Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Pharmacists’ Attitudes Towards Long-Term Use of Nasal Decongestants: A Cross-Sectional Study
Authors Mokhatrish MM, Almatrafi SD, Aldrees TM, Aldriweesh TA, AlGhamdi FM, Al-Dosary AS, Alhumaydani NK, Aldakkan OZ, Alrudian N, Ali AH
Received 26 November 2023
Accepted for publication 6 March 2024
Published 13 March 2024 Volume 2024:17 Pages 1079—1090
DOI https://doi.org/10.2147/JMDH.S451835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mohammad M Mokhatrish,1 Sharif D Almatrafi,1 Turki M Aldrees,1 Turki A Aldriweesh,1 Fahad M AlGhamdi,2 Abdullah S Al-Dosary,3 Naif K Alhumaydani,3 Osamah Z Aldakkan,3 Naif Alrudian,3 Ali Hassan Ali3
1Department of Otolaryngology-Head and Neck Surgery, College of Medicine, Prince Sattam bin Abdulaziz University, Alkharj, Saudi Arabia; 2Department of Family Medicine & Polyclinics, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia; 3College of Medicine, Prince Sattam Bin Abdulaziz, Alkharj, Saudi Arabia
Correspondence: Mohammad M Mokhatrish, Department of Otolaryngology-Head and Neck Surgery College of Medicine, Prince Sattam bin Abdulaziz University, AlKharj, 5721, Saudi Arabia, Tel +966500689852, Email [email protected]
Background: Rhinitis medicamentosa is a nonallergic inflammation of the nasal mucosa caused by topical decongestants overuse. It mainly affects young and middle-aged adults. Therefore, the aim of this study was to investigate the attitudes of pharmacists regarding the utilization of over-the-counter intranasal decongestants.
Methods: An online cross-sectional study was conducted from November 2021 to January 2022. The target population of the study included pharmacists who work in community pharmacies in Saudi Arabia. Binary logistic regression analysis was used to identify predictors of having positive attitude towards controlling the use of decongestant.
Results: A total of 220 participants were included in this study. Around 15.0% of them reported that ND come with a physician prescription. The majority of the participants (87.3%) reported that the less than 5 days is the maximum safe duration for the use of NDs. Overall, the study participants demonstrated moderately positive attitude towards controlling the use of decongestant with a mean attitude score of 2.5 (standard deviation: 1.2) out of 5; which represents 50.0% of the maximum score. Binary logistic regression analysis identified that pharmacists aged 31– 40 years were two-folds more likely to have positive attitude towards controlling the use of decongestant compared to others (p< 0.05). Around 45.9% of them reported that they recommend other over-the-counter treatments like nasal irrigation, nasal steroids, or antihistamine if they see a patient with RM asking for ND with or without prescription.
Conclusion: The majority of pharmacists in Saudi Arabia demonstrated sufficient awareness and understanding on the adverse effects associated with the excessive use of NDs. Rhinitis medicamentosa can be avoided by appropriate measures, highlighting the importance of raising awareness about the excessive use of decongestants among healthcare professionals and patients alike.
