Back to Journals » Clinical Interventions in Aging » Volume 19
Percutaneous Curved Vertebroplasty Decrease the Risk of Cemented Vertebra Refracture Compared with Bilateral Percutaneous Kyphoplasty in the Treatment of Osteoporotic Vertebral Compression Fractures
Authors Zhou Q , Wan Y, Ma L, Dong L, Yuan W
Received 31 August 2023
Accepted for publication 21 January 2024
Published 17 February 2024 Volume 2024:19 Pages 289—301
DOI https://doi.org/10.2147/CIA.S438036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Qiang Zhou, Yanlin Wan, Le Ma, Liang Dong, Weijian Yuan
Department of Orthopaedics, Tianjin First Central Hospital, Tianjin, People’s Republic of China
Correspondence: Qiang Zhou, Department of Orthopaedics, Tianjin First Central Hospital, Fukang Road 24#, Tianjin, People’s Republic of China, Email [email protected]
Purpose: The purpose of this study is to compare the refracture rate of the cemented vertebral body of percutaneous curved vertebroplasty (PCVP) and bilateral percutaneous kyphoplasty (PKP) in the treatment of osteoporotic vertebral compression fractures (OVCF).
Methods: Ninety-four patients with single segment thoracolumbar OVCF were randomly divided into two groups (47 patients in each) and underwent PCVP or bilateral PKP surgery, respectively. Refracture of cemented vertebral body, bone cement injection volume and cement pattern, cement leakage rate, total surgical time, intraoperative fluoroscopy time, preoperative and postoperative Cobb angles and anterior vertebral height, Oswestry disability index questionnaire (ODI) and visual analog scales (VAS) were recorded.
Results: The PCVP group had significantly lower refracture incidence of the cemented vertebral than the bilateral PKP group (p< 0.05). There was a significant postoperative improvement in the VAS score and ODI in both group (p< 0.01), and no significant difference was found between two groups. The operation time and intraoperative fluoroscopy times were significantly less in the PCVP group than in the bilateral PKP group (p< 0.01). The mean kyphosis angle correction and vertebral height restoration in the PCVP group was significantly less than that in the bilateral PKP group (p< 0.01).
Conclusion: Both PCVP and PKP were safe and effective treatments for OVCF. The PCVP had lower refracture rate of the cemented vertebral than the bilateral PKP group, and PCVP entailed less exposure to fluoroscopy and shorter operation time than bilateral PKP.
Keywords: osteoporosis, osteoporotic vertebral compression fractures, percutaneous curved vertebroplasty, percutaneous kyphoplasty
Introduction
For the treatment of osteoporotic vertebral compression fractures (OVCF), percutaneous kyphoplasty (PKP) and vertebroplasty (PVP) produce better outcomes in pain relief, functional recovery, health-related quality of life and lower mortality compared with non-surgical or sham treatment.1–4
However, complications such as refracture of previously operative vertebral body have been reported.5–10 Refracture of augmented vertebral body may result in decreased vertebral body height, aggravation of the kyphotic deformity, and even compression of the spinal cord, which usually requires further treatment.
The commonly used puncture approach for PKP or PVP is a bipedicular approach. Unipedicular approach can get the same pain relief as bipedicular approach, and it has been shown to have lower operation time, X-ray irradiation, wound degree and better cost-effectiveness.11,12 However, in the traditional unilateral straight approach, bone cement likely only fills the same side of application, while the contralateral vertebrae are poorly filled, which increases the risk of re-collapse of the non-augmented side.13 Furthermore, compared to the bipedicular approach, the unipedicular approach requires a more aggressive, lateral-to-medial insertion angle, which increases the risk of nerves or paravertebral vessels injury.
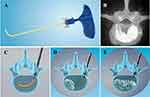
Percutaneous curved vertebroplasty (PCVP) provides a new approach for the treatment of OVCF, in which the curved injection cannula can easily reach the contralateral hemivertebra body and achieve a uniform bone cement distribution in vertebral body through unilateral approach14,15 (Figure 1). Compared to the traditional unipedicular approach, the insertion angle of PCVP is less aggressive and lateral-to-medial which lowers the risk of injury to paravertebral vessels or nerves. With the cannula withdrawn point-by-point, the cement is injected into the vertebra at each point through the ventral side openings of the cannula, which makes the cement distribution in vertebra more uniform (Figure 1).
It is unknown whether PCVP can reduce refracture incidence of cemented vertebra compared to bilateral PKP. In this study, we focused our research on the refracture of cemented vertebra compared PCVP with bilateral PKP in the treatment of OVCF.
