Back to Journals » Journal of Blood Medicine » Volume 14
Patient Blood Management and Its Role in Supporting Blood Supply
Authors Gammon RR , Dubey R, Gupta GK, Hinrichsen C, Jindal A , Lamba DS , Mangwana S, Radhakrishnan Nair A, Nalezinski S, Bocquet C
Received 2 July 2023
Accepted for publication 6 November 2023
Published 30 November 2023 Volume 2023:14 Pages 595—611
DOI https://doi.org/10.2147/JBM.S387322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Richard R Gammon,1,* Rounak Dubey,2,* Gaurav K Gupta,3,* Colleen Hinrichsen,4,* Aikaj Jindal,5,* Divjot Singh Lamba,6,* Sadhana Mangwana,7,* Amita Radhakrishnan Nair,8,* Shaughn Nalezinski,9,* Christopher Bocquet10
1Scientific, Medical and Technical Department, OneBlood, Orlando, FL, USA; 2Department of Transfusion Medicine, All India Institute of Medical Sciences, Nagpur, India; 3Department of Pathology and Laboratory Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA; 4Department of Transfusion Medicine, Princeton Medical Center at Penn Medicine, Plainsboro, NJ, USA; 5Department of Transfusion Medicine, Mohandas Oswal Hospital, Ludhiana, India; 6Department of Transfusion Medicine, Post Graduate Institute of Medical Education and Research, Chandigarh, India; 7Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, New Delhi, India; 8Department of Transfusion Medicine Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvanathapuram, Kerala, India; 9Department of Laboratory Medicine - Transfusion Services, Concord Hospital, Concord, NH, USA; 10Association for the Advancement of Blood and Biotherapies, Bethesda, MD, USA
*These authors contributed equally to this work
Correspondence: Richard R Gammon, OneBlood, 8669 Commodity Circle, Orlando, FL, 32819, USA, Tel +1407 947 7963, Fax +1 407 264 8265, Email [email protected]
Abstract: Blood donors and voluntary blood donations are essential for ensuring the blood supply that can be maintained by good patient blood management (PBM) practices. This review article explores the role of blood donation in PBM and highlights the importance of donor screening and selection processes in different regions worldwide. The donor health questionnaires and the focused physical examination guidelines have changed in the last decade to increase donor and recipient safety. This article also discusses the status of transfusion practices, including the challenges of ensuring a safe blood supply. Significant among these are the effects of the COVID-19 pandemic on the blood supply chain and the impact of an aging donor population, especially. Promoting autologous donations and other blood conservation strategies are suggested to mitigate these issues. The role of replacement donors and the upper age limit for voluntary blood donation may be decided based on the demography and donor pool. The involvement of C-suite executives is also critical in implementing and running a successful PBM program. The review highlights how these different aspects of blood donation are integral to a successful PBM program and the safety of patients who receive blood transfusions.
Keywords: donors, blood centers, transfusion service
Introduction
Ensuring that the blood supply is adequate to support the healthcare system and ensure blood is available to meet patient needs is essential.1 Ten years of declining blood use and changes in healthcare delivery place the current system’s sustainability at risk.2 Short-lived blood component shortages are common and managed with short-term solutions.3 A long-term shortage requires additional palliation strategies.4 Today, the blood donor population is aging (Table 1). Data from the US National Blood Collection and Utilization Survey between 2017 and 2019 showed a decline in all donor age groups with the exception of those 65 and older.5 New ways to engage younger generations of blood donors must be found.1
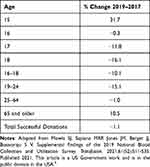 |
Table 1 Percent Change in Donors by Age in the US Between 2017 and 2019 |
PBM has grown and has become the accepted standard of care for patients.6 This evidence is supported by the rapid growth of publications since 2006.6 The global definition of PBM is as follows:
Patient blood management is a patient-centered, systematic, evidence-based approach to improve patient outcomes by managing and preserving a patient’s blood while promoting patient safety and empowerment.6
PBM involves vein-to-vein safety.
The purpose of this manuscript is three-fold. First, it will review the donation of blood process both in the United States (US) and globally. Second, it will discuss PBM’s importance for patient safety and ensuring the judicious use of blood. Finally, the manuscript shows how having an adequate supply of blood donors and blood donations is essential for ensuring good PBM practice for those patients, even with all of the alternative therapies available, who continue to require blood transfusions to support their care.7
Materials and Methods
This review article is part of a thematic series that explores the importance of blood donation and the role of PBM in supporting the blood supply. A panel of experts in PBM and transfusion medicine were invited to contribute to this article. The initial online meeting occurred in October 2022, when the article’s outline was finalized. Each contributing author was assigned specific sections to write based on their respective areas of interest and expertise. The compiled manuscript underwent thorough revisions, edits, and approval by all authors before its final submission.
Selection of Allogeneic Blood Donors
Registration of Blood Donors
Blood donor registration is collecting information from prospective volunteer blood donors. The essential information required for donor registration typically includes personal details, including the donor’s name, gender, date of birth, address, and telephone number. In the blood donor registration process, donors are assigned a unique donor identification number, receive educational material on blood donation, and complete a donor health questionnaire to determine eligibility. Donor health questionnaires are required to be completed for each donation. Donor registration creates a database of potential donors, whom the donor center can contact to replenish the blood supply and determine if a specific blood product is needed for a particular patient.
Educational Materials and Donor Acknowledgement and Consent
The donor selection process begins with public outreach and donor education. This will increase the likelihood of acceptance.8 Donors should be allowed to self-defer after being informed about the health issues and high-risk behaviors that would prevent blood donation.8 Strategies to promote voluntary blood donation, including donor education and information, must be practiced to create a secure and long-lasting voluntary donor base.9
Pre-donation information should be presented at the donation session in an easy-to-understand manner and in the appropriate languages8 Donors should be educated regarding the donor selection criteria, blood donation process, and screening tests performed. This lets the donors know the importance of disclosing any medical illness or relevant transfusion-transmitted infectious (RTTI)-associated risks and the unsuitability to donate blood and make a choice to self-defer themselves. Pre-donation information should include knowledge about blood and its components, the importance of blood donation, and the consequences of leading a healthy lifestyle.8 It should inform the donor regarding the pre-donation questionnaire, medical history, risk assessment, venipuncture, blood collection, post-donation care, and screening tests done. It should provide the donor with a rationale for the donor questionnaire and pre-donation health evaluation. It should inform the donor about the option to withdraw or self-defer at any moment during or after the donation procedure and to do so without being unduly embarrassed or questioned.8 The donor should be informed about routes of transmission, natural history, and ways to prevent RTTIs such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), as well as the window period of infection and alternate testing locations for them to know their infection status.8 Donors must be informed that when RTTI test results are abnormal, there are implications for donors and the blood they donate. There is also a mechanism for notifying donors of abnormal test results, providing post-donation counseling, assuring confidentiality, and, if necessary, referring donors for additional testing, and medical treatment.8
Donors should be educated regarding the possibility of adverse donor reactions as well. Evaluating the capacity to comprehend blood donation and informed consent is essential.8 Donors will be comforted that their welfare is vital to the blood center. They may be motivated to become repeat donors if they understand that providing accurate and complete information about their health is in their best interests. Verifying that the donor understands the questionnaire presented and offers precise information is crucial. This is especially important if the donor is a first-time donor. Responding to the donor’s inquiries and offering assurance if necessary is essential. A prospective donor must voluntarily agree to the following: blood donation, testing for RTTI; transfusion of donated blood to patients; and, if necessary, using the blood for additional tests, quality assurance, or research.8 To obtain informed consent from the donor, they should be informed about the tests (RTTI and others) conducted on the blood samples given, the justifications for these tests, and assured that all personal data, including test results, are treated confidentially. The process includes the donor’s signature and providing informed consent. It indicates agreement to permitting post-donation notification and counseling should a positive RTTI marker or any other abnormality be found and that the donor is aware of the risks of RTTI transmission through donated blood.8 In countries where donors may face legal consequences for delivering false information, they must comprehend the questionnaire and its implications. In countries where those under the age of majority may donate, a parent or guardian’s written consent must be sought per domestic laws.8
