Back to Journals » Vascular Health and Risk Management » Volume 20
Pericardiectomy for Constrictive Pericarditis with or without Cardiopulmonary Bypass
Received 9 November 2023
Accepted for publication 31 January 2024
Published 8 February 2024 Volume 2024:20 Pages 39—46
DOI https://doi.org/10.2147/VHRM.S439292
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Jing-Bin Huang, Yun-Tian Tang
Department of Cardiothoracic Surgery, The People’s Hospital of Guangxi Zhuang Autonomous Region, and Guangxi Academy of Medical Sciences, Nanning, Guangxi, People’s Republic of China
Correspondence: Jing-Bin Huang; Yun-Tian Tang, Tel +86-771-2188205, Fax +86-771-2188214, Email [email protected]; [email protected]
Aim: We aim to access the effect of pericardiectomy for constrictive pericarditis with or without cardiopulmonary bypass.
Methods: This was a review of pericardiectomy for constrictive pericarditis.
Results: Cardiopulmonary bypass is actually an important maneuver to attain complete relief of the constriction. The short additional time of cardiopulmonary bypass during the procedure has very little effect on the risk of morbidity of the main operation.
Conclusion: Incomplete pericardiectomy perhaps was the cause of postoperative remnant constriction and high diastolic filling pressure leading to multiorgan failure. Complete pericardiectomy (removal of phrenic-to-phrenic and the postero-lateral and inferior wall pericardial thickening) using cardiopulmonary bypass should be the routine for total relief of the constriction of the heart.
Keywords: pericardiectomy, constrictive pericarditis, cardiopulmonary bypass
Introduction
Constrictive pericarditis is the late-stage chronic inflammatory process characterized by thickening and calcification of pericardial fibers, damage to diastolic filling, decrease in cardiac output, and heart failure in the end.
The normal pericardium comprises a serous inner layer and a fibrous outer layer. The pericardial space normally contains about 20 to 50 mL of serous fluid. The pericardium has multiple functions, including mechanical effects (maintaining cardiac geometry, limiting dilation, promoting ventricular coupling interactions, achieving frictionless motion, and serving as an infection barrier), vasodilation, immunity, paracrine, and fibrinolytic activity.1–3
At present in the Western countries, viral or idiopathic pericarditis is the predominant cause of constrictive pericarditis (CP) followed by postcardiotomy irritation and mediastinal irradiation. While tuberculosis is still a main cause of pericarditis in developing countries.4–7
Diagnosis of Constrictive Pericarditis
Diagnosis of constrictive pericarditis is based on clinical manifestations, echocardiography, chest computed tomography, cardiac catheterization, surgical and pathological criteria. The clinical symptoms of constrictive pericarditis stem from a decrease in cardiac output and fluid overload. Physical examination results may include Beck’s triad (hypotension, paradoxical pulse, and deep heart sounds), jugular vein dilation secondary to high venous pressure, Kussmaul’s sign, a pericardial knock, edema, ascites, or cachexia.
Transthoracic echocardiography, (Figure 1) computed tomography, and cardiac magnetic resonance imaging each can reveal increased pericardial thickness and severe diastolic dysfunction. Cardiac catheterization reveals elevated end-diastolic pressure and the “square root sign” of right ventricular pressure tracing and can help to exclude restrictive cardiomyopathy and confirm a diagnosis of diastolic dysfunction secondary to pericardial constriction. A thickened pericardium of more than 4 mm on cardiac CT is supportive of the diagnosis and the best modality for the evaluation of pericardial calcification (Figure 2). Surgical and pathological findings can confirm the preoperative diagnosis. Surgical and pathological findings can confirm the preoperative diagnosis. Histopathologic features of tuberculosis in histopathologic studies of pericardium tissue from patients include the presence of typical granuloma and caseous necrosis (Figure 3). Histopathologic studies of pericardium tissue from patients with idiopathic or viral pericarditis show chronic nonspecific inflammatory changes (Figure 4).8–11
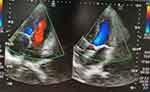 |
Figure 1 Transthoracic echocardiography showed the significantly thickened pericardium. |
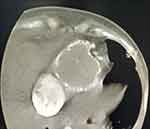 |
Figure 2 Chest computed tomographic scan showed the noticeably thickened and calcified ring of the pericardium. |
 |
Figure 3 Histopathologic studies of pericardium tissue from a patient showed histopathologic features of tuberculosis including the presence of typical granuloma and caseous necrosis. |
 |
Figure 4 Histopathologic studies of pericardium tissue from a patient showed chronic nonspecific inflammatory changes. |
Pericardiectomy early with complete relief of constriction has good symptomatic relief and is the good treatment of choice for constrictive pericarditis, before severe constriction and myocardial atrophy occur.