Patients and Methods
Patient Population
This study was designed to be a prospective and controlled study of 94 patients who underwent surgical treatment of OVCF at our hospital by the same operation team. In this study, cases with a BMD T-score < −2.5 at the lumbar spine, total hip or femoral neck as measured by dual-energy X-ray absorptiometry (DXA) device (Hologic, Inc. Discover Wi, USA) were diagnosed with osteoporosis. Lumbar vertebra DXA measured the lumbar 1–4 levels and excluded any vertebral body with fracture. This study was conducted in our orthopedic department from May 2020 to September 2021. This study was approved by the ethics committee of our hospital. Written informed consent was obtained from each patient prior to the study. All methods were carried out in accordance with relevant guidelines and regulations, and the study was performed in accordance with the Helsinki II declaration.
Inclusion and Exclusion Criteria
The inclusion criteria for the study were as follows: (1) patients aged > 60 years, both men and women; (2) local back pain in the midline without neurologic deficits; (3) OVCF at only one level as determined by magnetic resonance imaging (MRI) with no pedicle fracture; (3) less than 50% compress of the vertebral height, as determined by lateral plain radiographs; (4) vertebral compression fracture within 3 weeks; (5) granted informed consent to enroll in the trial.
The exclusion criteria were as follows: (1) vertebral compression fracture caused by other reasons than osteoporosis such as tumor, vertebra tuberculosis, bacterial infection, etc.; (2) largely incomplete vertebral posterior margin bone destruction; (3) >50% compression of vertebral height; (4) more than one vertebrae compression fractures; (5) patients have Kummel disease, which is defined as if the radiographs or CT scans revealed air or fluid within the vertebra; (6) burst fractures due to high energy trauma; (7) vertebral fracture more than 3 weeks; (8) Patient who had poor compliance and could not complete the study as required.
Patient Randomization and Sample Size Calculation
There were 112 patients initially identified. In which, 12 patients did not meet inclusion criteria, 6 patients refused to participate, leaving 94 patients included in this study. These cases included 17 males and 77 females, aged 61–92 years (average, 72.57 years). Using a random distribution software, patients were numbered consecutively from 1 to 94 at the time of admission and randomly divided into a PCVP group (n = 47) and a PKP group (n = 47).
Surgical Procedures
The patient was placed in a prone position. All procedures were guided by G-arm Fluoroscopy (Siemens Healthcare, Erlangen, Germany) and completed under local anesthesia. All the operations were performed by the same spinal surgeon who was a senior orthopedist.
PCVP Group
The device used in this study was designed and manufactured by Hicren (China), while polymethylmethacrylate (PMMA) was produced by Heraeus (Germany). After local anesthesia, a unilateral pedicle puncture was performed through the standard transpedicular approach, and the tip of puncture needle was punctured to the posterior 1/3 of the vertebral body (Figures 1 and 2). Pull out the puncture needle core and place the curved catheter into the vertebra through the straight cannula. Then insert the curved injection cannula to reach the contralateral hemivertebra body. Anteroposterior fluoroscopy showed that the end of the curved catheter passed through the centrum midline to reach the contralateral side of the vertebra, while the lateral fluoroscopy reached 1/3 of the anterior middle vertebra. Then, the inner nitinol needle was pulled out, and the outer polyether-ether-ketone injection cannula was retained. The bone cement was injected at each point when the cannula was withdrawn point-by-point through the side openings near the tip of the injection cannula. The injection process was strictly monitored under anteroposterior and lateral fluoroscopy. When the cement was uniformly distributed and close to the posterior wall of the vertebral body, the injection was stopped. Then, the injection cannula was pulled out. When the cement outside is completely hardened, the needle is rotated and the cannula sleeve is pulled out to prevent tailing phenomenon.
PKP (Percutaneous Kyphoplasty) Group
PKP was performed with a bilateral approach. The specialized instruments used in surgery were manufactured by Kinetic (China), and PMMA cement was produced by Tianjin Synthetic Material Research Institute (China). Preoperative localization, disinfection and anesthesia were identical to PCVP. The puncture needles were inserted into the collapsed vertebral body through bilateral pedicles and punctured to the anterior third of the vertebral body with G-arm fluoroscopic guidance. After withdrawing the core of the puncture needles, the guide wires, expansion pipe, and working channel were sequentially inserted. Then, the inflatable balloons were placed into the vertebra. The contrast medium in the balloon was extracted after the balloon was sufficiently inflated. Then, the balloon was withdrawn. The bone cement was mixed with the reagent and injected into the fractured vertebral body at the wiredrawing stage. When the filling cement was approached to the posterior wall of the vertebra under fluoroscopy, the injection was stopped.
Self-ambulation was permitted the second day after surgery. All patients wore back braces after surgery for 1 month and took oral vitamin D and calcium postoperatively. All the patients were discharged 1–7 days after operation. The patients were followed up at 1 month, 3 months and every 6 months.
