in Debre Markos, Ethiopia. |
Eight of the 14 S. aureus isolates were confirmed to be MRSA based on resistance to cefoxitin and PCR-based detection of the mecA gene (for detailed gel image analysis, see Figure S1. The occurrence rate of MRSA in S. aureus isolates was 57.1% in the present study. All (100%) of the cefoxitin-resistant isolates yielded positive results for the mecA gene, as shown in Table 2.
 |
Table 2 Phenotypic, Genotypic, and Antimicrobial Resistance Profiles of MRSA-Positive Isolates |
Six MRSA isolates were obtained from bulk milk samples, including 1 from a family (10%), 3 from an enterprise (13.6%), and 2 from private farms (11.1%). Two (9%) MRSA isolates were also detected from hand swabs collected from an enterprise farm (Table 1). Regarding ventilation systems, MRSA strains were most commonly detected in poor ventilation systems that lacked proper maintenance and cleaning practices. Detection of MRSA was more frequent in isolates from farms (80%) that did not practice hand washing before and between milking compared to isolates from farms that followed proper hand hygiene protocols (Table 1).
The antimicrobial susceptibility test revealed that all MRSA isolates showed 100% resistance to penicillin (Figure 3). Resistance to tetracycline was detected at a higher rate in six (75%) isolates. Only one isolate was resistant to gentamycin (12.5%). A high rate of antibiotic sensitivity was demonstrated against trimethoprim-sulfamethoxazole (87.5%) and gentamycin (87.5%), followed by erythromycin (75%) (Figure 3). Moreover, 75% of the isolates showed resistance to three or more antibiotic classes and were determined as multidrug-resistant (MDR).
 |
Figure 3 Summary of the antibiotic susceptibility test results of MRSA isolates. |
Discussion
Drug resistance is increasing at an alarming rate globally, which worsens the treatment and control of microbial diseases. The problem is more pronounced in developing countries, where infectious disease is more prevalent. This is due to the illegal and misused access to drugs in drug stores, local traders, and non-governmental organizations without the prescription of physicians and veterinarians.42 Among the global reports on antimicrobial resistance, MRSA is one of the MDR strains with a wide range of resistance to various groups of antibiotics. The occurrence rates of S. aureus and MRSA contamination in raw bovine milk have been widely reported and potentially threaten human health. In Ethiopia, the prevalence of MRSA has been reported in different regions, which implies an increasing spread of this multidrug-resistant pathogen in humans and animals.29,43
This study assessed the occurrence of S. aureus and MRSA on 50 dairy farms. Overall, 100 samples containing 50 bulk milk samples and 50 hand swabs were evaluated for methicillin resistance by cefoxitin disk diffusion testing and mecA-specific PCR. In this study, the overall occurrence of S. aureus on the investigated farms was 22%. Similar findings were reported by other authors that revealed the presence of 21.46% and 22.14% S. aureus in dairy farms located in the Bishoftu and Assosa towns, respectively.33,36 These agreements may be due to close similarities between the agroecological zones of the regions, and the laboratory procedures used to identify the target bacterium. The rate of contamination found in the present study was lower than that reported by,44 who investigated the 85% detection of S. aureus in dairy farms in Greece. Other studies on dairy farms in different countries have reported an occurrence rate ranging from 2.16% to 62%.45–48 The variations might be associated with the difference in sample size, different risk factors, management problems, the level of awareness of the disease among the farm owners, the species affected, and environmental variations.
MRSA was found in 12% of the sampled farms, comparable to the 13.2% reported by49 in a similar study conducted in selected dairy farms around Addis Ababa, Ethiopia. The low reports of MRSA suggest that disease management on farms is good, including regular cleaning and disinfection of dairy equipment and proper antibiotic use. Nonetheless, the present study disagrees with31 and,50 who presented 61% and 31% farm-wise occurrences of MRSA in Mekelle and Sebeta, Ethiopia, respectively. The differences in the reports are primarily due to the type of milk sample used; in this case, samples were taken from clinically mastitic milk, which can contain high levels of S. aureus. However, the current results showed a higher detection rate than reports from other countries, with percentages of 4.4% and 4.8%.2,51 This variation is typically associated with the sample size and type, the methods used to identify the pathogen, the species of animal, and the overall hygienic status of dairy farms and personnel.