Keywords: rhinitis medicamentosa, pharmacist, attitude, nasal decongestants, Saudi Arabia
Introduction
Nasal decongestants (ND)s are often used for alleviating nasal and sinus congestion, the common cold, seasonal rhinitis, and allergic rhinitis.1 Allergic rhinitis impacts around 10% to 20% of the global population, with a prevalence of 15% to 25% among children and adolescents.2 For certain individuals, fluctuations in humidity, temperature, or exposure to cold or dry air can trigger nasal symptoms like congestion and rhinitis, including nasal blockage, sneezing, a runny nose, and irritation.3 OTC nasal decongestants are commonly used to alleviate symptoms such as sneezing and a runny nose. Decongestants enhance nasal airflow and drainage by inducing vasoconstriction, which decreases congestion and swelling of the nasal mucosa.1 The diagnosis of rhinitis is indicated by the existence of one or more of the subsequent symptoms: nasal congestion, anterior and posterior runny nose, sneezing, and itching.4 In the management of rhinitis symptoms, it is customary to incorporate NDs as part of the therapeutic regimen. Nevertheless, the prolonged utilization of these medications may result in the development of a medical illness known as rhinitis medicamentosa (RM).5,6 The start of RM exhibits variability, with documented occurrences ranging from three days to six weeks after sustained usage of intranasal decongestants.4
Rhinitis medicamentosa, also known as rebound congestion, is within the category of non-allergic rhinitis and encompasses a diverse range of non-IgE-mediated illnesses characterized by various symptoms including nasal congestion, rhinorrhea, sneezing, and postnasal discharge.7 The prevalence of this condition is highest among individuals in the young and middle-aged adult population, with a similar occurrence rate observed between males and females. The prevalence of this particular condition has been reported to range up to 9% in patients attending otolaryngology clinics.6 The initial phase of therapy involves the cessation of the implicated medications. However, the subsequent care of the condition is deficient in terms of comprehensive controlled investigations.8,9 Pharmacists assume a crucial duty in elucidating and assuring the secure use of drugs. A previous research study conducted in the United States investigated pharmacists’ attitudes towards pediatric cough and cold products, including decongestants. The study revealed that most pharmacists were confident in recommending cough and cold products based on its safety and effectiveness.10 There are limited studies in this area in the middle east and Saudi Arabia. Therefore, the aim of this study was to investigate the attitudes of pharmacists regarding the utilization of over-the-counter intranasal decongestants. This might get insights that could aid in addressing the inappropriate use of decongestants and prevent their adverse effects.
Methodology
Study Design
An online cross-sectional study was conducted from November 2021 to January 2022.
Study Population
The target population of the study included pharmacists who work in community pharmacies in all regions of Saudi Arabia. Inclusion criteria were pharmacists who are currently working in Saudi Arabia in community pharmacies setting. There was no restriction on work shift, years of experience or gender. Pharmacists who are working in other pharmaceutical fields such as medical promotion were excluded from the study.
Sample Size
A minimum of 206 participants was required to ensure an 85% confidence level and a 5% margin of error. The sample size of this study was 220 pharmacists.
Data Collection
An online questionnaire was established based on previous literature review11 and distributed via social media platforms (Facebook, WhatsApp, Instagram, and Snap Chat) to pharmacists in Saudi Arabia. Convenience sampling technique was applied to recruit the study participants. This sampling technique is based on the participation of the study participants based on their willingness and availability. Pharmacists who meet the inclusion criteria were asked to read the cover letter of the questionnaire which highlights the importance of the study. They were informed that their completion of the questionnaire is considered as written consent for participation.
The questionnaire segments included demographic information (region of practice, age, gender, qualification, nationality, duration of experience, and practice settings), pharmacists’ practices regarding the prescription of NDs, pharmacists’ awareness regarding the prescription of NDs, pharmacists’ attitudes toward long-term use of NDs (using five questions of 5-point Likert scale format), pharmacists’ perceptions and awareness regarding RM (asking the participants on number of RM cases encountered, action performed if they see patient with RM asking for ND with or without prescription, and what is the best medical specialty that might help patients with RM). The pharmacists’ perception of ND prescription was assessed by 5-point Likert scale questions. The attitude score for the study participants was calculated by summing the scores from each question. The lower the score the more the attitude towards recommending the use of NDs. Besides, the participants were asked about the most prevalent symptoms of patients asking for decongestants and methods of improving public awareness regarding NDs.
A pilot study was done by sending the questionnaire to a selected group of pharmacists who had been omitted from the main study and asked to provide comments and feedback based on their knowledge and attitudes to establish validity. Electronic consent was obtained from the participants at the beginning of the questionnaire.
Ethical Approval
The study was reviewed and approved by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University with the ethical no- (IF-PSAU- 2021/03/18264) and written informed consent was obtained from participants.
Data Analysis
Data were extracted, revised, coded, and analyzed using the statistical software IBM SPSS version 22 (SPSS, Inc. Chicago, IL). All statistical analyses were conducted using two-tailed tests. A P-value less than 0.05 was statistically significant. Descriptive analysis based on frequency and percent distribution was done for all variables, including the pharmacists’ personal data, education level, work setting, and years of experience in the pharmacy field. In addition, pharmacists’ knowledge, attitudes, and practices regarding ND use, prescriptions, and effects were also tabulated. Crosstabulation was used to assess factors associated with pharmacists’ attitudes toward ND prescription. Relations were tested using Pearson’s chi-square test and the exact probability test for small frequency distributions. Binary logistic regression analysis was used to identify predictors of having positive attitude towards controlling the use of decongestant. The dummy variable used to define the dependent variable in the regression model was the mean attitude score for the study participants (which was 2.5 (sd: 1.2)). The significance level was assigned as p-value less than 0.05.