Outcome Examinations
Clinical parameters such as age, sex, bone mineral density, trauma history, recollapse levels and chemotherapy history were reviewed pre-operatively. VAS scores and Oswestry Disability Index (ODI) were recorded before the operation and 1 day, 3 months, 6 months and 1 year postoperatively; vertebral body compression rates (CRs) were measured as the ratio of the anterior height of the fractured vertebral body to the average anterior height of the upper and lower vertebral bodies. The restoration rate of vertebral height was calculated using the difference between preoperative and postoperative CRS. The posterior convex angles (KAs) were measured by Cobb method, which is the angle between the upper end plate of the upper vertebra and the lower end plate of the lower vertebra. The difference between preoperative and postoperative KAs was used to evaluate the correction of kyphosis angle. Whether there is a non PMMA area between the bone cement and the endplate was recorded. Non-PMMA-endplate-contact (NPEC) was defined as that the injected PMMA was not in contact with any portion of the endplate in postoperative plain X-ray film.
All patients underwent plain film and MRI to determine vertebral refracture and to exclude other reasons of pain, such as intervertebral disc herniation. The refracture of cemented vertebral body was diagnosed as the final anterior vertebral body height more than 1.0 mm lower than the postoperative anterior vertebral body height.9,16
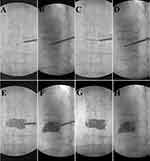
Radiological evaluation of X-ray plain films was performed immediately after the operation to evaluate the cement distribution pattern in cemented vertebral body. Based on the distribution of bone cement in cancellous bone, it was divided into two patterns: group 1: mass pattern; group 2: sponge pattern.
The surgical parameters were also assessed such as: (1) operation time (2) number of fluoroscopic images during operation (3) bone cement injection volume (4) the incidence of cement leakage.
Safety indices include the incidence of nerve and vascular injuries and complications. Serious complications occurred during or after the operation were recorded.
Statistical Analysis
The data analysis was processed using SPSS Statistics 19.0 statistical software (IBM, Chicago, USA). Continuous variables were analyzed using independent t-tests.
However, paired sample t-test and independent t-test were used to evaluate the inter-group and intra-group differences between VAS and ODI data before and after surgery. Categorical variables were analyzed using Pearson chi-square test or Fisher’s exact test. The statistical significance level was set as P < 0.05.
Results
Clinical Outcomes
There is no statistical difference in age, sex, BMD, BMI, vertebral region treated and mean time from injury between the PCVP and PKP groups (P>0.05) (Table 1). In terms of the preoperative VAS scores and ODI, there were no differences between the PCVP and PKP groups (P>0.05). VAS scores and ODI were significantly improved in both groups 1 day after operation (P<0.05). In addition, there was no difference in VAS score and ODI between the two groups on the first day, 6 months and 1 year after surgery (P>0.05) (Table 2).
 |
Table 1 Pre-Operative Demographic Data of Patients of Two Groups |
 |
Table 2 Pre- and Post-Operative VAS and ODI of Two Groups |
Safety
There were no puncture-related complications during or after operation in both PCVP and PKP groups. There was no significant difference in cement leakage rate between the two groups (P>0.05). There were five cases of leakage in the PCVP group (two cases in the intervertebral space, three cases in the paravertebral segment vessels), eight cases of leakage in the PKP group (three cases in the intervertebral space, three cases in the paravertebral segment vessels and two cases in the front of the fractured vertebral body). There were no serious complications such as spinal cord compression or nerve injury, spinal stenosis or pulmonary embolism at 1 year after operation.
Surgical Parameters
The surgical time in PCVP group was 23.04±3.45 min, which is significantly less than that in traditional PKP group (42.62±3.05, P<0.01). The number of intraoperative fluoroscopy in PCVP group (17.77±2.38) was significantly less than that in traditional PKP group (27.55±2.26, P<0.01) (Table 3).
 |
Table 3 Surgical Parameters of Two Groups |
Perfusion and Distribution of Bone Cement
In PCVP group, the average volume of injected bone cement was 4.61±0.88 mL. The average volume of bone cement perfusion in bilateral PKP group (6.78 ± 1.48 mL) was higher than that in PCVP group (P<0.01) (Table 3).
The cement distribution in PCVP group was more even than bilateral PKP group. In PCVP group, there were only two cases (4.25%) with mass cement distribution. Correspondingly, in PKP group, there were 26 cases (55.32%) of mass distribution.
Radiographic Results
Before operation, the anterior height of the vertebral body in PCVP group and traditional PKP group were 20.24 ± 3.57mm and 21.07 ± 3.21mm, respectively, and there was no statistically significant difference (P>0.05%). After operation, the mean AVH restoration in the PCVP group (6.39 ± 2.77%) was less than that in the PKP group (16.16 ± 3.16%, P<0.01). Before operation, there was no statistically significant difference in Cobb’s angles between the PCVP group (17.53 ± 2.25°) and the traditional PKP group (18.74 ± 2.53°, P>0.05%). After operation, the mean kyphosis restoration in the PCVP group (7.5 ± 2.25°) was significant less than that of the PKP group (11.35 ± 2.37°, P<0.01). There were 9 and 11 cases with NPEC in PCVP and PKP groups, respectively, and there was no significant difference between the two groups (P>0.05%) (Table 3).
