Back to Journals » Journal of Blood Medicine » Volume 14
Pharmacokinetic-Pharmacodynamic Comparison of Recombinant and Plasma-Derived von Willebrand Factor in Patients with von Willebrand Disease Type 3
Authors Bauer A, Friberg-Hietala S, Smania G, Wolfsegger M
Received 14 January 2023
Accepted for publication 26 May 2023
Published 13 June 2023 Volume 2023:14 Pages 399—411
DOI https://doi.org/10.2147/JBM.S395845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Alexander Bauer,1 Sofia Friberg-Hietala,2 Giovanni Smania,2 Martin Wolfsegger1
1Statistical and Quantitative Sciences, Baxalta Innovations GmbH, a Takeda Company, Vienna, Austria; 2Pharmetheus AB, Uppsala, Sweden
Correspondence: Alexander Bauer, Baxalta Innovations GmbH, a Takeda company, Donau-City-Straße 7, Vienna, A-1220, Austria, Tel +43 1 201002472997, Email [email protected]
Background: Recombinant von Willebrand factor (rVWF, vonicog alfa, Vonvendi/Veyvondi, Takeda Pharmaceuticals USA, Lexington, MA) and several plasma-derived VWF/factor VIII (pdVWF/FVIII) concentrates are available for treating bleeding episodes in patients with von Willebrand disease (VWD).
Purpose: To develop population pharmacokinetic (PK)/pharmacodynamic (PD) models that describe VWF:ristocetin cofactor (VWF:RCo) activity and its relationship with FVIII activity (FVIII:C) over time following intravenous administration of either rVWF or a pdVWF/FVIII concentrate (VWF:RCo/FVIII:C 2.4:1) in patients with VWD; to use the final PK/PD models for an in silico comparison of rVWF and pdVWF/FVIII.
Methods: The population PK model for rVWF was based on data from four clinical studies in which rVWF was administered to adult patients with VWD type 1, 2 or 3 (phase 1: NCT00816660; phase 3: NCT01410227 and NCT02283268) or severe hemophilia A (phase 1: EudraCT 2011– 004314-42). The PK and PK/PD models for pdVWF/FVIII were based on data from the phase 1 study (NCT00816660) in patients with type 3 VWD who received either rVWF plus recombinant FVIII (rFVIII, octocog alfa, ADVATE®, Takeda Pharmaceuticals USA, Lexington, MA, USA) or pdVWF/FVIII.
Results: There was a marked difference in clearance following rVWF administration compared with pdVWF/FVIII in type 3 VWD, leading to a ~1.75 longer mean residence time (ie, persistence of VWF:RCo activity in the body) and half-life for rVWF versus pdVWF/FVIII. Simulations showed that following repeated administration of rVWF (50 IU/kg), a FVIII:C activity of > 40 IU/dL can be maintained for the full 72 h dosing interval.
Conclusion: The slower elimination of VWF:RCo following rVWF administration results in a prolonged effect on FVIII turnover compared with pdVWF/FVIII administration.