In this study, the highest proportion of MRSA was recovered from the raw milk sample (75%), compared to the hand swab (25%), which is a comparable finding with,33 who identified higher detection from raw milk (25.5%) than milk handlers (19.23%). This finding is also consistent with a study conducted in Indonesia,52 where MRSA detection was more common in pooled milk than in close-contact humans. This result may be affected by the hygienic status of the milk handlers since antiseptic usage may compromise the recovery rate of the pathogen.
The antibiotic sensitivity result of this study showed that MRSA isolates were susceptible to antibiotics like trimethoprim-sulpha (87.5%), gentamycin (87.5%), erythromycin (75%), chloramphenicol (62.5%), and ciprofloxacin (62.5%), which is in line with studies conducted by53 in Assam, India, and54 in Italy. In contrast, a study from Ethiopia29 found a high level of trimethoprim-sulfamethoxazole resistance (76.9%) in MRSA isolates. The variation in the resistance profile of the bacteria may be due to differences in drug availability and the diversity of the source isolates. Conversely, 100% resistance isolates were recorded against cefoxitin and penicillin antibiotics, which is highly consistent with the studies conducted in Assam, India,53 and Libya.55 High multidrug resistance in bacteria can have significant consequences for both human and animal health.56 Infection caused by MDR MRSA results in increasing healthcare costs, mortality rate, and limited treatment options. Overall, it has a significant impact on disease management. Therefore, the development of new antibiotics and the exploration of alternative treatment strategies are needed to provide solutions. In this study, 75% of the isolates were MDR, indicating an alarming issue in the dairy sector and humans. This aggressive increment in the resistance rate can be related to the ability of pathogens to accumulate antibiotic-resistant genes coupled with the indiscriminate use of antibiotics.57
Conclusions
The present study investigated the occurrence of MRSA in bulk milk and dairy farms in northwestern Ethiopia, which could pose an increased risk to consumers of dairy products. Our results underline the need for continued comprehensive research and mentoring programs to better understand the prevalence and spreading of multidrug-resistant MRSA in smallholder dairy farming systems. Our detection of MRSA in both the milk of farms and farmworkers suggests the potential transmission of these pathogens between animals and humans in agricultural settings. Thus, efforts to better understand the pathogen are needed, with molecular typing investigations giving us clues regarding the genetic relatedness and transmission dynamics of MRSA in dairy farms. The study has limitations concerning the mecC gene, a novel variant of the mecA gene (formerly mecALGA251). Despite this limitation, the study demonstrates the occurrence of MRSA and the presence of multidrug-resistant MRSA in dairy farms for the first time in north-western Ethiopia and the potential role of animal-to-human transmission in the spread of MRSA.
Data Sharing Statement
The datasets generated for this study are available upon request of the corresponding author.
Ethical Approval and Informed Consent
Informed consent was obtained from the owner or farm workers during enrollment in the study. The study was ethically approved by the Ethical Review Committee of the College of Agriculture and Natural Resources, Debre Markos University approved the study.
Acknowledgments
The authors would like to thank all farm owners and the Debre Markos town livestock resource development office.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors received no specific funding for this work.
Disclosure
The authors declare no conflicts of interest concerning this work.
References
1. Yehia HM, Al-Masoud AH, Alarjani KM, Alamri MS. Prevalence of methicillin-resistant (mecA gene) and heat-resistant Staphylococcus aureus strains in pasteurized camel milk. J Dairy Sci. 2020;103:5947–5963. doi:10.3168/jds.2019-17631