Refracture Occurrence Rate
In this study, eight patients (17.02%) in PKP group and one patient (2.13%) in PCVP group appeared refractures at the cemented vertebra, respectively. The refracture rate of PCVP group was significantly less than that of PKP group (P<0.05) (Table 3).
Discussion
Vertebral osteoporotic fracture can seriously affect the daily life of patients. Conservative treatment requires long-term bed rest and fixation, which leads to further aggravated osteoporosis, hypostatic pneumonia, deep venous thrombosis, bedsore and other complications. The PKP and PVP using PMMA is widely accepted as a safe and effective treatment of OVCF,17 which can provide rapid pain relief, biomechanical stability and partial vertebral height recovery. Early out-of-bed activity after operation can also reduce complications in bed.
Complications associated with previous PKP or PVP, such as compression fractures of adjacent vertebral bodies, have been reported.18–20 However, there are few reports of refracture or recollapse of cemented vertebral bodies after PKP or PVP, and there are some controversies about the mechanism of refracture.8,21 In the present study, the rate of cemented vertebral refracture was 2.13% in the PCVP group and 17.02% in the PKP group, which was generally consistent with the results of previous researches, ranging from 0.56% to 27.63%.5–7,22,23
Clinical manifestations of refracture include new pain consistent with the previous surgical site, progression of vertebral height loss after PKP or PCVP, and MR imaging results showing recurrence or increased bone marrow edema in the treated vertebra. In the present study, the refracture of the augmented vertebral body was defined as the final anterior height being more than 1.0 mm lower than the postoperative anterior height.9,16 Studies show that, when in injured vertebra with Kummell osteonecrosis or intravertebral cleft (IVC), bone cement will be distributed in a cleft pattern or a solid lump rather than a trabecular pattern, which will increase the refracture rate of the augmented vertebra.6,24 In order to eliminate the interference of Kummell disease or IVC on the research results, patients with Kummell disease or IVC were excluded in this study.
The results of this study show that PKP and PVCP can dramatically relieve pain immediately after surgery and no significant difference was found in VAS scores and ODI between two groups. No puncture-related complications occurred in both groups. The clinical outcomes showed that both the PVCP and the PKP were effective and safe operations for the treatment of OVCF.
However, PKP (17.02%) has a higher rate of cemented vertebral refracture than PCVP (2.13%, P<0.05). Studies have shown that PKP was an important risk factor for refracture of cemented vertebra after percutaneous vertebral augmentation (PVA).25,26 The biomechanical studies of Kim et al25 using cadaver vertebrae treated with PVP and PKP showed that the average vertebral height loss of PKP was significantly greater than that of PVP, and the distribution of bone cement in PVP was more even than that in PKP.
In PKP, we think that the inflated balloon squeezes cancellous bone around during expansion, which makes the adjacent cancellous bone to be more dense and form a “cavity.” Cement tends to firstly fill this cavity left after balloon is withdrawn rather than infiltrating into the surrounding cancellous bone. After the cavity is gradually filled, the bone cement becomes viscous and has poor fluidity. By this time, it is difficult for the cement to diffuse into the outside of the “cavity” to form a good cross-linking with the surrounding cancellous bone. Therefore, two masses are formed instead of interdigitated cement distribution in PKP surgery (Figure 3), and this mass pattern cement distribution had also been shown to be a risk factor for refracture.27 Studies suggest that cement distribution patterns may be an important predisposing factor for refracture.5,27,28 Yu et al reported that after PVP treatment of OVCF with intervertebral cleft, the distribution pattern of solid block bone cement could lead to a 12.5-fold increase in the risk of vertebral refracture.29 Compared with mass pattern, spongy pattern can better maintain vertebral body height and reduce the risk of cemented vertebral body refracture.30 In this study, 95.75% of the PCVP cases were with spongy pattern and 55.32% of the PKP cases were with mass pattern.
In PCVP, the cement fills the cancellous bone of the vertebral body in an interdigitated pattern, which makes the stress distribution of the cemented vertebrae more balanced. When the loading is transmitted through the augmented vertebra, the sponge pattern of cement distribution in PCVP causes less stress concentration than mass pattern in PKP, resulting in less refracture. Excessive recovery of vertebral body height is an important risk factor for cemented vertebral body refracture after operation.6,7,10 In this study, the mean vertebral height restoration and mean kyphosis angle restoration in the PCVP group was less than that in the PKP group after operation (P<0.01). And the refracture rate of cemented vertebra of PCVP group was less than that of PKP group. PKP can better restore the height of fractured vertebrae, but at the cost of partial destruction of cancellous bone. Theoretically, the more the vertebral body height and kyphosis recovers, the greater the cancellous bone injury. The damage to cancellous bone of PCVP was significantly less than that of PKP, which was one of the reasons for the lower rate of refracture in PCVP. On the other hand, preoperative severe kyphosis with significant reduction in vertebral height is suitable for PKP. Therefore, only cases with vertebral collapse less than 50% were included in this study. The results of this study suggested that PCVP could significantly restore the anterior vertebral height and correct kyphosis, which was consistent with the previous studies using PVP.6,30 We believe that the recovery of vertebral body height in PCVP is mainly due to body position reduction after taking prone position.