Surgical Technique
Pericardectomy can be performed through a median sternotomy or a left anterior lateral thoracotomy. The median sternotomy can effectively provide access to the right ventricle, great vessels, and right atrium, allowing the thickened pericardium to be cleared from the phrenic nerve to the phrenic nerve. Left anterior thoracotomy is mainly used for infectious suppurative pericarditis to avoid postoperative sternal infection. Femoral access sites are usually kept open.
Femoral blood vessels are usually prepared or intubated before thoracotomy, depending on the degree of postoperative adhesion of the sternum, the proximity of the heart structure to the sternum, the degree of heart failure, and the necessity of additional cardiac intervention. In order to evaluate the hemodynamic impact of the pericardiectomy intraoperatively, comprehensive monitoring of patients was conducted.12–15
By using sharp and blunt dissection techniques, dissection starts from the midline. It is important to carefully locate the dissection plane between the fibrotic-constricted parietal pericardium and epicardium while paying attention to the coronary artery. To avoid them and ensure sufficient depth in the dissection plane, the arteries should be visible.
When access to the correct dissection plane is attained, good cardiac relaxation can be achieved after removing the fibrous parietal pericardium. In order to remove all hard pericardial tissue, further dissection is performed between the pericardium of left and right ventricular walls, as well as between the left and right atrial walls.
Sometimes very thick local adhesions are encountered and difficult to remove. In this case, adhesion should be left untreated to prevent accidental damage to the underlying heart cavity. All small local bleeding will be treated immediately. Waffler surgery is another option for patients with extensive epicardial involvement, with multiple transverse and longitudinal incisions made in the epicardial layer.16–18
Pericardiectomy on Cardiopulmonary Bypass (Complete Pericardiectomy)
Pericardectomy involves cardiopulmonary bypass through a median sternotomy, with mild hypothermia (32–34°C) applied to the beating heart. Cardiopulmonary bypass is instituted through catheterization of the aorta, superior and inferior vena cava. Without cardioplegia, the aorta is unclamped and the heart remains beating throughout the entire extracorporeal circulation process.
After the median sternotomy, palpation of the pericardium is performed to identify an area that is not calcified and relatively soft, and the thymus is removed laterally. Dissection is started from the base of the aorta and extends downwards to the lateral and posterior walls of the left ventricle, followed by the diaphragmatic pericardium. The pericardium over the right atrium and vena cava is resected finally. If there are calcified plaques penetrating the epicardium, small islands of calcified pericardial tissue will be left untreated. Complete pericardiectomy is performed, followed by cardiopulmonary bypass weaned. In order to completely alleviate cardiac constrictions, the pericardium was completely removed during cardiopulmonary bypass (removal of phrenic-to-phrenic and the postero-lateral and inferior wall pericardial thickening).19–21
Pericardiectomy without Cardiopulmonary Bypass (Incomplete Pericardiectomy)
Pericardiectomy is conducted without extracorporeal circulation through sternotomy. Pericardectomy is performed between two phrenic nerves and from the large blood vessels to the base of the heart. Dissection was started at the base of the aorta, extended downward to the lateral walls of the left ventricle. The pericardium over the right atrium and vena cava was removed last.22–24 Radical pericardiectomy provided superior 10-year survival and clinical functional improvement in patients with chronic constrictive pericarditis compared to sub-total pericardiectomy.25
Operative Mortality
In 1913, German surgeon Ludwig Rehn successfully completed the first pericardiectomy for constrictive pericarditis, which was later considered a treatment method.2 Different surgical techniques and methods, such as partial pericardiectomy and total pericardiectomy, the necessity of extracorporeal circulation, and sternotomy and lateral thoracotomy, are still under debate. The median sternotomy approach enables a more radical clearance of pericardium. In cases of purulent pericarditis and effusive-constricted constrictive pericardial disease, the left anterior lateral thoracotomy approach should be preferred, as there is a risk of both empyema and sternal infection.26–29
Despite successful pericardiectomy, residual and prolonged constriction, or myocardial atrophy following myocardial processes can lead to long-term heart failure. The causes of death are multiple organ failure, heart failure, and respiratory insufficiency.28,30–32
Low Cardiac Output Syndrome
The etiology of low cardiac output syndrome is related to incomplete resection of thickened pericardium, excessive ventricular dilation after pericardial dissection, myocardial weakness, unsatisfactory relief of left ventricular compression, and heart failure. Incomplete pericardial dissection is associated with low cardiac output syndrome after pericardiectomy.