Health History Assessment- AABB DHQ
A blood center’s role is both donor and recipient safety.8 A blood donor should be in good health. The WHO defined “Health” as “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity”.10 Good health can be determined using specific associated parameters ascertained from a brief medical history, observation, and simple tests.8 The donor assessment aims to determine suitability so that the donation is safe and the products will not harm the recipients. This provides an opportunity to assess the donor’s fitness.8
The assessment of donor suitability and deferral are excluding donations from those at risk of RTTI, especially those that may have occurred recently and may not be detected by routine screening tests or for which no practical blood screening tests are available.11
The WHO has given recommendations for donor assessment and acceptance and deferral of donors based on selection criteria.8 These include lower and upper age limits, donor appearance and inspection, minor illnesses, weight, gender for pregnancy, lactation, breastfeeding, decreasing transfusion-associated acute lung injury (TRALI) risk, vital signs, donor iron status to be determined by hemoglobin (Hb) screening, frequency of donation and iron supplementation, occupation and leisure activities and special considerations for apheresis donations.8
The Association for the Advancement of Blood and Biotherapies (AABB) published an Individual Risk Assessment (IRA) Donor History Questionnaire Example Model in 2022 using Canada’s eligibility criteria to help the Food and Drug Administration (FDA) and the transfusion community visualize the implementation this system. The FDA approved an updated questionnaire including IRA in June 2023.12,13 The US FDA issued a final guidance document, “Recommendations for Evaluating Donor Eligibility Using Individual Risk-Based Questions to Reduce the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Product”, in May 2023 in which FDA’s recommendations for donor deferral and re-entry are summarized.14
Blood donation is one of the most highly controlled practices globally. Safeguarding the safety and efficacy of blood components is critical, as blood is an irreplaceable part of modern clinical therapies.11 The World Health Organization (WHO) and AABB have defined donor health questionnaires. Norway changed its guidelines and procedures in 2019 to include new donors at the Oslo Blood Center. With this change, only anaphylactic reactions provide a reason for deferral, while people with other allergic symptoms can donate. Similarly, those with β -blockers and antihypertensive medications could not donate, while those taking other antihypertensive medications in the revised guidelines remain eligible.10 The upper age limit has been removed, allowing some healthy elderly individuals to continue to donate. The national guidelines of Norway were revised to defer persons taking permanently or who had been taking injectable drugs. Cannabis use and oral/nasal intake more than one year prior are currently acceptable.10 Another welcome change is the shorter deferral of 4 months rather than 12 months of people traveling to malaria-endemic countries and donors from South/Latin America.
In Korea, the Blood Management Act was enacted in 1970, and the national blood service was consigned to the Korean Red Cross (KRC) in 1981.15 In 1998, a 100% voluntary blood donor system was achieved.15 Donations can be accessed anywhere in the country via the Electronic Health History Assessment System on the KRC website (https://bloodinfo.net).15 Potential donors, who were deferred in the past, are added to this system and deemed ineligible nationally.16
Uganda is an East African low-income country (LIC), with nearly 20% of its population below the poverty line.17 Although it has a national blood transfusion service, it needs more blood available for patients. In a study by Checkley et al on “Assessment of Blood Donation and Transfusion”, the authors found that > 50% of participants were unaware of the minimum age and weight requirements for blood donation and the greatest blood collection volume.17 There was an incorrect knowledge of eligibility criteria regarding pregnancy, menstruation, breastfeeding women, and diabetics. Education about donation through informational messaging may, it is hoped, will increase the safe blood supply.17
In India, blood transfusion services are governed under the Drugs and Cosmetics Rules, initiated in 1945 and amended periodically. In 2011, the upper age limit was increased from 60 years to 65 years. Through another amendment in the act in 2020, donor selection criteria were revised in which after whole blood donation, a plateletpheresis donor shall not be accepted before 28 days.18
Donor Qualification by Focused Physical Examination
The general health of a donor is evaluated before accepting an individual for donation, and this process is commonly referred to as a focused or mini-physical examination. The broad criteria for donor qualification include vital parameters like temperature, pulse, blood pressure, and Hb/hematocrit (Hct) levels.19 These criteria are essential to ensure the quality of the blood components and also the donor’s safety.19 There are additional criteria for the apheresis donors, which must meet the regulatory requirements. The AABB Standards for Blood Banks and Transfusion Services (BB/TS) and WHO guidelines for donor examination are two of the most widely followed documents for these criteria.8,19 These guidelines define the specific physical requirements and the level of medical supervision required for the assessment. For example, the WHO guidelines state that first-time donors above 60 years of age and repeat donors above 65 years may be accepted if found fit on examination.8 The AABB BB/TS also addresses these issues similarly; however, it does not require additional evaluation for older donors. The commonly used vital parameters and their acceptance criteria across different geographical locations are discussed here.20
Pulse
The criteria for measuring pulse before blood donation varies significantly from one country to another. As per the AABB BB/TS and the US FDA regulations, the donor’s pulse should be regular and, in the range, of 50 −100 beats per minute.19 Suppose the pulse is irregular or outside these values. In that case, the designated physician must determine if the donor may be accepted after validating that donating would not adversely affect the donor’s health. The WHO guidelines have mentioned that a regular pulse in the range of 60–100 beats per minute indicates good health but suggested assessing its role in blood donation.9 The National Health Service (NHS, United Kingdom) blood program states that checking the pulse of the blood donor is a good practice. Still, it should not be considered an absolute criterion for donor qualification.
Blood Pressure
The AABB BB/TS and the US FDA regulations (21 Code of Federal Regulations (CFR) 630.10(f)(2)) state that the systolic blood pressure (BP) should be in the range of 90 −180 mm of mercury (mm Hg) and diastolic in 50–100 mm Hg.10 A donor with BP outside these values may be accepted after evaluation by the responsible physician along similar lines for a pulse, as stated earlier. Studies done on this topic, including a systematic literature review, could not establish any association between raised systolic blood pressure and adverse donor reactions.20,21 Given these experiences, some blood centers, no longer defer blood donors for BP (either high or low).11
Weight
The weight norms for that population should be considered for establishing the lower weight limit for donors. In the US, 50 kg (110 lbs.) has been set as the lower limit for collecting 500 mL of whole blood. In most other countries, the donor must weigh at least 45 kg to donate 350 mL of blood and at least 50 kg to donate 450 mL of blood. For double red blood cell (RBC) apheresis donations, the donor’s weight should be more than 70 kg.8
Local Examination and General Appearance
The focused examination also includes an inspection of the antecubital area of the donor for scars or other skin lesions. Common and mild skin disorders are usually not considered a cause for the deferral of the donor. Still, if there is evidence of superimposed bacterial infection, the donor may be deferred. Also, if the lesion interferes with the skin disinfection of the phlebotomy area or there is evidence of intravenous drug use, the donor may be deferred. The general condition of the donor should be observed to rule out malnutrition and the influence of alcohol/intoxication. The donor should not have a fever, breathing difficulties, or persistent cough.
Hemoglobin/Hematocrit Measurement
Hb or Hct measurement before the donation is essential to ensure that blood is not collected from a donor already anemic and that the RBC has an adequate Hb content and Hct to be effective in the recipient. It is also a required parameter for collecting blood using automated platforms. The method for estimating Hb/Hct should be rapid, easy to use, and cause minimum discomfort to the donor. The US FDA has set a lower acceptable limit for Hb at 12.5 g/dL for females and 13.0 g/dL for males.19 Acceptable ranges vary amongst other countries from 12.5 −13.5 g/dL, while some countries have kept a single cut-off level for all donors. Non-invasive methods of Hb measurement have recently been approved in some countries, which do not involve a blood sample but estimate Hb levels through the skin. Hb is measured by following optical changes after a brief blood flow occlusion in a finger (occlusion spectroscopy).