The bilateral transpedicular approach is a standard technique for traditional PKP or PVP surgery. However, the bilateral transpedicular approach also has disadvantages, such as a long operation time, more punctures and more intraoperative fluoroscopy times.31 In recent years, many scholars proposed using unilateral pedicle puncture.13,14,32,33 Compared to the bipedicular approach, the unipedicular approach has obvious advantages such as less surgery time, trauma, device cost, and radiation exposure, as well as lower risk of cement leakage.11,12 However, compared with the bipedicular approach, it requires a more aggressive puncture approach angle from the lateral side to the medial side, which leads to penetration of the inner wall of the pedicle and increases the risk of spinal cord and nerve root injury.31,34,35
Studies have shown that the clinical efficacy of the unilateral approach is similar to that of the bilateral approach.12 However, using the traditional straight unipedicular approach in PVP or PKP, the cement is likely confined to the ipsilateral side of the vertebral body and cannot effectively diffuse through the midline to the collateral side. The distribution of bone cement is “O” shaped, and the uneven distribution of bone cement may increase the risk of recollapse of the non-cemented side, especially when lateral bending, resulting in refracture.15,36,37
In PCVP, a curved injection cannula is used through a standard unilateral transpedicular approach, which allows easy access to the contralateral hemivertebra body. Compared to the traditional straight unipedicular approach, it is safer because it requires less aggressive and lateral-to-medial, which lowers the risk of spinal cord or nerve roots injury. Surgeons who master the basic technique of transpedicular puncture can achieve a uniform distribution of bone cement on both sides. In this study, no puncture-related complications occurred either during or after the operation. In PCVP, as the curved cannula is withdrawn point by point, bone cement is delivered into the vertebral body at each point through the lateral opening of the ventral tip of the cannula. This also makes the bone cement distribution more even in both the ipsilateral and contralateral sides, unlike localization around the puncture channel in the traditional PKP or PVP. Chen et al found that the symmetric distribution of bone cement was closely related to the stiffness of the vertebral bodies.13 In theory, with multiple points of cement delivery, the injection pressure is lower than the traditional straight unipedicular approach or bipedicular approach PKP or PVP, and which also minimizes the risk of cement leakage. However, although the number of cement leakage cases in PCVP group (five cases) was lower than that in PKP group (eight cases), there was no significant difference between PCVP and PKP group in cement leakage (P>0.05). In PKP surgery, bone cement becoming viscous with poor fluidity after filling the cavity left by balloon withdrawal is a likely reason why the cement leakage rate is not high.
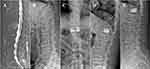
In PCVP, bone cement is dispersed in the anterior and middle of the vertebral body. But in PKP, bone cement is mainly filled on the side of the vertebral body.
Unlike the H-type of PKP or the O-type of PVP through traditional unilateral approach, the bone cement after PCVP showed kidney-shaped and mainly located in the anterior and middle of the vertebral body which is the main area of osteoporotic compression fracture (Figure 1). The distribution of bone cement is more consistent with the biomechanics of fractured vertebral body and can provide better support for vertebral body.38 Meanwhile, PCVP also has the same advantages as unilateral approaches, such as less operation time, puncture risk and X-ray irradiation.
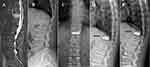
In this study, the bone cement did not contact the upper or lower endplates of the treated vertebral body in 20 cases, including 9 cases in PCVP group and 11 cases in PKP group (p>0.05). In PKP group, 6 of these 11 cases with Non-PMMA-endplate-contact (NPEC) appeared as refractures of the cemented vertebra. In the PCVP group, the only case that appeared to be refractured was not with NPEC, but the bone cement did not contact the upper endplate (Figure 4). It suggested that bone cement not in contact with both the endplates was more likely to refracture, which was consistent with other studies.16,23,36 Bone cement in contact with both endplates can provide better support in the vertical direction for the load can be transmitted through the upper and lower endplates, which can better restore the strength of the vertebral body.39 When bone cement contacts two endplates at the same time, the strength of vertebral body will increase about 8–12 times, and the stress transmission will be significantly improved; However, when the bone cement contacts only one of the endplates (upper or lower), the vertebral strength increases only about twofold.40 If the bone cement is not in contact with either the upper or the lower endplate, there is a non-cement area between the bone cement mass and two endplates. The non-cemented cancellous bone will be crushed progressively under Cyclic loading when the load cannot transfer through it, and the refracture of the injured vertebral body will occur.25,41 Studies have shown that refracture mainly occurs in the cementless part of the vertebral body.6,9
In present study, there were nine patients with NPEC in PCVP group, but none of them had refracture in the cemented vertebra. In these nine patients, all the distribution of bone cement had a spongy pattern. However, in the six cases of refracture with NPEC in PKP group, all the distribution of bone cement had a mass pattern. This may be related to the fact that the mass distribution of bone cement is more likely to cause local uneven stress than the sponge distribution, which will lead to refracture of cancellous bone around the mass cement under cyclic loading. These results indicate that the distribution pattern of bone cement is important for the refracture of the cemented vertebra, even more important than NPEC.