The relief of left ventricular constriction is crucial for the recovery of cardiac function after pericardiectomy. Apex adhesions should be free enough to restore normal ventricular contraction and rotational function. Incomplete resection of thickened pericardium and insufficient relief of left ventricular compression are associated with low cardiac output syndrome and operative deaths after pericardiectomy. Removing the pericardium from the phrenic nerve to the phrenic nerve without extracorporeal circulation usually results in insufficient removal of the pericardium to alleviate the constriction, especially in cases of complete encirclement of the heart. The most common is a severely thickened calcified ring around the base. In these cases, sometimes posterior lateral and inferior wall pericardial thickening associated with severe cardiac constriction are left untreated. Therefore, in this severe constrictive pericarditis, the textbook method of phrenic nerve to phrenic nerve resection of the pericardium is usually not sufficient to alleviate constriction. Perhaps it is precisely for this reason that the proportion of patients with low cardiac output syndrome and operative deaths after pericardiectomy is so high. Without extracorporeal circulation, severe pericardial contraction is usually not effectively eliminated. In cases of severe myocardial infiltration with calcification, without cardiopulmonary bypass or left ventricular ventilation, these lesions cannot be safely and effectively removed. Therefore, if the main purpose is to completely alleviate the constriction of the heart, then extracorporeal circulation is actually a crucial operation. The brief additional cardiopulmonary bypass time during the surgical process hardly increases the risk of morbidity of the main surgery.33–35
Cardiopulmonary bypass clarifies the appropriate dissection plane through emptying the ventricular cavities, which facilitates surgical dissection and the management of accidental heart injury. Complete pericardiectomy (diaphragmatic to diaphragmatic resection, as well as resection of posterior lateral and inferior wall pericardial thickening) for complete relief of cardiac constriction during cardiopulmonary bypass should be routine.36–42
Postoperative Acute Kidney Injury
In Huang et al’s study, serum creatinine was used as the diagnostic standard for acute kidney injury (AKI). According to KDIGO classification, if serum creatinine increases by ≥ 26.5 μmol/L (0.3 mg/dL) within 48 hours, serum creatinine is 50% higher than baseline within the first 7 days, or urine output is <0.5 mL/kg/h for 6 hours, the patient is considered to have acute kidney injury.43,44
Acute renal injury after pericardiectomy is a serious postoperative complication, leading to a significant increase in perioperative morbidity and mortality rates. The incidence of postoperative acute kidney injury in the study was 27.2%. The predictive factors for acute kidney injury include intubation time, thoracic drainage, serum creatinine at 24 and 48 hours after surgery, fresh-frozen plasma, and packed red cells.
The following factors have been found to contribute to the development of acute renal injury after cardiac surgery: age, female, obesity, blood transfusion, low cardiac output, valve replacement surgery, myocardial infarction in the last 30 days, heart failure, peripheral artery disease, chronic obstructive pulmonary disease, prolonged cardiopulmonary bypass, use of intra-aortic balloon pump, use of inotropic or vasoconstrictor drugs, diabetes mellitus, systemic arterial hypertension, and chronic kidney disease.