Results
Participants’ Demographic and Practice Settings Characteristics
The survey was completed by 220 pharmacists. Around 30.0% of them were from the Eastern region. The majority of the participants (71.4%) were aged 20–30 years. More than half of them (59.1%) were males. The majority of them (81.8%) reported that they hold bachelor’s degree. The vast majority of them (95.0%) were Saudis. The majority of them (71.4%) reported that they have been practicing pharmacy for less than 5 years. Almost half of the participants (52.7%) reported that they practice in government hospital pharmacy, Table 1.
 |
Table 1 Personal Data of the Study’s Pharmacists in Saudi Arabia |
Pharmacists’ Practices Regarding the Prescription of Nasal Decongestants
Almost one-third of the study participants (34.5%) reported that they receive an average of 3–5 NDs prescriptions per day. Around 15.0% of them reported that ND come with a physician prescription, Table 2. The participating pharmacists reported that nasal obstruction is the most prevalent symptoms of patients asking for decongestants, Figure 1.
 |
Table 2 Pharmacists’ Practices Regarding the Prescription of Nasal Decongestants |
 |
Figure 1 Most prevalent symptoms of patients asking for NDs. |
Pharmacists’ Awareness Regarding the Prescription of Nasal Decongestants
The majority of the participants (87.3%) reported that the less than 5 days is the maximum safe duration for the use of NDs, Table 3.
 |
Table 3 Pharmacists’ Awareness Regarding the Prescription of Nasal Decongestants |
Predictors of Pharmacists’ Positive Attitude Towards Controlling the Use of Decongestant
Participants responses to items that examined their attitude toward long-term use of NDs are shown in Table 4. Overall, the study participants demonstrated moderately positive attitude towards controlling the use of decongestant with a mean attitude score of 2.5 (sd: 1.2) out of 5; which represents 50.0% of the maximum score. Binary logistic regression analysis identified that pharmacists aged 31–40 years were two-folds more likely to have positive attitude towards controlling the use of decongestant compared to others (p<0.05), Table 5.
 |
Table 4 Pharmacists’ Attitudes Toward Long-Term Use of Nasal Decongestants |
 |
Table 5 Predictors of Positive Attitude Towards Controlling Use of Decongestant |
Pharmacists’ Perceptions and Awareness Regarding Rhinitis Medicamentosa
Table 6 presents pharmacists’ perceptions and awareness regarding RM in Saudi Arabia. Around half of the participants (52.7%) reported that they see in their practice on average less than 10 cases of RM per year. Around 45.9% of them reported that they recommend other over-The-counter treatments like nasal irrigation, nasal steroids, or antihistamine if they see a patient with RM asking for ND with or without prescription. The most commonly reported methods of improving public awareness regarding ND in Saudi Arabia was pharmacist education, Figure 2.
 |
Table 6 Pharmacists’ Perceptions and Awareness Regarding Rhinitis Medicamentosa in Saudi Arabia |
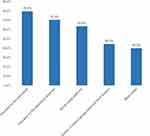 |
Figure 2 Methods of improving public awareness regarding NDs. |
Discussion
According to the findings of our study, it was determined that around 34.5% of the participants reported receiving an average of 3–5 prescriptions for NDs on a daily basis. In the general population of Saudi Arabia, approximately 45.1% reported utilizing NDs.12 Furthermore, within the participants of this study, around 15.0% indicated obtaining NDs through a prescription from a physician. However, although there exists a minority of physicians who prescribe these medications, it has been observed that they are the primary prescribers of NDs, constituting over half of the users, while pharmacists advise approximately one-fifth of the cases.12
Our study findings revealed that around 20.5% of the participants reported a regular practice of checking for RM when prescribed NDs. Moreover, there has been a discernible rise in the global prevalence of RM associated with congestion.13 Consequently, pharmacists frequently include ND medications in prescriptions for rhinitis due to their extensive utilization in the field of rhinology, where they are commonly recommended for the general population.2 Furthermore, the pharmacists who took part in our study indicated that nasal obstruction is the most commonly observed symptom among patients seeking decongestants. In fact, it was found that nasal obstruction accounted for the highest percentage (62.7%) of reported reasons for using NDs among the general population in Saudi Arabia.12 It is worth noting that NDs are recommended for the treatment of acute rhinosinusitis in order to alleviate the distressing consequences of frequent nasal congestion and obstruction.1,8
Furthermore, a significant majority of the participants (87.3%) indicated that a duration of less than five days is considered the maximum safe period for the utilization of NDs. It has been observed that prolonged usage of decongestants can result in histologic alterations in the nasal respiratory mucosa, potentially leading to sinusitis.14–16 Additionally, there are potential risks associated with the improper use of NDs, including serious conditions such as heart attacks and strokes.17,18 However, it is important to note that discontinuing prolonged ND treatment may trigger a rebound congestion, characterized by the return of nasal congestion induced by rhinosinusitis.8 Consequently, it has been suggested that the duration of decongestant therapy should be limited to 3 days for patients with a history of rebound nasal congestion episodes.11