2. Locatelli C, Cremonesi P, Caprioli A, et al. Occurrence of methicillin-resistant Staphylococcus aureus in dairy cattle herds, related swine farms, and humans in contact with herds. J Dairy Sci. 2017;100(1):608–619. doi:10.3168/jds.2016-11797
3. Fisher EA, Paterson GK. Prevalence and characterisation of methicillin-resistant staphylococci from bovine bulk tank milk in England and Wales. J Glob Antimicrob Resist. 2020;22:139–144. doi:10.1016/j.jgar.2020.01.013
4. Nyokabi S, Luning PA, de Boer IJM, et al. Milk quality and hygiene: knowledge, attitudes and practices of smallholder dairy farmers in central Kenya. Food Control. 2021;130:1–10. doi:10.1016/j.foodcont.2021.108303
5. Kivaria FM, Noordhuizen JPTM, Kapaga AM. Evaluation of the hygienic quality and associated public health hazards of raw milk marketed by smallholder dairy producers in the Dar es Salaam region, Tanzania. Trop Anim Health Prod. 2006;38(3):185–194. doi:10.1007/s11250-006-4339-y
6. Knight-Jones TJD, Hang’ombe MB, Songe MM, et al. Microbial contamination and hygiene of fresh cow’s milk produced by smallholders in Western Zambia. Int J Environ Res Public Health. 2016;13(7):737. doi:10.3390/ijerph13070737
7. Haag AF, Fitzgerald JR, Penadés JR. Staphylococcus aureus in animals. Microbiol Spectr. 2019;7(3):7. doi:10.1128/microbiolspec.gpp3-0060-2019
8. Poutrel B, Vialard J, Groud K, et al. Sources of contamination of bovine milk and raw milk cheese by Staphylococcus aureus using variable number of tandem repeat analysis. J Vet Sci Technol. 2015;06:10–4172. doi:10.4172/2157-7579.1000246
9. Li T, Lu H, Wang X, et al. Molecular characteristics of Staphylococcus aureus causing bovine mastitis between 2014 and 2015. Front Cell Infect Microbiol. 2017;7. doi:10.3389/fcimb.2017.00127
10. Abebe R, Hatiya H, Abera M, et al. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12(1):1–11. doi:10.1186/s12917-016-0905-3
11. Kümmel J, Stessl B, Gonano M, et al. Staphylococcus aureus entrance into the dairy chain: tracking S. aureus from dairy cow to cheese. Front Microbiol. 2016;7:1–11. doi:10.3389/fmicb.2016.01603
12. Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and Staphylococcal food-borne disease: an ongoing challenge in public health. Biomed Res Int. 2014;2014:1–9. doi:10.1155/2014/827965
13. Le HHT, Dalsgaard A, Andersen PS, et al. Large-scale Staphylococcus aureus foodborne disease poisoning outbreak among primary school children. Microbiol Res. 2021;12(1):43–52. doi:10.3390/MICROBIOLRES12010005
14. Mutua F, Masanja H, Chacha J, et al. (2021) A rapid review of foodborne disease hazards in East Africa. ILRI Discussion Paper.
15. Gomes F, Henriques M. Control of bovine mastitis: old and recent therapeutic approaches. Curr Microbiol. 2016;72(4):377–382. doi:10.1007/s00284-015-0958-8
16. Sharma C, Rokana N, Chandra M, et al. Antimicrobial resistance: its surveillance, impact, and alternative management strategies in dairy animals. Front Vet Sci. 2018;4:1–27. doi:10.3389/fvets.2017.00237
17. Dhungel S, Rijal KR, Yadav B, et al. Methicillin-Resistant Staphylococcus aureus (MRSA): prevalence, antimicrobial susceptibility pattern, and detection of mec a gene among cardiac patients from a tertiary care heart center in Kathmandu, Nepal. Infect Dis. 2021;14:1–10. doi:10.1177/11786337211037355
18. Fuda C, Suvorov M, Vakulenko SB, Mobashery S. The basis for resistance to β-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J Biol Chem. 2004;279(39):40802–40806. doi:10.1074/jbc.M403589200
19. Chen C, Wu F. Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) colonisation and infection among livestock workers and veterinarians: a systematic review and meta-analysis. Occup Environ Med. 2021;78(7):465–471. doi:10.1136/oemed-2020-106418
20. Larsen J, Stegger M, Andersen PS, et al. Evidence for human adaptation and foodborne transmission of livestock-associated methicillin-resistant Staphylococcus aureus: table 1. Clinl Infect Dis. 2016;63(10):1349–1352. doi:10.1093/cid/ciw532
21. Fetsch A, Etter D, Johler S. Livestock-associated methicillin-resistant Staphylococcus aureus—current situation and impact from a one health perspective. Curr Clin Microbiol Rep. 2021;8(3):103–113. doi:10.1007/s40588-021-00170-y