As evident from the above discussion, the selection criteria for voluntary donors have undergone a significant change, especially in the last decade. In many countries, blood collection centers follow the guidelines set by their regional or national regulatory agencies, and these guidelines often change with time as new evidence emerges. These changes aim to maintain the blood supply and are often aligned with demographical factors. For example, relatively less stringent criteria for blood pressure and age will enable more donors in regions with an aging population to donate blood. A more stringent deferral criteria in the past for certain groups, including men who had sex with men (MSM), had limited the available donor pool. The deferral criteria were changed to 3 months from 1 year by the US FDA in 2020 to expand the donor pool. The 2023 policy guidance by the US FDA has advocated an IDA approach along the lines adopted by various countries and a step towards a more inclusive blood supply.22
Blood-Center-Defined Donor Eligibility Criteria
Cancer
With the advent of better diagnostic and therapeutic options available to patients suffering from malignancy, these patients often present as prospective donors with a history of cured or “in-remission” malignancy. The deferral period for such donors depends upon the type of cancer and the treatment history. WHO and the American Red Cross (ARC) permanently defer individuals with current or cured hematological malignancies from donating blood.8,23 These include patients with a history of leukemia or lymphoma, including conditions such as Hodgkin lymphomas, polycythemia rubra vera, essential thrombocythemia, and paroxysmal nocturnal hemoglobinuria.
There is wide variation in accepting recovered patients as blood donors regarding solid tumors. While the ARC takes specific donors who have no cancer for a period, there are certain speculations regarding theoretical risks of transfusion-transmission of tumor cells or oncogenic viruses, which have warranted permanent deferral in some blood transfusion services.24–26
The WHO recommends accepting recovered solid malignant tumors after five years of successful curative treatment and accepting “in-situ” malignant diseases like basal cell carcinoma or cervical carcinoma in situ.8 However, as per Indian Blood Transfusion Services, there is a blanket permanent deferral for any malignant condition.
Bleeding Conditions or Blood Diseases
The WHO permanently defers individuals with coagulation factor deficiencies (inherited or acquired).8 The Indian Blood Transfusion Services permanently defer all with a history of bleeding disorders and unexplained bleeding tendencies. Hemoglobinopathies such as thalassemia major and sickle cell disease are permanently deferred, while the thalassemia trait can be accepted if the donor meets the minimum Hb threshold for blood donation.8,26 Those with sickle cell trait are allowed for whole blood donation by WHO; however, with certain limitations on its use, including to neonates and recipients with sickle cell disease. Red cell enzyme deficiencies with a known history of hemolysis, including the most commonly seen glucose-6–phosphate dehydrogenase (G6PD) deficiency, are permanently deferred.
Heart and Lung Conditions
According to the WHO, cardiovascular disease requires written permission from a cardiologist or physician before donating.8 In addition to symptomatic ischemic heart disease, a history of myocardial infarction, symptomatic peripheral vascular disease, and rheumatic fever with evidence of chronic heart disease, several cardiac conditions can lead to permanent blood donation deferral. When assessing the eligibility of individuals with heart disease, consideration should be given to how the disease impacts their ability to tolerate hemodynamic changes caused by blood donation.8
The WHO permanently defers donations from donors with severe obstructive airway disease, including those on long-term oral steroid therapy, chronic or recurrent respiratory infections, and breathlessness at rest or with minimal exertion.8 Asthmatic patients who are asymptomatic and receiving a nonsteroidal and inhaled steroid maintenance dose may be eligible for blood donation. However, there are differences regarding deferral for asthma patients taking oral or injectable steroids. WHO provides deferral for 14 days after recovery or the last dose; the ARC does not mention deferral for such cases.8 The Indian Blood Transfusion Service permanently excludes such patients from blood donation.
Tuberculosis patients are accepted two years after confirmation of cure, while the contacts of tuberculosis individuals are deferred until they are confirmed clear of infection. When assessing those with respiratory disease, the health status of the donor and the risk of transfusion-transmission to the recipient must be considered.8
Medications
Usually, most over-the-counter drugs are acceptable for blood donation. For aspirin, there is a deferral period ranging from 72 hours to 5 days (if the blood is used for making platelets). Certain teratogenic medications such as etretinate, acitretin, isotretinoin, and fetotoxic drugs that include dutasteride and finasteride warrant deferral for a specific period (Table 2). It should be noted that not all medication deferrals are evidence-based, and some are based upon the precautionary principle.
 |
Table 2 A Selection of Medications and Deferral Periods for Blood Donors |
Recipient-Specific Designated or Directed Blood Donation
Exceptional Medical Need
Sometimes a recipient may benefit from a specific donor.11 The recipient may have multiple antibodies or antibodies to high-prevalence antigens.11 Multiple short-interval donations by a designated individual with a medical need require that the blood collection facility have a policy that requires a request from the patient’s doctor and authorization by the blood center’s medical director.11 The donor must meet all other allogeneic donor requirements with the expectation of frequency. If an urgent need arises, blood can be released before RTTIs results are complete but must be labeled and managed per the CFR.11,28
Directed Blood Donations
Patients can request the blood center collect directed donors, usually family members and friends. While directed donations have declined in recent years, this practice remains and is often driven by an inaccurate perception of the risk of RTTIs.29 There is no evidence of improved safety. These donors may feel coerced to donate, which could reduce blood safety. The confidentiality of positive test results may be challenging.11
Directed donors must meet all criteria as allogeneic donors, and their blood can be transfused to others if not used by the intended recipient.19 The blood center should convey its directed donation procedures so expectations of unit availability are clear to the hospital, ordering physician, and patient. This should include the mandated interval between collection and availability to the patient. The hospital should have a defined policy for the release of directed donations to other patients should the unit not be used.11
Autologous Blood Donations
In the 1980s, when the awareness that allogenic transfusions are associated with several adverse effects, such as the transmission of RTTIs and hemolytic transfusion reactions, autologous blood transfusions (ABT) gained popularity since it was safer and offered the best compatibility for the patient.30
Pre-procedure autologous blood donation, perioperative hemodilution, perioperative salvaging of blood, and recombinant erythropoiesis-stimulating agents (ESA) therapy and combinations of these approaches come under the purview of autologous donations.31 ABT is not a recent concept; blood shed during surgery was reinfused as early as 1818, and preoperative donation of autologous blood was promoted since the beginning of blood banking in the 1930s.32
Less stringent criteria are used for autologous donation selection compared to allogeneic donation. The AABB BB/TS mandates no age or weight restrictions, and the minimum Hb level for the donor-patient should be 11.0 g/dL and hematocrit 33%.19 Donations may be made more than once per week.11,19
Autologous blood, however, is not necessarily 100% safe. Transfusion errors, transfusion reactions, adverse reactions during blood donation, bacterial contamination during processing and storage, and incompatible transfusions due to clerical errors continue to occur.33 In a hospital-based study, autologous blood units were implicated in 2.1% of all adverse transfusion reactions.34 Furthermore, autologous transfusions are logistical and resource-intensive.
A successful ABT requires an organized commitment between the surgical and blood center teams to maximize the donated blood’s utilization. Blood loss must be anticipated individually to determine the number of blood units to be collected, and the surgery schedule must be decided early enough to avoid the outdating of collected units.35 The cost per unit of autologous blood tends to be significantly higher than that for allogeneic blood units.36 Data reveals that patients who had autologous blood deposited tend to be transfused at higher hematocrits than patients receiving allogeneic transfusions.37 More than half of all units collected are discarded.
Strategies for making autologous transfusions cost-effective have been proposed, which include developing a “maximum surgical blood order schedule” or MSBOS for autologous blood, similar to allogenic units, not screening autologous blood units for RTTIs, using whole blood instead of processing into components, and requiring patients to pay for the additional costs of collecting and storage.38,39 However, the cost-benefit of these actions still needs to be investigated and proven.