In our study, the mean attitude score was determined to be 2.5, with a standard deviation of 1.2, out of five. This score suggests that the participants exhibited a moderately positive attitude towards regulating the usage of decongestants, accounting for around 50.0% of the maximum attainable score. There are several factors that may contribute to the moderate attitude score observed in this context. One such factor is the availability of NDs for consumption without a prescription, which is influenced by patient-related factors.19 Additionally, there is a lack of general population awareness regarding the use of NDs and their associated side effects in Saudi Arabia. This suggests that there is room for improvement in terms of public knowledge and understanding.20,21
The study findings indicate that binary logistic regression analysis revealed a significant association between age and pharmacists’ attitudes towards regulating decongestant use. Specifically, pharmacists between the ages of 31 and 40 exhibited a twofold higher likelihood of possessing a positive attitude compared to their counterparts. Furthermore, the study suggests that pharmacist attitudes towards regulation of decongestant use tend to increase with greater professional experience.22 This finding aligns with previous research highlighting the importance of effective pharmacist-patient communication in optimizing treatment outcomes.23 Conversely, the study also found that future health professionals may not be adequately prepared to promote health education regarding the rational use of NDs. This lack of preparedness may contribute to insufficient awareness about certain medications, potentially leading to self-medication and associated risks.24
The findings of the study indicate that approximately 52.7% of the participants reported encountering RM in their clinical practice, with an average of less than 10 cases per year. Rhinitis medicamentosa is a prevalent condition that arises as a consequence of excessive and prolonged utilization of topical NDs.6,25 This condition is characterized by a significant obstruction of the nasal airway.26 The study findings indicate that approximately 45.9% of the participants expressed a preference for recommending alternative over-The-counter treatments such as nasal irrigation, nasal steroids, or antihistamines when confronted with a patient seeking ND for RM, with or without a prescription. Previous research has demonstrated that the use of topical nasal corticosteroids can effectively reduce nasal airway resistance in individuals with RM.27,28 Additionally, other treatment approaches, including saline rinses, oral steroids, and antihistamines, are believed to be beneficial in the treatment and management of RM.8,29 However, the primary approach for treating RM is the cessation of ND usage.29 Referral to otolaryngologists, who are specialized clinicians responsible for treating various diseases including RM, is also recommended.11 In our study, we found that 56.4% of participating pharmacists reported otorhinolaryngology as the best medical specialty to assist patients with RM. It is important to note that certain measures can impact and prevent the occurrence of RM, such as the use of warning labels. While warning labels play a crucial role in ensuring patient safety, it is unfortunate that many of these labels are inadequately positioned, small in size, and lack contrast compared to other information. This can contribute to the excessive and prolonged use of NDs.29
Furthermore, the study findings revealed that the predominant method employed to increase public awareness regarding NDs in Saudi Arabia was through pharmacist education. This can be attributed to the fact that community pharmacists in Saudi Arabia offer patient-centered care services.30 Additionally, pharmacists are able to effectively educate their patients during outpatient consultations.31
Our study’s findings underscore the importance of implementing regulatory measures and conducting vigilant oversight of ND administration, in light of the possible hazards that may arise from extended use, including paranasal sinusitis and RM. Physicians should exercise caution in prescribing these medications, placing emphasis on accurate diagnosis and providing patients with comprehensive information regarding the associated risks. Additionally, pharmacists should assume a pivotal role in patient education, offering guidance on appropriate usage and advocating for alternative treatments such as nasal irrigation and steroids. In contrast, it is recommended that public awareness campaigns, which involve the participation of pharmacists, prioritize the dissemination of information regarding the potential hazards associated with excessive utilization. This can be achieved through the implementation of conspicuous warning labels on packaging, as well as the establishment of improved communication channels between pharmacists and patients. These measures are crucial in promoting the safe and responsible use of NDs, thereby contributing to improved health outcomes.