In this study, both PKP and PCVP groups had cases in which bone cement did not contact the endplate. The cancellous bone below the upper endplate and above the lower endplate became condensed when the endplate collapsed after vertebra compressed. When injected, bone cement always fills the low-density area first.
The bone cement is difficult to diffuse to the collapsed dense area, especially near the endplate. Even if the operator intentionally penetrates the puncture needle into the collapsed dense area during the operation, the bone cement likely does not diffuse to the collapsed dense area, but to the non-dense area.
The results of this study indicated that the volume of bone cement injected in PCVP (4.61±0.88 mL) was less than that of PKP (6.78±1.48 mL), and the refracture rate of PCVP group was significantly lower than that of PKP group (P<0.01). The results indicate that the more bone cement injection, the lower the rate of refracture, which is not correct. The results of this study showed that there was no difference in pain relief between PCVP and PKP groups. Molloy et al reported that 16.2% and 29.8% cement filling were required for the restoration of vertebral strength and stiffness, respectively.42 The other study using finite element demonstrated that 15% volume of bone cement augmentation was required to recover stiffness of the fractured vertebrae to its pre-injury value and that excessive bone cement did not provide greater benefit but increase the risk of cement leakage.43 Related studies also confirm the above view.44,45
Some scholars found that most of the refracture of cemented vertebral body occurred within 3 months after surgery.9 In this study, most of the refracture (9 of 12 cases) of cemented vertebral occurred within 1 month after operation, suggesting that there is a higher risk of refracture within 1 month after surgery, and more attention should be given to avoid the occurrence of cemented vertebral refracture during this period.
There are several limitations to our study. A major limitation of this study is the small number of cases. The follow-up period was relatively short (1 year). Therefore, the conclusions of this study still need to be validated by larger prospective randomized controlled clinical trials and longer follow-up.
Conclusion
Both PCVP and PKP were safe and effective treatment for OVCF. The PCVP had lower refracture rate of the cemented vertebral than the bilateral PKP group, and PCVP entailed less exposure to fluoroscopy and shorter operation time than bilateral PKP.
Abbreviations
OVCFs, Osteoporotic vertebral compression fractures; PKP, Percutaneous kyphoplasty; PCVP, Percutaneous curved vertebroplasty; PVA, Percutaneous vertebral augmentation; ODI, Oswestry disability index; VAS, Visual analogue scale.
Data Sharing Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study has been approved by Ethics Committee of Tianjin First Central Hospital. Written informed consent was obtained from each patient prior to the study.
Acknowledgments
The authors thank all their colleagues for their valuable assistance during this study.
Disclosure
The authors declare that they have no competing interests.
References
1. Li L, Ren J, Liu J, et al. Results of vertebral augmentation treatment for patients of painful osteoporotic vertebral compression fractures: a meta-analysis of eight randomized controlled trials. PLoS One. 2015;10(9):e0138126. doi:10.1371/journal.pone.0138126
2. Anderson PA, Froyshteter AB, Tontz WL. Meta-analysis of vertebral augmentation compared with conservative treatment for osteoporotic spinal fractures. J Bone Miner Res. 2013;28(2):372–382. doi:10.1002/jbmr.1762
3. Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011;26(7):1617–1626. doi:10.1002/jbmr.353
4. Edidin AA, Ong KL, Lau E, Kurtz SM. Morbidity and mortality after vertebral fractures: comparison of vertebral augmentation and nonoperative management in the medicare population. Spine. 2015;40(15):1228–1241. doi:10.1097/BRS.0000000000000992
5. Kang SK, Lee CW, Park NK, et al. Predictive risk factors for refracture after percutaneous vertebroplasty. Ann Rehabil Med. 2011;35(6):844–851. doi:10.5535/arm.2011.35.6.844
6. Heo DH, Chin DK, Yoon YS, Kuh SU. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2009;20(3):473–480. doi:10.1007/s00198-008-0682-3