Patients with complications such as diabetes, chronic obstructive pulmonary disease, congestive heart failure and existing chronic kidney disease often take various nephrotoxic drugs, including nonsteroidal anti-inflammatory drugs, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers, all of which further adversely affect glomerular perfusion.
Preoperative heart injury is a risk factor associated with pre-, peri-, or post-operative low cardiac output syndrome, and increases the likelihood of acute kidney injury after surgery due to reduced perfusion pressure and total renal ischemia injury. Renal toxicity, vasoconstriction, hemodynamic instability, inotropic drugs, and systemic inflammation are important factors that affect the development of postoperative acute kidney injury. In addition, due to the reduced oxygen carrying capacity of red blood cells and ischemic damage to the renal system, the development of pre existing anemia or postoperative anemia may also lead to acute kidney injury. Therefore, preventing the development of acute renal injury after cardiac surgery, optimizing pre-, intra- and post-operative factors, to reduce acute renal injury, contribute to better postoperative results, and reduce the rate of acute renal injury, morbidity, and mortality.45,46
Strengths and limitations of pericardiectomy with or without cardiopulmonary bypass were showed in Table 1.
 |
Table 1 Pericardiectomy with or without Cardiopulmonary Bypass |
Conclusions
Cardiopulmonary bypass is actually an important maneuver to attain complete relief of the constriction. The short additional time of cardiopulmonary bypass during the procedure has very little effect on the risk of morbidity of the main operation. Incomplete pericardiectomy perhaps was the cause of postoperative remnant constriction and high diastolic filling pressure leading to multiorgan failure. Complete pericardiectomy (removal of phrenic-to-phrenic and the postero-lateral and inferior wall pericardial thickening) using cardiopulmonary bypass should be the routine for total relief of the constriction of the heart.
Funding
This work was supported by the Natural Science Foundation of China (No: 81360014), the Natural Science Foundation of Guangxi (No: 2014GXNSFAA118234), the Guangxi key scientific and technological project (No: 2013BC26236), and the Projects in Guangxi Health Department (No: GZPT13-27).
Disclosure
The authors declare no competing interests in this work.
References
1. Shabetai R. The Pericardium. Norwell (MA): Kluwer Academic Publishers; 2003:1–50.
2. Ghavidel AA, Gholampour M, Kyavar M, et al. Constrictive pericarditis treated by surgery. Tex Heart Inst J. 2012;39(2):199–205.
3. Clare GC, Troughton RW. Management of constrictive pericarditis in the 21st century. Curr Treat Options Cardiovasc Med. 2007;9(6):436–442. doi:10.1007/s11936-007-0038-x
4. Kosmopoulos M, Liatsou Ε, Theochari C, et al. Updates on the global prevalence and etiology of constrictive pericarditis: a systematic review. Cardiol Rev. 2023. doi:10.1097/CRD.0000000000000529
5. Barua A, Cosbey L, Jeeji R, Balacumaraswami L. Early life threatening constrictive pericarditis following off-pump CABG. J Surg Case Rep. 2023;2023(10):rjad602.
6. Claire D, Etienne M, Chalard A, et al. Recurrent pericarditis: current challenges and future prospects. Res Rep Clin Cardiol. 2016;7:99–108. doi:10.2147/RRCC.S87827
7. Tchana-Sato V, Ancion A, Ansart F, et al. Constrictive pericarditis following cardiac transplantation: a report of two cases and a literature review. Acta Cardiol. 2023;78(7):763–772. doi:10.1080/00015385.2023.2209405
8. Benjamin SR, Mohammad A, Shankar R, et al. Does tuberculosis affect surgical outcomes following pericardiectomy for chronic constrictive pericarditis? Twelve years’ experience from a tertiary care center in India. Indian J Thorac Cardiovasc Surg. 2022;38(3):241–250. doi:10.1007/s12055-021-01313-y
9. Seifert FC, Miller DC, Oesterle SN, et al. Surgical treatment of constrictive pericarditis: analysis of outcome and diagnostic error. Circulation. 1985;72(3 Pt 2):264–273.