Conclusion
Most pharmacists in Saudi Arabia showed adequate awareness and knowledge about the complications of ND overuse. Rhinitis medicamentosa is a preventable condition; therefore, awareness of decongestant overuse among both healthcare professionals and patients is essential. Diagnosis of this condition can be challenging, and the literature lacks a detailed approach to treating these patients.
Data Sharing Statement
Data are available upon request from the author.
Ethics Approval and Consent to Participate
The study was reviewed and approved by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University with the ethical no- (IF-PSAU- 2021/03/18264) and written informed consent was obtained from participants.
Acknowledgments
The authors extended their appreciation to the deputyship for research and innovation, ministry of education in Saudi Arabia for funding this research work through the project number (IF-PSAU- 2021/03/18264).
Funding
This study is supported via funding from Prince Sattam bin Abdulaziz University project number (IF-PSAU- 2021/03/18264).
Disclosure
The authors declare no competing interests in this work.
References
1. Meltzer EO, Caballero F, Fromer LM, Krouse JH. Scadding G: treatment of congestion in upper respiratory diseases. Int J Gene Med. 2010;3:69–91. doi:10.2147/ijgm.s8184
2. Lenz D, Cardoso K, Bitti A, Andrade T. Evaluation of the use of topic nasal decongestants in university students from health sciences courses. Braz J Pharm Sci. 2011;47(4):761–767. doi:10.1590/S1984-82502011000400013
3. Schroer B, Pien LC. Nonallergic rhinitis: common problem, chronic symptoms. Cleveland Clin j med. 2012;79(4):285–293. doi:10.3949/ccjm.79a11099
4. Dykewicz MS, Wallace DV, Amrol DJ, et al. Steven GC: rhinitis 2020: a practice parameter update. J Allergy Clin Immunol. 2020;146:721–767. doi:10.1016/j.jaci.2020.07.007
5. Graf P. Rhinitis medicamentosa: a review of causes and treatment. Treat Respir Med. 2005;4:21–29. doi:10.2165/00151829-200504010-00003
6. Wahid NWB, Shermetaro C. Rhinitis Medicamentosa. StatPearls. StatPearls Publishing Copyright © 2023, StatPearls Publishing LLC., Treasure Island (FL) ineligible companies; 2023. Disclosure: Carl Shermetaro declares no relevant financial relationships with ineligible companies.
7. Shah R, McGrath KG. Chapter 6: nonallergic rhinitis. Allergy Asthma Proc. 2012;33(Suppl 1):19–21. doi:10.2500/aap.2012.33.3536
8. Mortuaire G, de Gabory L, François M, et al. Rebound congestion and rhinitis medicamentosa: nasal decongestants in clinical practice. Critical review of the literature by a medical panel. Eur Ann Otorhinolaryngology. 2013;130:137–144. doi:10.1016/j.anorl.2012.09.005
9. Varghese M, Glaum MC, Lockey RF. Drug-induced rhinitis. Clin Exp Allergy. 2010;40(3):381–384. doi:10.1111/j.1365-2222.2009.03450.x
10. Huston SA, Porter KB, Clements T, Shepherd G. Pharmacists’ attitudes towards pediatric cough and cold products and behind the counter status. j Pediatric Pharmacol Therapeutics. 2010;15:126–137.