7. Chen LH, Hsieh MK, Liao JC, et al. Repeated percutaneous vertebroplasty for refracture of cemented vertebrae. Arch Orthop Trauma Surg. 2011;131(7):927–933. doi:10.1007/s00402-010-1236-7
8. Leslie-Mazwi T, Deen HG. Repeated fracture of a vertebral body after treatment with balloon kyphoplasty: case illustration. J Neurosurg Spine. 2006;4(3):270. doi:10.3171/spi.2006.4.3.270
9. Kim YY, Rhyu KW. Recompression of vertebral body after balloon kyphoplasty for osteoporotic vertebral compression fracture. Eur Spine J. 2010;19(11):1907–1912. doi:10.1007/s00586-010-1479-6
10. Xiong YC, Guo W, Xu F, et al. Refracture of the cemented vertebrae after percutaneous vertebroplasty: risk factors and imaging findings. BMC Musculoskelet Disord. 2021;22(1):459. doi:10.1186/s12891-021-04355-w
11. Chang WS, Lee SH, Choi WG, Choi G, Jo BJ. Unipedicular vertebroplasty for osteoporotic compression fracture using an individualized needle insertion angle. Clin J Pain. 2007;23(9):767–773. doi:10.1097/AJP.0b013e318154b6c3
12. Zhang L, Liu Z, Wang J, et al. Unipedicular versus bipedicular percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a prospective randomized study. BMC Musculoskelet Disord. 2015;16:145. doi:10.1186/s12891-015-0590-6
13. Chen B, Li Y, Xie D, Yang X, Zheng Z. Comparison of unipedicular and bipedicular kyphoplasty on the stiffness and biomechanical balance of compression fractured vertebrae. Eur Spine J. 2011;20(8):1272–1280. doi:10.1007/s00586-011-1744-3
14. Cheng Y, Liu Y. Percutaneous curved vertebroplasty in the treatment of thoracolumbar osteoporotic vertebral compression fractures. J Int Med Res. 2019;47(6):2424–2433. doi:10.1177/0300060519836917
15. Zhong R, Liu J, Wang R, et al. Unilateral curved versus bipedicular vertebroplasty in the treatment of osteoporotic vertebral compression fractures. BMC Surg. 2019;19(1):193. doi:10.1186/s12893-019-0653-y
16. Tan L, Wen B, Guo Z, Chen Z. The effect of bone cement distribution on the outcome of percutaneous Vertebroplasty: a case cohort study. BMC Musculoskelet Disord. 2020;21(1):541. doi:10.1186/s12891-020-03568-9
17. Chang X, Lv YF, Chen B, et al. Vertebroplasty versus kyphoplasty in osteoporotic vertebral compression fracture: a meta-analysis of prospective comparative studies. Int Orthop. 2015;39(3):491–500. doi:10.1007/s00264-014-2525-5
18. Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226(1):119–124. doi:10.1148/radiol.2261011911
19. Yang S, Liu Y, Yang H, Zou J. Risk factors and correlation of secondary adjacent vertebral compression fracture in percutaneous kyphoplasty. Int J Surg. 2016;36(Pt A):138–142. doi:10.1016/j.ijsu.2016.10.030
20. Li H, Yang DL, Ma L, Wang H, Ding WY, Yang SD. Risk Factors Associated with Adjacent Vertebral Compression Fracture Following Percutaneous Vertebroplasty After Menopause: a Retrospective Study. Med Sci Monit. 2017;23:5271–5276. doi:10.12659/msm.907364
21. Wagner AL, Baskurt E. Refracture with cement extrusion following percutaneous vertebroplasty of a large interbody cleft. AJNR Am J Neuroradiol. 2006;27(1):230–231.
22. Yu WB, Jiang XB, Liang D, Xu WX, Ye LQ, Wang J. Risk factors and score for recollapse of the augmented vertebrae after percutaneous vertebroplasty in osteoporotic vertebral compression fractures. Osteoporos Int. 2019;30(2):423–430. doi:10.1007/s00198-018-4754-8
23. Zhang L, Wang Q, Wang L, Shen J, Zhang Q, Sun C. Bone cement distribution in the vertebral body affects chances of recompression after percutaneous vertebroplasty treatment in elderly patients with osteoporotic vertebral compression fractures. Clin Interv Aging. 2017;12:431–436. doi:10.2147/CIA.S113240
24. Kim YJ, Lee JW, Kim KJ, et al. Percutaneous vertebroplasty for intravertebral cleft: analysis of therapeutic effects and outcome predictors. Skeletal Radiol. 2010;39(8):757–766. doi:10.1007/s00256-009-0866-8
25. Kim MJ, Lindsey DP, Hannibal M, Alamin TF. Vertebroplasty versus kyphoplasty: biomechanical behavior under repetitive loading conditions. Spine. 2006;31(18):2079–2084. doi:10.1097/01.brs.0000231714.15876.76
26. Wilke HJ, Mehnert U, Claes LE, Bierschneider MM, Jaksche H, Boszczyk BM. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethyl methacrylate or calcium phosphate cement under cyclic loading. Spine. 2006;31(25):2934–2941. doi:10.1097/01.brs.0000248423.28511.44