Perioperative hemodilution, which includes acute normovolemic hemodilution, is a type of autologous donation where the autologous blood unit is collected either just before or soon after induction of anesthesia, and intravenous crystalloid or colloid solution is used to sustain the blood volume. Since perioperative hemodilution does not involve processing, it may be more cost-effective and feasible as one constituent of a multifaceted approach to bringing down blood use.40
In a survey conducted at the Cleveland Clinic among patients undergoing ABT, only 20% cited fear of infection, and 68% had opted for an autologous donation because their physician had recommended it.41 Even though an autologous donation is recognized as a part of PBM, perioperative strategies to minimize blood loss, based on two Cochrane reviews of good quality, it is not recommended for routine use because the risk of receiving RBC transfusion (allogenic and autologous) increases due to lowered Hb levels.42,43 Autologous blood donation is recommended only in certain situations, such as a patient with a rare blood group when transfusion requirements cannot be met with allogeneic blood.
Significant developments in window period reduction, enhanced sensitivity of the screening assays, and pathogen reduction technology (PRT) have improved blood safety considerably. This has led to a decline in ABT. In many parts of the world, particularly in high-income countries, autologous donations have all but disappeared. Autologous donation can result in potential harm to the patient, who can develop iron deficiency with or without anemia).
Replacement Donors
In high-income countries (HIC) with well-established blood transfusion services, the demand for blood and blood products is met entirely from blood donated voluntarily, while in low and middle-income countries (LMIC), blood shortages are common and chronic.44 Families and friends often provide blood for patients requiring transfusion or paid donation supplements inventory in countries with low rates of voluntary blood donation.45
A replacement blood donation occurs mainly when the hospital staff asks the patient’s relatives to provide a specified number of blood donors for every patient who requires a transfusion or undergoes surgery.46 Patients may also prefer blood from family members or friends rather than “strangers” because they supposedly “know” their family members or friends (FRD).46 Their keenness to donate blood either to save the life of a loved one or their fear of distressing or displeasing family may cause them to withhold information about their health status or lifestyle behavior.46 Studies have also shown higher rates of discard in the blood units from replacement donors due to the presence of RTTI markers.47–50 Another impact of asking for blood on a replacement basis is on its subsequent use. Physicians are pressured by the patient and their family members for irrational transfusions, regardless of patient need and risks associated with transfusion. This widens the gap between supply and demand and deprives blood and blood products for patients who require it. The issue of blood safety was addressed at the first World Health Assembly resolution (WHA28.72) a half-century prior.51
The WHO Global Database on Blood Safety has identified several challenges that need to be addressed to promote voluntary blood donation,45 including commitment and support of the government towards infrastructure, human and financial resources, acknowledgment of transfusion as an essential part of the health care, development, and enactment of a national blood policy and a plan with the distribution of supplies are all crucial to achieving and sustaining a 100% voluntary blood donation program.44 Public trust in the blood center’s ability to support transfusion needs can be earned only over an extended amount of time. Still, it can be lost very swiftly, adversely affecting the trustworthiness and support of donors, partner organizations, and the public.44 In short, sustaining a 100% voluntary blood donation program is financially and resource-intensive.
Recent studies conducted in four sub-Saharan African countries indicate FRDs and first-time voluntary blood donors (VBD) were epidemiologically undistinguishable regarding the RTTI marker status.52–55 The prevalence of RTTI markers was significantly lower only in VBDs who donated within a 12-month interval.52
The hospital-based FRD system has been in existence for many years. It is providing a maintainable blood inventory, especially in communities and countries without access to a centralized, voluntary donor system.56 Instead of completely neglecting FRDs, the safety of the donated units from FRDs can be enhanced by creating awareness about safe blood and enhanced pre-donation counseling, including the option for self-deferral, as well as adherence to donor selection criteria and employing sensitive screening assays for RTTIs. FRDs can be considered a viable, sustainable, and cost-effective source of blood for transfusion as a bridge until a committed voluntary blood donation program is established.
Aging Populations (US)
The number of transplants performed in Europe and associated countries continues to rise, with 47,468 hematopoietic stem cell transplants in 42,901 patients [19,630 allogeneic (41%) and 27,838 autologous (59%)] reported by 701 centers in 50 countries in 2018.57–59
Blood transfusion is a transfer of blood from donors to recipients. These two groups differ considerably in their demographic structure.58 Donors are an essential “powering” element for blood services.58 The donor population is young and healthy, while most patients requiring blood are of an older demographic.58 These significant age differences lead to demographic trends important for future blood supply.58
Globally, the population is shifting to an older demographic due to increased life expectancy and the recent decrease in birth rates with stabilization at a current low level.60 Adequate blood supply of a population requires the right number of blood donations and the demand for blood transfusions. Because most blood donors are 18 (or 16 years in some countries) to 65 years, and most blood recipients are >65 years, the ratio between these two population groups is relevant for the blood supply and demand relationship.59 This the number of recipients will change substantially in all European and North American countries.
It is evident from the population pyramids of 2023 of various continents (Figure 1) that the demography of North America, Europe, and the United Kingdom (UK) is changing towards an aging population; which means the donor pool is shrinking with an increase in the recipient population while in population pyramid of Africa, the base is broad indicating predominance of the younger, pediatric population which are yet to enter donor pool with lesser of the elderly population. Asia and particularly India has an expansive donor pool with a less elderly recipient population. These different demographics in different continents will affect the blood supply, more in western counties, in the next ten years.
 |
Figure 1 It is evident from population pyramids of 2023 that the demography of Europe is changing towards an aging population, which means the donor pool is shrinking with an increase in the recipient population while in the population pyramid of Africa, the base is broad indicating a predominance of the younger, pediatric population which are yet to enter donor pool with lesser of the elderly population. These different demographics will affect the blood supply in the next ten years. Reprinted from Population Pyramids of the World from 1950 to 2100. Available from: https://www.populationpyramid.net/world/2023/. |
In the last few decades, more studies on PRT have been done, but changing the demography of HIC poses a much higher risk for the safety of blood supply than PRT which is currently available for plasma and platelets in some countries and under investigation for RBCs in others. In 1994, Vamvakas & Taswell forecasted future US blood requirements to increase by 64% from 1989 until 2030, while blood collection would increase by only 12%, suggesting a shortfall of nearly 4 million RBC units by 2030 in US.61
A study by Zou et al for the ARC found that repeat donations by donors over the age of 50 increased from 22.1% to 34.5% from 1996 through 2005, while repeat donations by donors of less than 50 years age group decreased from 59% to 48% during the same period.62 They predicted a severe shortage of blood components unless measures are taken to increase the inventory or reduce the use considerably. The European blood donor population data tends to be sparser, possibly related to underreporting studies or language issues.62
Currie et al predicted a future increase in transfusions for Wales in 2004.63 It would increase over the next quarter century, achieving a 29% increase in demand by 2026 compared with 1999, primarily due to an aging population with higher transfusion rates.63 Requests for RBCs are increasing in Taiwan, and authors anticipated that this would endure due to the aging population.64 In Finland, there is a marked increase in RBC use beginning at around age 50.65 Those patients age 70 and older have an eightfold higher RBC consumption than their counterparts ages 20–40 years.65
With pronounced demographic changes occurring globally and different medical practices between countries, it is essential to monitor the donor and recipient age distributions regularly. Some radical actions such as extending the age limit for donations, reversing certain donor deferrals, the impact of changing demography on blood demand and supply, and improving various PBM strategies to prevent blood shortages or wastage due to overproduction will need to be explored.