22. Silva V, Monteiro A, Pereira JE, et al. MRSA in humans, pets and livestock in Portugal: where we came from and where we are going. Pathogens. 2022;11(10):1110. doi:10.3390/pathogens11101110
23. Graveland H, Duim B, van Duijkeren E, et al. Livestock-associated methicillin-resistant Staphylococcus aureus in animals and humans. Int J Med Microbiol. 2011;301(8):630–634. doi:10.1016/j.ijmm.2011.09.004
24. Anjum MF, Marco-Jimenez F, Duncan D, et al. Livestock-associated methicillin-resistant Staphylococcus aureus from animals and animal products in the UK. Front Microbiol. 2019;10:1–7. doi:10.3389/fmicb.2019.02136
25. Wendlandt S, Schwarz S, Silley P. Methicillin-resistant Staphylococcus aureus: a food-borne pathogen? Annu Rev Food Sci Technol. 2013;4(1):117–139. doi:10.1146/annurev-food-030212-182653
26. Cuny C, Wieler LH, Witte W. Livestock-associated MRSA: the impact on humans. Antibiotics. 2015;4(4):521–543. doi:10.3390/antibiotics4040521
27. Anand KB, Agrawal P, Kumar S, Kapila K. Comparison of cefoxitin disc diffusion test, oxacillin screen agar, and PCR for mecA gene for detection of MRSA. Indian J Med Microbiol. 2009;27(1):27–29. doi:10.1016/S0255-0857(21)01748-5
28. R S, P V, D’Souza AO, Vinod R. Comparison of phenotypic and genotypic characterization methods for the detection of methicillin-resistant Staphylococcus aureus. Cureus. 2022;14:1–5. doi:10.7759/cureus.23396
29. Gebremeskel FT, Alemayehu T, Ali MM. Methicillin-resistant Staphylococcus aureus antibiotic susceptibility profile and associated factors among hospitalized patients at Hawassa University Comprehensive Specialized Hospital, Ethiopia. IJID Regions. 2022;3:129–134. doi:10.1016/j.ijregi.2022.03.015
30. Tefera S, Awoke T, Mekonnen D. Methicillin and vancomycin-resistant staphylococcus aureus and associated factors from surgical ward inpatients at Debre Markos referral hospital, northwest Ethiopia. Infect Drug Resist. 2021;14:3053–3062. doi:10.2147/IDR.S324042
31. Girmay W, Gugsa G, Taddele H, et al. Isolation and Identification of Methicillin-Resistant Staphylococcus aureus (MRSA) from Milk in Shire Dairy Farms, Tigray, Ethiopia. Vet Med Int. 2020;2020(1):1–7. doi:10.1155/2020/8833973
32. Mekuriya E, Manilal A, Aklilu A, et al. Methicillin-resistant Staphylococcus aureus colonization among medicine and health science students, Arba Minch University, Ethiopia. Sci Rep. 2022;12(1):1–11. doi:10.1038/s41598-022-14212-y
33. Tibebu L, Belete Y, Tigabu E, Tsegaye W. Prevalence of Staphylococcus aureus, methicillin-resistant Staphylococcus aureus and potential risk factors in selected dairy farms at the interface of animal and human in Bishoftu, Ethiopia. Vet Med. 2021;12:241–251. doi:10.2147/vmrr.s331968
34. Desta K, Aklillu E, Gebrehiwot Y, et al. High levels of methicillin-resistant Staphylococcus aureus carriage among healthcare workers at a teaching hospital in Addis Ababa Ethiopia: first evidence using mecA detection. Infect Drug Resist. 2022;15:3135–3147. doi:10.2147/IDR.S360123
35. Asmelash T, Mesfin N, Addisu D, et al. Isolation, identification and drug resistance patterns of methicillin resistant Staphylococcus aureus from mastitic cows milk from selected dairy farms in and around Kombolcha, Ethiopia. J Vet Med Anim Health. 2016;8(1):1–10. doi:10.5897/jvmah2015.0422
36. Tassew A, Aki A, Legesse K. Isolation, identification and antimicrobial resistance profile of Staphylococcus aureus and occurrence of methicillin resistant S. aureus isolated from mastitic lactating cows in and around Assosa Town, Benishangul Gumuz Region, Ethiopia. J Dairy Vet Anim Res. 2017;6:1–7. doi:10.15406/jdvar.2017.06.00180
37. Issa G, Aksu H. Detection of methicillin-resistant Staphylococcus aureus in milk by PCR-based phenotyping and genotyping. Acta Veterinaria Eurasia. 2020;46(3):120–124. doi:10.5152/ACTAVET.2020.20011
38. Gómez P, Lozano C, González-Barrio D, et al. High prevalence of methicillin-resistant Staphylococcus aureus (MRSA) carrying the mecC gene in a semi-extensive red deer (Cervus elaphus hispanicus) farm in Southern Spain. Vet Microbiol. 2015;177(3–4):326–331. doi:10.1016/j.vetmic.2015.03.029
39. CLSI. Performance standards for antimicrobial susceptibility testing, 29th ed. In: CLSI Supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2019.