10. George TJ, Arnaoutakis GJ, Beaty CA, et al. Contemporary etiologies, risk factors, and outcomes after pericardiectomy. Ann Thorac Surg. 2012;94(2):445–451. doi:10.1016/j.athoracsur.2012.03.079
11. Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108(15):1852–1857. doi:10.1161/01.CIR.0000087606.18453.FD
12. Gopaldas RR, Dao TK, Caron NR, et al. Predictors of in-hospital complications after pericardiectomy: a nationwide outcomes study. J Thorac Cardiovasc Surg. 2013;145(5):1227–1233. doi:10.1016/j.jtcvs.2012.03.072
13. Villavicencio MA, Dearani JA, Sundt TM. Pericardiectomy for constrictive or recurrent inflammatory pericarditis. Oper Tech Thorac Cardiovasc Surg. 2008;13(1):2–13. doi:10.1053/j.optechstcvs.2008.02.001
14. Chowdhury UK, Seth S, Reddy SM. Pericardiectomy for chronic constrictive pericarditis via left anterolateral thoracotomy. Oper Tech Thorac Cardiovasc Surg. 2008;13(1):14–25. doi:10.1053/j.optechstcvs.2008.01.004
15. Tokuta Y, Miyata H, Motomura N, et al. Outcome of pericardiectomy for constrictive pericarditis in Japan: a nationwide outcome study. Ann Thorac Surg. 2013;96(2):571–576. doi:10.1016/j.athoracsur.2013.04.054
16. Maisch B, Seferovic PM, Ristic AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; the task force on the diagnosis and management of pericardial diseases of the European Society of Cardiology. Eur Heart J. 2004;25(7):587–610.
17. Yadav S, Shah S, Iqbal Z, et al. Pericardiectomy for Constrictive Tuberculous Pericarditis: a Systematic Review and Meta-analysis on the Etiology, Patients’ Characteristics, and the Outcomes. Cureus. 2021;13(9):e18252. doi:10.7759/cureus.18252
18. Fang L, Yu G, Huang J, et al. Predictors of postoperative complication and prolonged intensive care unit stay after complete pericardiectomy in tuberculous constrictive pericarditis. J Cardiothorac Surg. 2020;15(1):148. doi:10.1186/s13019-020-01198-9
19. Diethrich EB, Tolls R. Bone around the heart: presentation of a case of chronic constrictive pericarditis and review of recent surgical literature. Cardiovasc Dis. 1977;4(1):37–48.
20. Huang JB, Wen ZK, Yang JR, et al. Analysis of risk factors of early mortality after pericardiectomy for constrictive pericarditis. Heart Surg Forum. 2022;25(1):E056–E064. doi:10.1532/hsf.4329
21. Karima T, Nesrine BZ, Hatem L, et al. Constrictive pericarditis: 21 years’ experience and review of literature. Pan Afr Med J. 2021;38:141. doi:10.11604/pamj.2021.38.141.22884
22. Fang L, Yu G, Ye B, et al. The optimal duration of anti-tuberculous therapy before pericardiectomy in constrictive tuberculous pericarditis. J Cardiothorac Surg. 2021;16(1):313. doi:10.1186/s13019-021-01691-9
23. Shi C, Dong C, Yao L, et al. Anesthesia management for pericardiectomy- a case series study. BMC Anesthesiol. 2023;23(1):191. doi:10.1186/s12871-023-02155-4
24. Matsuura K, Mogi K, Takahara Y. Off-pump waffle procedure using an ultrasonic scalpel for constrictive pericarditis. Eur J Cardiothorac Surg. 2015;47(5):e220–e222. doi:10.1093/ejcts/ezu554
25. Nozohoor S, Johansson M, Koul B, Cunha-Goncalves D. Radical pericardiectomy for chronic constrictive pericarditis. J Card Surg. 2018;33(6):301–307. doi:10.1111/jocs.13715
26. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43(8):1445–1452. doi:10.1016/j.jacc.2003.11.048
27. Depboylu BC, Mootoosamy P, Vistarini N, et al. Surgical treatment of constrictive pericarditis. Tex Heart Inst J. 2017;44(2):101–106. doi:10.14503/THIJ-16-5772
28. Bertazzo B, Cicolini A, Fanilla M, Bertolotti A. Surgical treatment of constrictive pericarditis. Braz J Cardiovasc Surg. 2023;38(3):320–325. doi:10.21470/1678-9741-2022-0302
29. Yeşiltaş MA, Kavala AA, Turkyilmaz S, et al. Surgical treatment of constrictive pericarditis at a single center: 10 years of experience. Acta Chir Belg. 2023;1–7. doi:10.1080/00015458.2023.2216377