Infrastructure Concerns (US)
For a successful blood center, achieving 100% voluntary, non-remunerated blood donors (VNRBD) are considered a landmark for the available resources. However, monetary allocations for rewards and the availability of modern infrastructure play a vital role in achieving the above status. Contrarily, blood donor programs are often perceived to be inexpensive to run, and they receive lower priority in funds allocations in governmental-run blood centers and more so in LMICs. This fund insufficiency affects donor recalls, the operation of mobile donor vehicles, and the maintenance of vehicles. While much work has been done globally on blood availability, blood and transfusion safety, and appropriate use of blood and blood components, each country’s approach to funding the blood supply and provision of transfusion needs to be standardized and is highly variable. Funding varies among countries, with some countries with no specific mechanism and others using cost recovery.
Within blood centers, gaps are well known, more in LMICs, concerning competent trained staff, which gets compounded in LMICs. There is a need for training and education to ensure adequate blood supplies and transfusion safety, especially in LMICs.
Blood should only be collected in settings with an appropriate number of trained staff to perform the procedures safely. Staff should be trained in communication skills for successful donor information, education, and motivation. Notifications and counseling, especially when adverse events occur, require qualified and trained staff with excellent interpersonal talents and the capability to provide support and care. Human resources are needed to strengthen infrastructure, particularly graphic design and marketing experts, to create effective posters and leaflets, organize traditional and social media campaigns, and hold public talks.
RBC serology and molecular testing are highly variable in many parts of the world. In some countries and regions, pre-transfusion anti-human globulin crossmatch is well established, while tile crossmatch is performed in other places. Extended red-cell phenotyping is performed only in some hospital-based transfusion services, more so in sub-Saharan African countries where there is often a lack of reagents to conduct tests or technical skills.66 The situation gets further compounded for multiply-transfused sickle cell patients who have developed multiple alloantibodies. Other infrastructure and resource constraints are faced with procuring sufficient screening for red cell antibodies, RTTIs, hemovigilance, and quality assurance.
Similarly, screening tests for RTTIs with low sensitivity led to the high prevalence of RTTIs. Due to a shortage of money and technical ability, in most hospitals in sub-Saharan Africa, the prevalence of HIV, HBV, HCV, and syphilis in blood donors is 10.9% in Nigeria, 9.4% in Kenya and 6.6% in Ethiopia.66 Only a few countries in Africa are using nucleic acid tests (NAT), namely Ghana, Egypt, Namibia, and South Africa.66
Using technology, the internet, various blood establishment computer systems, or implementing blood center services based on cloud computing increases efficiency and safety. However, it is a significant infrastructure challenge for resource-constrained countries where basic component production and fractionation is a distant dream. In sub-Saharan African countries, some blood centers have no constant electricity supply. Generators can make electricity supply for blood bank refrigerators. Still, the energy required for deep freezers to store plasma and cryoprecipitate often exceeds their capacity, or freezers break down due to frequent electricity failures, power outages, and fluctuating voltage. This leads to the thawing of plasma several times within a few weeks, requiring it to be discarded.67
Implementation science, a term coined by Custer et al in 2018, evaluates the acceptability, affordability, appropriateness, feasibility, and sustainability of interventions to improve the availability of blood and blood components in LMICs.68 Where applicable, every government, federal or state, and private organization needs to allocate adequate budgets to have effective and safe blood donations and transfusions.
Increased Demand
In the US, though around 6.8 million donors attempt to donate, only approximately 10% of that population is eligible.23 Furthermore, global crises, such as the recent SARS-CoV-2 pandemic, have further hindered blood and blood product collection. It was estimated by the ARC that during the height of the pandemic, there was a 62% drop in blood donations, specifically from school drives, which supply most of their blood products.23 The pressure for more blood donations has witnessed a significant upsurge in recent years, influenced by various factors. The expanding population and the increasing number of medical procedures requiring blood transfusions are the primary factors behind this surge. As more individuals undergo surgeries and medical treatments, the demand for blood products intensifies. Natural disasters and emergencies also contribute to the elevated demand, as they often result in substantial loss of life and injuries, necessitating a greater supply of blood. The aging population is another contributing factor to the augmented need for blood donations. As individuals age, they become more prone to developing health conditions that require blood transfusions, such as anemia or cancer. Moreover, advancements in medical technology have introduced novel treatments and procedures that rely on utilizing blood products, further propelling the demand.
New donors are constantly needed to meet the increasing demand for blood donations. Blood centers rely on the generosity of volunteer donors to keep their supplies at a level that can meet the needs of patients. Regular blood donations are essential to ensure that a sufficient supply is available when it is needed. Therefore, individuals must consider donating blood regularly to help others in need and meet the growing demand.
Role of Patient Blood Management
Blood is a common therapeutic intervention during hospitalization. More than 15 million blood components (red blood cells, platelets, and plasma) are transfused annually in the US.5 Both blood product utilization and collection have decreased over the past decade; however, the decline is now appearing to slow down,59 and keeping a steady balance of supply and demand is crucial. Blood product utilization declined primarily due to the advancement of less invasive surgical techniques and revised transfusion guidelines from professional medical societies.
PBM is a comprehensive, evidence-based, multidisciplinary process focused on patients for optimal utilization of blood products and limiting blood transfusion risks. The core principles of PBM are appropriate preoperative anemia management and optimization of RBC mass, limiting blood loss during surgeries, and rational transfusion of blood products.69 PBM was primarily aimed at surgical patients; however, its scope has also gradually evolved for medical conditions. Reduced blood product utilization has been shown in several studies with PBM implementation. A retrospective study of 605,046 patients showed a 41% reduction in transfused blood product units (RBC, fresh frozen plasma, platelets) per admission and improved patient outcomes after initiating a health systemwide PBM program.70 Another study showed a significant reduction in RBC (39%) and platelet (35%) transfusions per admission in patients on intense chemotherapy for acute leukemia or hematopoietic stem cell transplant.71
A comprehensive PBM program not only focuses on optimal blood product utilization but also has a component for reducing blood product wastage. Although a fraction of blood product wastage is inevitable, minimizing avoidable wastage can play a crucial role in blood product supply conservation. With an average rate of 1 to 5% blood product wastage, a few hundred thousand blood products are discarded annually in the US. A multidisciplinary approach, including implementing effective inventory management for tracking and storing blood products and communication with ordering providers, can be used to minimize blood product wastage. Heitmiller et al used an interdisciplinary team and the Lean Six Sigma methodology. They reduced overall RBC wastage by approximately 50% (4.4% to less than 2%).72 A recent study by Levin et al showed that even a low-cost initiative such as physician-to-physician communication could significantly reduce blood product wastage.73 In a dwindling donor supply setting, there is a greater need to promote PBM as a standard of care.
Promote Understanding of the Importance and Culture of Patient Blood Management
According to data from clinical trials and published clinical guidelines, a limited blood transfusion strategy is recommended as the best practice for hospitalized, stabilized patients. A crucial process improvement for lowering variability in transfusion practices and clinical outcomes is creating and implementing PBM programs.
It can be challenging to improve practice, especially when there is little enthusiasm for change. Utilizing practice development teams that have already been established inside the organization and employees who are knowledgeable about the technique should be considered.
It requires collaborative and team effort to practice change and implementation. It may work locally with the help of short-term project teams but needs organizational, structural, and permanent process change to be effective at hospital /institute or multi-hospital levels.
Understanding and utilizing hospitals’ unique settings and cultures can help bring about change.
Especially from within the organization groups, leaders are essential. Executive leadership is crucial to support and promote a space for PBM inside the hospital. The most important resources for getting physicians to pay attention to the research and the need for practice change are reputed clinicians in the institute. It is essential to start thinking about clinical champions in the affected groups as soon as possible. Local doctors must be involved in the co-design process to ensure projects’ relevance to a specific environment and increase their chances of success. Emphasis should also be made to engage non-clinical stakeholders as altered processes may also affect them. Patients are kept at the center of care when changes are made, a widely accepted principle in PBM and all models of care.