40. Stegger M, Andersen PS, Kearns A, et al. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin Microbiol Infect. 2012;18(4):395–400. doi:10.1111/j.1469-0691.2011.03715.x
41. Xie Y, He Y, Gehring A, et al. Genotypes and toxin gene profiles of staphylococcus aureus clinical isolates from China. PLoS One. 2011;6(12):1–11. doi:10.1371/journal.pone.0028276
42. Hart CA, Kariuki S. Antimicrobial resistance in developing countries. BMJ. 1998;317(7159):647–650. doi:10.1136/bmj.317.7159.647
43. Eshetie S, Tarekegn F, Moges F, et al. Methicillin resistant Staphylococcus aureus in Ethiopia: a meta-analysis. BMC Infect Dis. 2016;16(1):1–9. doi:10.1186/s12879-016-2014-0
44. Papadopoulos P, Papadopoulos T, Angelidis AS, et al. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol. 2018;69:43–50. doi:10.1016/j.fm.2017.07.016
45. Carfora V, Giacinti G, Sagrafoli D, et al. Methicillin-resistant and methicillin-susceptible Staphylococcus aureus in dairy sheep and in-contact humans: an intra-farm study. J Dairy Sci. 2016;99(6):4251–4258. doi:10.3168/jds.2016-10912
46. Haran KP, Godden SM, Boxrud D, et al. Prevalence and characterization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J Clin Microbiol. 2012;50(3):688–695. doi:10.1128/JCM.05214-11
47. Giacinti G, Carfora V, Caprioli A, et al. Prevalence and characterization of methicillin-resistant Staphylococcus aureus carrying mecA or mecC and methicillin-susceptible Staphylococcus aureus in dairy sheep farms in central Italy. J Dairy Sci. 2017;100(10):7857–7863. doi:10.3168/jds.2017-12940
48. Schmidt T, Kock MM, Ehlers MM. Diversity and antimicrobial susceptibility profiling of staphylococci isolated from bovine mastitis cases and close human contacts. J Dairy Sci. 2015;98(9):6256–6269. doi:10.3168/jds.2015-9715
49. Mekuria A, Asrat D, Woldeamanuel Y, Tefera G. Identification and antimicrobial susceptibility of Staphylococcus aureus isolated from milk samples of dairy cows and nasal swabs of farm workers in selected dairy farms around Addis Ababa, Ethiopia. Afr J Microbiol Res. 2013;7:3501–3510. doi:10.5897/AJMR12.2060
50. Kalayu AA, Woldetsadik DA, Woldeamanuel Y, et al. Burden and antimicrobial resistance of S. aureus in dairy farms in Mekelle, Northern Ethiopia. BMC Vet Res. 2020;16(1):1–8. doi:10.1186/s12917-020-2235-8
51. Kreausukon K, Fetsch A, Kraushaar B, et al. Prevalence, antimicrobial resistance, and molecular characterization of methicillin-resistant Staphylococcus aureus from bulk tank milk of dairy herds. J Dairy Sci. 2012;95(8):4382–4388. doi:10.3168/jds.2011-5198
52. Rafif Khairullah A, Rehman S, Agus Sudjarwo S, et al. Detection of mecA gene and methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and risk factors from farms in Probolinggo, Indonesia. F1000Res. 2022;11:722. doi:10.12688/f1000research.122225.1
53. Saikia L, Nath R, Choudhury B, Sarkar M. Prevalence and antimicrobial susceptibility pattern of methicillin-resistant Staphylococcus aureus in Assam. Indian J Crit Care Med. 2009;13:156–158. doi:10.4103/0972-5229.58542
54. Blanco AR, Sudano Roccaro A, Spoto CG, Papa V. Susceptibility of methicillin-resistant staphylococci clinical isolates to netilmicin and other antibiotics commonly used in ophthalmic therapy. Curr Eye Res. 2013;38(8):811–816. doi:10.3109/02713683.2013.780624
55. Aetrugh S, Aboshkiwa M, Husien W, et al. Antimicrobial resistance profile and molecular characterization of methicillin-resistant staphylococcus isolates in Tripoli Central Hospital, Libya. Libyan Int Med Univ J. 2017;02(01):74–83. doi:10.21502/limuj.010.02.2017
56. Talukder M, Islam M, Ievy S, et al. Detection of multidrug resistant salmonella spp. From healthy and diseased broilers having potential public health significance. J Adv Biotechnol Exp Ther. 2021;4(2):248–255. doi:10.5455/JABET.2021.D125
57. Sommer MOA, Dantas G. Antibiotics and the resistant microbiome. Curr Opin Microbiol. 2011;14(5):556–563. doi:10.1016/j.mib.2011.07.005
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