Keywords: factor VIII, pharmacodynamics, pharmacokinetics, plasma-derived, recombinant, von Willebrand factor
Introduction
Von Willebrand disease (VWD) is an inherited bleeding disorder characterized by qualitative or quantitative defects in von Willebrand factor (VWF), a large multimeric glycoprotein that mediates platelet adhesion and stabilizes factor VIII (FVIII) in the circulation.1–4 Recombinant VWF (rVWF, vonicog alfa, Vonvendi® [USA, Japan, Australia] / VEYVONDI™ [EU, UK, Switzerland], Takeda Pharmaceuticals USA, Lexington, MA, USA) has demonstrated efficacy with a favorable safety profile when used for on-demand treatment of hemorrhage and the prevention and treatment of surgical bleeding in adults with VWD.5–8 The efficacy and safety of prophylaxis with rVWF in patients with severe VWD has also been assessed in a phase 3, open-label, nonrandomized trial.9 In that study, rVWF reduced the rate of treated, spontaneous bleeding events in patients previously receiving on-demand VWF therapy by 91.5%.9 Several human plasma-derived VWF (pdVWF)/FVIII concentrates have been approved for the treatment of bleeding episodes in patients with VWD, and their efficacy, safety, and pharmacokinetic (PK) data are published.10–12 Of these, Haemate P®/Humate-P® (pdFVIII/VWF complex [Human]; CSL Behring GmbH, Marburg, Germany: VWF: ristocetin cofactor [RCo]/ factor VIII activity [FVIII:C] 2.4:1) is a widely used pdVWF/FVIII concentrate for the treatment of VWD that is effective in the prevention and control of bleeding in patients with VWD.12–14
Intravenous administration of rVWF corrects VWF platelet binding activity (as measured by VWF:RCo), and increases FVIII:C to hemostatically effective levels within 6 hours (h) in most patients.5,6,8 rVWF replaces VWF without necessarily requiring the coadministration of exogenous FVIII in most of the cases.15 All currently licensed pdVWF products, except for Wilfactin®/Willfact® (LFB, France), contain FVIII, with a ratio of VWF:RCo/FVIII:C varying from 1.1 to 2.54.16 As a result, the pdVWF from these products will stabilize both exogenously administered FVIII (if the product contains it), as well as the endogenous FVIII pool. This may result in FVIII accumulation, especially in patients undergoing surgery who have increased levels of FVIII:C postoperatively as part of the physiological response to the procedure.17,18
The objective of this work was to develop population PK/pharmacodynamic (PD) models that describe VWF:RCo activity and its relationship with FVIII:C over time following intravenous administration of either rVWF or pdVWF/FVIII (VWF:RCo/FVIII:C 2.4:1) in patients with VWD. Then, use the final PK/PD models for an in silico comparison of rVWF and pdVWF/FVIII. An improved understanding of the PK and PK/PD of these products should help with the individualized treatment of patients with VWD based on PK/PD-guided dosing strategies.
Methods
Patients and Data Collection
The population PK model for rVWF was based on four clinical studies in which rVWF was administered in adult patients with VWD type 1, 2 or 3 (phase 1: NCT00816660;19 phase 3: NCT014102276 and NCT02283268)7 or severe hemophilia A (phase 1: EudraCT 2011–004314-42) (Table 1 and Table 2). Patients who developed inhibitory antibodies to VWF or FVIII (titer ≥0.6 Bethesda units using the Bethesda assay) or were co-administered plasma-derived replacement therapy (treatment with pdVWF/FVIII in NCT00816660)19 were excluded from the analysis. In addition, observations associated with a bleeding event were excluded. VWF:RCo and FVIII:C were measured before dosing and over various time points up to 120 h after intravenous rVWF infusion. Because patients in the phase 1 studies received concomitant exogenous FVIII concentrates, the population PK/PD model for rVWF was developed using data from the two phase 3 studies. FVIII activity was quantified by the one-stage clotting assay in all studies.
 |
Table 1 Clinical Studies Contributing Data to the Development of the Population PK/PD Models for rVWF and pdVWF/FVIII |
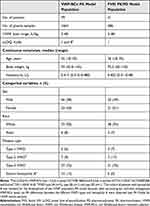 |
Table 2 rVWF Population PK and PK/PD Models: Patient Baseline Characteristics |
The population PK and PK/PD models of pdVWF/FVIII were based on data in patients with type 3 VWD from a single cohort (n=22) of a phase 1, dose escalation study (NCT00816660)19 (Table 1 and Table 2). Patients received single doses of either rVWF and recombinant FVIII (rFVIII; octocog alfa, ADVATE®, 50 IU/kg VWF:RCo/38.5 IU/kg FVIII:C) or pdVWF/FVIII (Humate-P, 50 IU/kg VWF:RCo, VWF:RCo/FVIII:C 2.4:1) in a random, crossover cohort design. VWF:RCo and FVIII:C were measured over various time points up to 96 h following intravenous pdVWF/FVIII infusion.19 Measurements following administration of rVWF and rFVIII were not included in the analysis. Measurements at screening and at the end of the study (30 days × 24 h from the last infusion) were also excluded due to uncertain dosing history at these times. The lower limit of quantification (LLOQ) for VWF:RCo and FVIII:C was 1 IU/dL for all included observations in study NCT00816660.