Support from C-Suite
Implementing a PBM program at a hospital/transfusion service requires input and support on many levels. The program depends on a multidisciplinary committee of doctors, nurses, laboratory scientists, and management. It is imperative to have executive support for the successful startup of a program. Starting a PBM program involves many changes that clinicians must abide by, as well as startup costs associated with supporting the program (eg, hospital information systems, education, and staff support).74
C-suite support is crucial for the success of PBM programs. The C-suite consists of top-level executives, such as the chief executive officer (CEO), chief operating officer (COO), and chief financial officer (CFO), who have the power to make crucial decisions and allocate resources within an organization. The C-suite can demonstrate its commitment to improving patient care and reducing healthcare costs by supporting PBM programs.
PBM programs aim to optimize the use of blood products to minimize blood transfusions, reduce the risk of transfusion-related adverse events, and improve patient outcomes. C-suite support for these programs can include providing financial resources to implement new protocols and technology and encouraging interdepartmental collaboration and staff education. In addition, C-suite leaders can advocate for the program to stakeholders, including other executives, physicians, and regulatory bodies, to help secure the necessary support and resources.74
Having the support of the C-suite is also crucial for the sustainability and growth of PBM programs. The C-suite can ensure that the program remains a priority for the organization, even as other initiatives are introduced. In addition, by providing ongoing support and resources, the C-suite can help ensure that the program successfully improves patient care and reduces healthcare costs over the long term. McKinney mentions an annual blood acquisition reduction of $1.6 million US dollars from their PBM program and a 10% decrease in cardiac surgery costs per case.75 They also provide annual education for providers, and their electronic ordering system allows for real-time alerts. Depending on the contract and current system set up for the ordering clinician, this can require startup capital. Annual education also needs funds for provider wages as well as educational content. Overall, cost savings from successful programs support the initial startup costs.
The Effects of COVID on the Blood Supply
Due to the impacts of COVID-19 on blood donation efforts, for several months in the first half of 2021, a severe national blood shortage plagued the US during the first half of 2021. On June 17, 2020, a joint statement released by AABB and the blood centers of the US urged hospitals to implement PBM strategies. In addition, the WHO issued a policy brief relating to implementing a PBM program. The three pillars of PBM include detecting and managing anemia and iron deficiency, minimizing blood loss and optimizing coagulation, and leveraging and optimizing the patient-specific physiological tolerance of anemia.76
A systematic review of the literature during March and April 2020 provided recommendations to consider, namely, the impact COVID-19 has on patient need for transfusion, donor and donation factors impacting supply, PBM implementation, and prioritization of patient transfusion needs.77 Lessons learned from the COVID-19 pandemic may assist transfusion facilities in future planning during similar supply shortages. These efforts highlight ways facilities can start or improve upon existing PBM programs.
With the cancellations of elective procedures early on in the COVID-19 pandemic, some facilities saw a concurrent reduction in blood utilization, so blood shortages were often avoided.78 Similarly, in one French institution Delabranche et al79 provided, a year-to-year retrospective analysis study showed that a reduction of patient influx due to the cancelation of elective surgeries despite an 11% reduction in blood donations, led to a manageable supply of blood available for patients, with no needed transfusions being canceled. Finally, for some facilities with pre-existing PBM programs before the pandemic, whether formalized or in-house implementations,80 were less impacted initially by the shortages.
International Society of Blood Transfusion (ISBT) survey researchers conducted a 37-question anonymous survey of transfusion medicine specialists across the US.81 Highlights of this study showed that 98% of respondents experienced a blood shortage, 35.3% of institutions altered their blood inventory number or composition of products as well as blood suppliers, 78% of respondents reported that blood conservation strategies resulted in reduced blood product usage, and 38.1% reported decreased blood product wastage.
The authors of one US proof of concept study concluded that an ultra-restrictive transfusion approach for non-bleeding, non-COVID, or non-cardiac ischemic patients (< 6 g/dL Hb threshold) led to adequate blood inventory management during the pandemic.82
As a result of shortages experienced during COVID-19, some hospitals modified existing massive transfusion protocols (MTPs). For example, one author’s (CH) institution modified quantities of each round while maintaining expected product ratios. Instead of four units of RBCs and plasma sent in each round, two units of each product were sent. After the pandemic, a decision was made to keep the reduced quantities as part of each MTP round, with better inventory management and reduction of wastage as factors to support this change. The National Transfusion Committee in the UK further describes a triage tool for patients requiring massive transfusion to ensure that the most significant number of life years can be saved ethically and equitably.83
PBM also has a role in emergency medicine. Beverina et al, describe how a hospital embraced PBM and broke with the tradition of transfusion therapy for treating severe iron deficiency anemia (IDA). In 2018 the Emergency Department and anemia clinic (AC) adopted a synchronized approach to treating patients with severe IDA with the objective of offering, as swiftly as possible, the most appropriate treatment at the first point of admission and subsequently proceeding with additional therapy and follow-up.84
Conclusion
This manuscript has shown that blood donation and PBM go hand in hand. With the many challenges blood centers face, there must be an adequate number of donors for the blood products needed. At the time of writing of this manuscript, blood can only be obtained from donors and not manufactured. As the population continues to age in certain regions, those who were previously donors may need to leave the pool and potentially become blood product recipients. In an effort to maximize patient safety, however, transfusion medicine practice culture needs to shift towards a PBM approach, with hospitals implementing it as an important tool to minimize the risks of allogeneic blood transfusion and optimize the precious gift of blood donation.85 In summary, a robust blood donor pool and good stewardship of the blood supply through PBM ensure evidence-based transfusions, and product wastage is minimized for a safe and adequate blood supply as we move into the second quarter of the 21 century.
Disclosure
All authors report no conflicts of interest in this work.
References
1. US Department of Health and Human Services Report to Congress on the Adequacy of the National Blood Supply 2020; 2020. Available from: https://www.aabb.org/docs/default-source/default-document-library/positions/hhs-report-to-congress-on-The-adequacy-of-The-national-blood-supply.pdf?sfvrsn=aeea20ff_4.
2. Mulcahy A, Kapinos K, Briscomb B, et al. Toward a Sustainable Blood Supply in the United States: An Analysis of the Current System and Alternatives for the Future. Rand Corporation; 2016.
3. Cohn CS, Pagano MB, Allen ES, et al. How do I manage long‐term blood component shortages in a hospital transfusion service? Transfusion. 2020:
4. McCarthy LJ. How do I manage a blood shortage in a transfusion service? Transfusion. 2007;47(5):760–762. doi:10.1111/j.1537-2995.2007.01187.x
5. Mowla SJ, Sapiano MRP, Jones JM, Berger JJ, Basavaraju SV. Supplemental findings of the 2019 National Blood Collection and Utilization Survey. Transfusion. 2021;61(S2):S11–S35. doi:10.1111/trf.16606
6. Shander A, Hardy JF, Ozawa S, et al. A Global definition of patient blood management. Anesth Analg. 2022;135(3):476–488. doi:10.1213/ANE.0000000000005873
7. Blanton K. What’s in a name: the role and impact of the Transfusion Safety Officer. In:
8. World Health Organization. Blood Donor Selection: Guidelines on Assessing Donor Suitability for Blood Donation. World Health Organization; 2012.
9. World Health Organization. The urgent need to implement patient blood management: policy brief; 2021. Available from: https://apps.who.int/iris/bitstream/handle/10665/346655/9789240035744-eng.pdf.
10. Nissen-Meyer LSH, Seghatchian J. Donor health assessment – when is blood donation safe? Transfus Apher Sci. 2019;58(1):113–116. doi:10.1016/j.transci.2018.12.016
11. Kessler D, Rossmann S. Allogeneic and autologous blood donor selection. In: Cohn C, Delaney M, Johnson S, Katz L, editors. Technical Manual.
12. Association for the Advancement of Blood and Biotherapies. Individual risk assessment donor history questionnaire example model using Canada’s eligibility criteria; 2023. Availabe from: https://www.aabb.org/docs/default-source/default-document-library/resources/individual-risk-assessment-dhq-example-model-using-canadas-eligibility-criteria.pdf.