27. He D, Lou C, Yu W, et al. Cement distribution patterns are associated with recompression in cemented vertebrae after percutaneous vertebroplasty: a retrospective study. World Neurosurg. 2018;120:e1–e7. doi:10.1016/j.wneu.2018.06.113
28. Lin WC, Lee YC, Lee CH, et al. Refractures in cemented vertebrae after percutaneous vertebroplasty: a retrospective analysis. Eur Spine J. 2008;17(4):592–599. doi:10.1007/s00586-007-0564-y
29. Yu W, Xiao X, Zhang J, et al. Cement distribution patterns in osteoporotic vertebral compression fractures with intravertebral cleft: effect on therapeutic efficacy. World Neurosurg. 2019;123:e408–e415. doi:10.1016/j.wneu.2018.11.181
30. Li Q, Long X, Wang Y, et al. Clinical observation of two bone cement distribution modes after percutaneous vertebroplasty for osteoporotic vertebral compression fractures. BMC Musculoskelet Disord. 2021;22(1):577. doi:10.1186/s12891-021-04480-6
31. Chen L, Yang H, Tang T. Unilateral versus bilateral balloon kyphoplasty for multilevel osteoporotic vertebral compression fractures: a prospective study. Spine. 2011;36(7):534–540. doi:10.1097/BRS.0b013e3181f99d70
32. Tang J, Guo WC, Hu JF, Yu L. Unilateral and bilateral percutaneous kyphoplasty for thoracolumbar osteoporotic compression fractures. J Coll Physicians Surg Pak. 2019;29(10):946–950. doi:10.29271/jcpsp.2019.10.946
33. Yang S, Chen C, Wang H, Wu Z, Liu L. A systematic review of unilateral versus bilateral percutaneous vertebroplasty/percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Acta Orthop Traumatol Turc. 2017;51(4):290–297. doi:10.1016/j.aott.2017.05.006
34. Sun H, Li C. Comparison of unilateral and bilateral percutaneous vertebroplasty for osteoporotic vertebral compression fractures: a systematic review and meta-analysis. J Orthop Surg Res. 2016;11(1):156. doi:10.1186/s13018-016-0479-6
35. He S, Zhang Y, Lv N, et al. The effect of bone cement distribution on clinical efficacy after percutaneous kyphoplasty for osteoporotic vertebral compression fractures. Medicine. 2019;98(50):e18217. doi:10.1097/MD.0000000000018217
36. Liang D, Ye LQ, Jiang XB, et al. Biomechanical effects of cement distribution in the fractured area on osteoporotic vertebral compression fractures: a three-dimensional finite element analysis. J Surg Res. 2015;195(1):246–256. doi:10.1016/j.jss.2014.12.053
37. Zhao WT, Qin DP, Zhang XG, Wang ZP, Tong Z. Biomechanical effects of different vertebral heights after augmentation of osteoporotic vertebral compression fracture: a three-dimensional finite element analysis. J Orthop Surg Res. 2018;13(1):32. doi:10.1186/s13018-018-0733-1
38. Wang C, Zhang Y, Chen W, Yan SL, Guo KJ, Feng S. Comparison of percutaneous curved kyphoplasty and bilateral percutaneous kyphoplasty in osteoporotic vertebral compression fractures: a randomized controlled trial. BMC Musculoskelet Disord. 2021;22(1):588. doi:10.1186/s12891-021-04469-1
39. Xu K, Li YL, Song F, Liu HW, Yang HD, Xiao SH. Influence of the distribution of bone cement along the fracture line on the curative effect of vertebral augmentation. J Int Med Res. 2019;47(9):4505–4513. doi:10.1177/0300060519864183
40. Chevalier Y, Pahr D, Charlebois M, Heini P, Schneider E, Zysset P. Cement distribution, volume, and compliance in vertebroplasty: some answers from an anatomy-based nonlinear finite element study. Spine. 2008;33(16):1722–1730. doi:10.1097/BRS.0b013e31817c750b
41. Jansen LE, Birch NP, Schiffman JD, Crosby AJ, Peyton SR. Mechanics of intact bone marrow. J Mech Behav Biomed Mater. 2015;50:299–307. doi:10.1016/j.jmbbm.2015.06.023
42. McKiernan F, Faciszewski T, Jensen R. Reporting height restoration in vertebral compression fractures. Spine. 2003;28(22):2517–21; disucssion 3. doi:10.1097/01.BRS.0000092424.29886.C9
43. Liebschner MA, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine. 2001;26(14):1547–1554. doi:10.1097/00007632-200107150-00009
44. Belkoff SM, Mathis JM, Jasper LE, Deramond H. The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine. 2001;26(14):1537–1541. doi:10.1097/00007632-200107150-00007
45. Nieuwenhuijse MJ, Bollen L, van Erkel AR, Dijkstra PD. Optimal intravertebral cement volume in percutaneous vertebroplasty for painful osteoporotic vertebral compression fractures. Spine. 2012;37(20):1747–1755. doi:10.1097/BRS.0b013e318254871c
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.