11. Fowler J, Chin CJ, Massoud E. Rhinitis medicamentosa: a nationwide survey of Canadian otolaryngologists. J Otolaryngol. 2019;48:70. doi:10.1186/s40463-019-0392-1
12. Alharthi AS, Alharthi SA, Altowairqi AF, Alswat SH, Alnofaie MF. Assessment of the Prevalence of the Use of Nasal Decongestants Among the General Population in Saudi Arabia. Cureus. 2022;14:e31892. doi:10.7759/cureus.31892
13. Schoenwetter WF. Allergic rhinitis: epidemiology and natural history. Allergy Asthma Proc. 2000;21(1):1–6. doi:10.2500/108854100778248971
14. Min YG, Kim HS, Suh SH, Jeon SY, Son YI, Yoon S. Paranasal sinusitis after long-term use of topical nasal decongestants. Acta oto-laryngologica. 1996;116(3):465–471. doi:10.3109/00016489609137874
15. Suh SH, Chon KM, Min YG, Jeong CH, Hong SH. Effects of topical nasal decongestants on histology of nasal respiratory mucosa in rabbits. Acta oto-laryngologica. 1995;115:664–671. doi:10.3109/00016489509139384
16. Shaikh N, Wald ER. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2014;2014(10):Cd007909. doi:10.1002/14651858.CD007909.pub4
17. Lafaurie M, Olivier P, Khouri C, et al. Myocardial infarction and ischemic stroke with vasoconstrictors used as nasal decongestant for common cold: a French pharmacovigilance survey. Eur J Clin Pharmacol. 2020;76(4):603–604. doi:10.1007/s00228-019-02807-w
18. Grimaldi-Bensouda L, Begaud B, Benichou J, et al. Decongestant use and the risk of myocardial infarction and stroke: a case-crossover study. Sci Rep. 2021;11(1):4160. doi:10.1038/s41598-021-83718-8
19. Stasa Petkovic IM, Djuric S, Dragutinovic N. Olivera Milovanovic: evaluation of Nasal Decongestants by Literature Review. Serb J ExpClin Res. 2019;1–7.
20. Almutairi A, Almutairi H, Althwiny FA, et al. Awareness of the Unaizah populations in Al-Qassim province in Saudi Arabia regarding nasal decongestant use for allergic rhinitis and their side effect. J Family Med Primary Care. 2022;11(3):1070–1076. doi:10.4103/jfmpc.jfmpc_1258_21
21. Alyahya AZ, Almubarak ZA, Al-Khalifah AA, Alawadh AH. Awareness of the Saudi population regarding nasal decongestants use for allergic rhinitis and their side effects. IJMDC. 2020;4:303–308. doi:10.24911/IJMDC.51-1571640524
22. Javadi M, Ashrafi N, Salari P. Assessment of Pharmacists Experiences and Attitudes Toward Professionalism and its Challenges in Pharmacy Practice. Iranian j Pharm Res. 2018;17(Suppl):168–177.
23. Svensberg K, Sporrong SK. Björnsdottir I: a review of countries’ pharmacist-patient communication legal requirements on prescription medications and alignment with practice: comparison of Nordic countries. Res Social Adm Pharm. 2015;11:784–802. doi:10.1016/j.sapharm.2015.01.002
24. Torquato A, Shima V, Araújo D. RISCOS ASSOCIADOS À PRÁTICA DE AUTOMEDICAÇÃO COM DESCONGESTIONANTE NASAL / RISKS ASSOCIATED WITH SELF-MEDICATION PRACTICE WITH NASAL DECONGESTANT. Braz J Dev. 2020;6(11):86899–86917. doi:10.34117/bjdv6n11-206
25. Doshi J. Rhinitis medicamentosa: what an otolaryngologist needs to know. Europ Archiv Oto-Rhino-Laryngol. 2009;266(5):623–625. doi:10.1007/s00405-008-0896-1
26. Black MJ, Remsen KA. Rhinitis medicamentosa. Can. Med. Assoc. J. 1980;122(8):881–884.
27. Bende M. Treatment of Rhinitis Medicamentosa. Am J Rhinol. 1996;10(5):323–326. doi:10.2500/105065896782159639
28. Segboer C, Gevorgyan A, Avdeeva K, et al. Intranasal corticosteroids for non-allergic rhinitis. Cochrane Database Syst Rev. 2019;2019.doi:10.1002/14651858.CD010592.pub2
29. Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Invest Allergol Clin Immunol. 2006;16:148–155.
30. Rasheed M, Hasan S, Babar Z-U-D. Community pharmacist’s knowledge, attitude, roles and practices towards patient-centred care in Saudi Arabia: a systematic review of the literature. J Pharm Health Serv Res. 2018;10. doi:10.1111/jphs.12264
31. Fisher RC. Patient education and compliance: a pharmacist’s perspective. Patient Educ Couns. 1992;19:261–271. doi:10.1016/0738-3991(92)90145-9
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