13. US Food and Drug Administration. Implementation of Acceptable FullLength and Abbreviated Donor History Questionnaires and Accompanying Materials for Use in Screening Donors of Source Plasma; 2023.
14. US Food and Drug Administration. Recommendations for Evaluating Donor Eligibility Using Individual Risk-Based Questions to Reduce the Risk of Human Immunodeficiency Virus Transmission by Blood and Blood Products. US Food and Drug Administration; 2023.
15. Cho JE. National blood management system and the direction of government policy in Korea. Korean J Hematol. 2010;45(2):81. doi:10.5045/kjh.2010.45.2.81
16. Kim HO. Current state of blood management services in Korea. Ann Lab Med. 2022;42(3):306–313. doi:10.3343/alm.2022.42.3.306
17. Checkley L, Motwani G, Wange IC, et al. Assessment of blood donation and transfusion in Eastern Uganda: a Mixed-Methods Study. Ann Glob Health. 2019;85(1). doi:10.5334/aogh.2426
18. Ministry of Health and Family Welfare. Drugs and Cosmetics Act, 1945. Final G.S.R. 166(E) _Amendment in Part X B & Part XII B pertains to Blood centre and Blood components. The Gazette of India: Extraordinary; 1945. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NTc2MQ.
19. Gammon RR, Boyd T, Chanez T. Standards for Blood Banks and Transfusion Services.
20. Roubinian NH, Plimier C, Woo JP, et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood J Am Soci Hematol. 2019;134(13):1003–1013.
21. De Kort W, Mayr W, Jungbauer C, et al. Blood donor selection in European Union directives: room for improvement. Blood Transfus. 2016;14(2):101. doi:10.2450/2015.0148-15
22. Miller AS, Cahill S, Mayer KH. FDA’s 2023 Policy Update—Promoting Safety and Inclusivity in Blood Donation. JAMA Health Forum. 2023;4(8):e232388. doi:10.1001/jamahealthforum.2023.2388
23. Blood Donor Eligibility Criteria. Red Cross blood services; 2023. Availabe from: https://www.redcrossblood.org/donate-blood/how-to-donate/eligibility-requirements/eligibility-criteria-alphabetical.html.
24. Memon A, Doll SR. A search for unknown blood-borne oncogenic viruses. Int J Cancer. 1994;58(3):366–368. doi:10.1002/ijc.2910580310
25. Dzik WH, Okayama A. Can blood transfusion transmit disease-producing genes? Transfusion. 1999;39(8):795–800. doi:10.1046/j.1537-2995.1999.39080795.x
26. NACO, NBTC. Guidel. Blood Donor Sel; 2017. Availabe from: http://nbtc.naco.gov.in/page/publications/.
27. Blood donor selection guideline on assessing donor suitability for blood donation; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138219/.
28. Government Publishing Office. 21 CFR 600-799. Code of Federal Regulations. Government Publishing Office; 2022.
29. Dorsey KA, Moritz ED, Steele WR, Eder AF, Stramer SL. A comparison of human immunodeficiency virus, hepatitis C virus, hepatitis B virus, and human T-lymphotropic virus marker rates for directed versus volunteer blood donations to the American Red Cross during 2005 to 2010. Transfusion. 2013;53(6):1250–1256. doi:10.1111/j.1537-2995.2012.03904.x
30. Etchason J, Petz L, Keeler E, et al. The cost effectiveness of preoperative autologous blood donations. N Engl J Med. 1995;332(11):719–724. doi:10.1056/NEJM199503163321106
31. Domen RE. Preoperative autologous blood donation: clinical, economic, and ethical issues. Cleve Clin J Med. 1996;63(5):295–300. doi:10.3949/ccjm.63.5.295
32. Goodnough LT. Autologous Blood Donation. Anesthesiol Clin North Am. 2005;23(2):263–270. doi:10.1016/j.atc.2004.07.003
33. Yomtovian R. Practical aspects of preoperative autologous transfusion. Am J Clin Pathol. 1997;107(4 Suppl 1):S28–35.
34. Domen R. Adverse reactions associated with autologous blood transfusion: evaluation and incidence at a large academic hospital. Transfusion. 1998;38(3):296–300. doi:10.1046/j.1537-2995.1998.38398222875.x
35. Letts M, Perng R, Luke B, Jarvis J, Lawton L, Hoey S. An analysis of a preoperative pediatric autologous blood donation program. Can J Surg. 2000;43(2):125–129.
36. Rock G, Berger R, Filion D, et al. Autologous blood programs: what is happening? Transfus Apher Sci. 2008;39(3):193–197. doi:10.1016/j.transci.2008.09.014
37. Axelrod F, Pepkowitz S, Goldfinger D. Establishment of a schedule of optimal preoperative collection of autologous blood. Transfusion. 1989;29(8):677–680. doi:10.1046/j.1537-2995.1989.29890020438.x
38. Birkmeyer JD, Goodnough LT, AuBuchon JP, Noordsij PG, Littenberg B. The cost-effectiveness of preoperative autologous blood donation for total Hip and knee replacement. Transfusion. 1993;33(7):544–551. doi:10.1046/j.1537-2995.1993.33793325048.x
39. Birkmeyer JD, AuBuchon JP, Littenberg B, et al. Cost-effectiveness of preoperative autologons donation in coronary artery bypass grafting. Ann Thorac Surg. 1994;57(1):161–168. doi:10.1016/0003-4975(94)90386-7
40. Stehling L, Zauder H. Controversies in transfusion medicine. Perioperative hemodilution: pro. Transfusion. 1994;34(3):265–268. doi:10.1046/j.1537-2995.1994.34394196628.x
41. Domen RE, Ribicki LA, Hoeltge GA. An analysis of autologous blood donor motivational factors. Vox Sang. 1995;69(2):110–113. doi:10.1111/j.1423-0410.1995.tb01679.x
42. Henry DA, Carless PA, Moxey AJ, O’Connell D, Ker K, Fergusson DA. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2001;2010(4). doi:10.1002/14651858.CD003602
43. Henry DA, Moxey AJ, Carless PA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. In: editor, Henry DA. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2001. doi:10.1002/14651858.CD001886.pub2
44. World Health Organization. Towards 100% voluntary blood donation: a global framework for action; 2010. Available from: https://www.ncbi.nlm.nih.gov/books/NBK305666.
45. National AIDS Control Organisation. Ministry of Health and Family Welfare. Government of India. Voluntary blood donation programme - an operational guideline; 2007.
46. Ad-Drees A. Attitude, belief and knowledge about blood donation and transfusion in Saudi population. Pak J Med Sci. 2008;24(1):74–79.
47. Matee MI, Magesa PM, Lyamuya EF. Seroprevalence of human immunodeficiency virus, hepatitis B and C viruses and syphilis infections among blood donors at the Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health. 2006;6(1):21. doi:10.1186/1471-2458-6-21
48. Sharma RR, Cheema R, Vajpayee M, et al. Prevalence of markers of transfusion transmissible diseases in voluntary and replacement blood donors. Natl Med J India. 2004;17(1):19–21.
49. Sultan F, Mehmood T, Mahmood MT. Infectious pathogens in volunteer and replacement blood donors in Pakistan: a ten-year experience. Int J Infect Dis. 2007;11(5):407–412. doi:10.1016/j.ijid.2006.10.004
50. Mremi A, Yahaya JJ, Nyindo M, Mollel E. Transfusion-transmitted infections and associated risk factors at the Northern Zone Blood Transfusion Center in Tanzania: a study of blood donors between 2017 and 2019. PLoS One. 2021;16(3):e0249061. doi:10.1371/journal.pone.0249061
51. Twenty-Eighth World Health Assembly. WHA28.72-Utilization and Supply of Human Blood and Blood Products. Geneva: Twenty-Eighth World Health Assembly; 1975.