30. Shumacker HB. The Evolution of Cardiac Surgery. Bloomington (IN): Indiana University Press; 1992:18.
31. Bozbuga N, Erentug V, Eren E, et al. Pericardiectomy for chronic constrictive tuberculous pericarditis: risks and predictors of survival. Tex Heart Inst J. 2003;30(3):180–185.
32. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10(2):165–193. doi:10.1093/ejechocard/jep007
33. Chowdhury UK, Subramaniam GK, Kumar AS, et al. Pericardiectomy for constrictive pericarditis: a clinical, echocardiographic, and hemodynamic evaluation of two surgical techniques. Ann Thorac Surg. 2006;81(2):522–529. doi:10.1016/j.athoracsur.2005.08.009
34. Syed FF, Schaff HV, Oh JK. Constrictive pericarditis--a curable diastolic heart failure. Nat Rev Cardiol. 2014;11(9):530–544. doi:10.1038/nrcardio.2014.100
35. Huang JB, Wen ZK, Lu WJ, et al. Fluid balance of the second day following operation is associated with early mortality and multiorgan failure after pericardiectomy for constrictive pericarditis. Heart Surg Forum. 2021;24(4):E700–E708. doi:10.1532/hsf.3939
36. Seidler S, Lebowitz D, Muller H. Chronic constrictive pericarditis [in French]. Rev Med Suisse. 2015;11(476):1166, 1168–71.
37. Huang JB, Wen ZK, Yang JR, et al. Incomplete pericardial dissection, fluid overload, delayed diagnosis and treatment, and tuberculosis pericarditis are associated with low cardiac output syndrome following pericardiectomy. Heart Surg Forum. 2022;25(5):E793–E803. doi:10.1532/hsf.4449
38. Lin Y, Zhou M, Xiao J, et al. Treating constrictive pericarditis in a Chinese single-center study: a five-year experience. Ann Thorac Surg. 2012;94(4):1235–1240. doi:10.1016/j.athoracsur.2012.05.002
39. Huang JB, Wen ZK, Lu WJ, et al. Preoperative pericardial effusion is associated with low cardiac output syndrome after pericardiectomy for constrictive pericarditis. Heart Surg Forum. 2021;24(3):E427–E432. doi:10.1532/hsf.3813
40. Goland S, Caspi A, Malnick SD. Idiopathic chronic pericardial effusion. N Engl J Med. 2000;342(19):1449–1450.
41. Szabo G, Schmack B, Bulut C, et al. Constrictive pericarditis: risks, aetiologies and outcomes after total pericardiectomy: 24 years of experience. Eur J Cardiothoracic Surg. 2013;44(6):1023–1028. doi:10.1093/ejcts/ezt138
42. Vistarini N, Chen C, Mazine A, et al. Pericardiectomy for constrictive pericarditis: 20 years of experience at the Montreal Heart Institute. Ann Thorac Surg. 2015;100(1):107–113. doi:10.1016/j.athoracsur.2015.02.054
43. Huang JB, Wen ZK, Lu CC, et al. Acute kidney injury: lessons from pericardiectomy. Heart Surg Forum. 2021;24(4):E656–E661. doi:10.1532/hsf.3869
44. Huang JB, Wen ZK, Yang JR, et al. Analysis of risk factors of multiorgan failure after pericardiectomy for constrictive pericarditis. J Cardiothorac Surg. 2022;17(1):244. doi:10.1186/s13019-022-02007-1
45. Wang J, Yu C, Zhang Y, et al. A prediction model for acute kidney injury after pericardiectomy: an observational study. Front Cardiovasc Med. 2022;9:790044. doi:10.3389/fcvm.2022.790044
46. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100(13):1380–1386. doi:10.1161/01.CIR.100.13.1380
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