52. Asenso-Mensah K, Achina G, Appiah R, Owusu-Ofori S, Allain JP. Can family or replacement blood donors become regular volunteer donors? Transfusion. 2014;54(3pt2):797–804. doi:10.1111/trf.12216
53. Allain JP, Sarkodie F, Asenso-Mensah K, Owusu-Ofori S. Relative safety of first-time volunteer and replacement donors in West Africa. Transfusion. 2010;50(2):340–343. doi:10.1111/j.1537-2995.2009.02444.x
54. Loua A, Nze Nkoure G. Relative safety of first-time volunteer and replacement donors in Guinea. Transfusion. 2010;50(8):1850–1851; author reply 1851–2. doi:10.1111/j.1537-2995.2010.02718.x
55. Mbanya DN, Feunou F, Tayou TC. Volunteer or family/replacement donations: are the tides changing? Transfusion. 2010;50(8):1849–1850. doi:10.1111/j.1537-2995.2010.02656.x
56. Bates I, Hassall O. Should we neglect or nurture replacement blood donors in sub-Saharan Africa? Biologicals. 2010;38(1):65–67. doi:10.1016/j.biologicals.2009.10.013
57. Passweg JR, Baldomero H, Chabannon C, et al. The EBMT activity survey on hematopoietic-cell transplantation and cellular therapy 2018: CAR-T’s come into focus. Bone Marrow Transplant. 2020;55(8):1604–1613. doi:10.1038/s41409-020-0826-4
58. Greinacher A, Fendrich K, Hoffmann W. Demographic changes: the impact for safe blood supply. Transfus Med Hemother. 2010;37(3):141–148. doi:10.1159/000313949
59. Jones JM, Sapiano MRP, Savinkina AA, et al. Slowing decline in blood collection and transfusion in the United States - 2017. Transfusion. 2020;60(Suppl 2):S1–S9. doi:10.1111/trf.15604
60. Rossmann SN, Townsend M, Simon TL. Recruitment and screening of donors and the collection of blood. Rossi’s Princ Transfus Med. 2022;2022:25–36.
61. Vamvakas E, Taswell H. Epidemiology of blood transfusion. Transfusion. 1994;34(6):464–470. doi:10.1046/j.1537-2995.1994.34694295059.x
62. Zou S, Musavi F, Notari EP, Fang CT. Changing age distribution of the blood donor population in the United States. Transfusion. 2007;071117010348009. doi:10.1111/j.1537-2995.2007.01517.x
63. Currie CJ, Patel TC, McEwan P, Dixon S. Evaluation of the future supply and demand for blood products in the United Kingdom National Health Service. Transfus Med. 2004;14(1):19–24. doi:10.1111/j.0958-7578.2004.00475.x
64. Liu W, Chen Y, Hsu L, Chen J, Wei S, Hou S. An imbalance in blood collection and demand is anticipated to occur in the near future in Taiwan. J Formosan Med Assoc. 2022;21(8):1610–1614. doi:10.1016/j.jfma.2021.07.027
65. Ali A, Auvinen MK, Rautonen J. Blood donors and Blood Collection: the aging population poses a global challenge for blood services. Transfusion. 2009;50(3):584–588. doi:10.1111/j.1537-2995.2009.02490.x
66. Ugwu A, Gwarzo D, Nwagha T, Gwarzo A, Greinacher A. Transfusion in limited infrastructure locations – where to go decades after safe blood initiative by World Health Organization? ISBT Sci Ser. 2020;15(1):118–125. doi:10.1111/voxs.12519
67. Erhabor O, Adias T, Mainasara A. Provisions of safe blood transfusion services in low income setting in West Africa, case study of Nigeria. Adv Med Biol. 2013;59:1–58.
68. Custer B, Zou S, Glynn SA, et al. Addressing gaps in international blood availability and transfusion safety in low-and middle-income countries: a NHLBI workshop. Transfusion. 2018;58(5):1307–1317. doi:10.1111/trf.14598
69. Sullivan HC, Roback JD. The pillars of patient blood management: key to successful implementation (Article, p. 2840). Transfusion. 2019;59(9):2763–2767. doi:10.1111/trf.15464
70. Leahy MF, Hofmann A, Towler S, et al. Improved outcomes and reduced costs associated with a health-system-wide patient blood management program: a retrospective observational study in four major adult tertiary-care hospitals. Transfusion. 2017;57(6):1347–1358. doi:10.1111/trf.14006
71. Leahy MF, Trentino KM, May C, Swain SG, Chuah H, Farmer SL. Blood use in patients receiving intensive chemotherapy for acute leukemia or hematopoietic stem cell transplantation: the impact of a health system-wide patient blood management program. Transfusion. 2017;57(9):2189–2196. doi:10.1111/trf.14191
72. Heitmiller ES, Hill RB, Marshall CE, et al. Blood wastage reduction using Lean Sigma methodology. Transfusion. 2010;50(9):1887–1896. doi:10.1111/j.1537-2995.2010.02679.x
73. Levin JH, Collins L, Adekunle O, et al. Blood product wastage reduction by utilising low-cost, low-impact multimodal physician-to-physician communication initiatives. Transfus Med. 2019;29(6):389–393. doi:10.1111/tme.12640
74. Morgan TO. Blood conservation: the CEO perspective. J Cardiothorac Vasc Anesth. 2004;18(4):S15–S17. doi:10.1053/j.jvca.2004.06.003
75. McKinney M. Maine hospital slashes transfusions, reducing patient risk, costs. Mod Healthc. 2014;25:30.
76. World Health Organization. The Urgent Need to Implement Patient Blood Management: Policy Brief. World Health Organization; 2021.
77. Stanworth SJ, New HV, Apelseth TO, et al. Effects of the COVID-19 pandemic on supply and use of blood for transfusion. Lancet Haematol. 2020;7(10):e756–e764. doi:10.1016/S2352-3026(20)30186-1
78. Flegel WA. COVID‐19 insights from transfusion medicine. Br J Haematol. 2020;190(5):715–717. doi:10.1111/bjh.17005
79. Delabranche X, Kientz D, Tacquard C, et al. Impact of COVID‐19 and lockdown regarding blood transfusion. Transfusion. 2021;61(8):2327–2335. doi:10.1111/trf.16422
80. Fede J, Hinrichsen C. Improving red blood cell usage at an academic medical center. Medical Laboratory Observer; 2022.
81. Jacobs JW, Karafin MS, Allen ES, et al. Blood conservation strategies at United States hospitals during the COVID ‐19 pandemic: findings from a multi‐institutional analysis ‐ International Society of Blood Transfusion survey. Transfusion. 2022;62(11):2271–2281. doi:10.1111/trf.17116
82. Kheirbeck T, Martin T, Wakely M, Lueckel S, Adams C. Safety and feasibility of ultra-restrictive transfusion protocol as a blood-preservation strategy during shortage crises. R I Med J. 2022;49–54. Available from: http://www.rimed.org/rimedicaljournal/2022/09/2022-09.pdf#page=49.
83. Doughty H, Green L, Callum J, Murphy MF. Triage tool for the rationing of blood for massively bleeding patients during a severe national blood shortage: guidance from the National Blood Transfusion Committee. Br J Haematol. 2020;191(3):340–346. doi:10.1111/bjh.16736
84. Beverina I, Ranzini M. A synchronized approach between emergency department and anemia clinic to intravenous irontreatmentforverysevere(Hb<7.0g/dL) and extreme (<5.0 g/dL) iron-deficiency anemia: short-, medium- and long-term efficacy and safety analysis. Blood Transfus. 2023;21(3):235–239. doi:10.2450/2023.0199-22
85. Bolcato M, Russo M, Trentino K, Isbister J, Rodriguez D, Aprile A. Patient blood management: the best approach to transfusion medicine risk management. Transfus Apher Sci. 2020;59(4). doi:10.1016/j.transci.2020.102779
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