Model Development
PK and PK/PD models were developed for rVWF (full details in the Supplementary Methods and Supplementary Table 1) and then used to develop models for pdVWF/FVIII. Model development and evaluation were performed in accordance with United States Food and Drug Administration and European Medicines Agency guidelines20,21 and were based on a prespecified statistical analysis plan. Population models were developed using nonlinear mixed-effects modeling as implemented in NONMEM software (NONMEM® version 7.3.0 or 7.4.0; ICON Development Solutions, Ellicott City, MD, USA).22 Data assembly and graphical analyses were performed using R version 3.5.3.23
The final population PK and PK/PD models for the respective products were used to simulate VWF:RCo or FVIII activity–time profiles following repeated administration of rVWF and pdVWF/FVIII (VWF:RCo/FVIII:C 2.4:1). For this analysis, a typical patient with type 3 VWD was defined as having a body weight of 75 kg and a hematocrit level of 0.4 L/L based on the central tendency of the study population.
Results
Population Characteristics
A total of 1664 VWF:RCo samples from 79 patients treated with rVWF in the four clinical studies were available for the PK analysis. Patients treated with rVWF in the two phase 3 VWD studies (n=41) provided 686 FVIII samples for the PK/PD analysis (Table 1). Data from seven patients with antibodies to VWF or FVIII were excluded from the analyses (n=4 binding antibodies to VWF; n=1 inhibitory antibodies to FVIII; n=2 binding antibodies to FVIII IgG). Four VWF:RCo and FVIII:C observations associated with a bleeding event and 308 VWF:RCo and FVIII:C observations collected after the coadministration of plasma-derived therapy were also excluded. The VWF:RCo and FVIII:C analysis populations were heterogenous with respect to demographics and clinical characteristics (Table 2).
A total of 281 samples were available for VWF:RCo and FVIII:C following administration of pdVWF/FVIII to 20 patients. Two patients were excluded from the analysis: one patient had no PK/PD measurements following administration of pdVWF/FVIII and one patient dropped out prior to receiving the pdVWF/FVIII dose. Details of patient baseline characteristics of the pdVWF/FVIII analysis population are shown in Table 3.
 |
Table 3 pdVWF/FVIII Population PK and PD Models: Patient Baseline Characteristics |
PK Model for rVWF
One- and two-compartment disposition models were evaluated for VWF:RCo PK, accounting for endogenous background in VWF:RCo. The final model was a two-compartment model, parametrized in terms of clearance, intercompartmental clearance, central volume of distribution, and volume of distribution for the peripheral compartment. Parameters were allometrically scaled to body weight. As expected, the volume of the central compartment (anticipated to represent blood volume) decreased with increasing hematocrit. Once the individual endogenous VWF:RCo levels were accounted for, no PK differences were observed between the different VWD types and hemophilia A (Supplementary Results).
PK/PD Model for rVWF
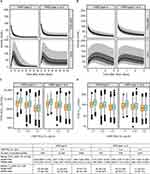
The population PK/PD model was an indirect response model in which VWF:RCo inhibited FVIII elimination, accounting for the delayed FVIII response in relation to rVWF levels (Supplementary Figures 1 and 2). Model evaluation demonstrated that there was good agreement between individual model predictions and observations of VWF:RCo and FVIII:C data after rVWF doses (Figure 1).
The simulated VWF:RCo and FVIII activities following a single rVWF dose of 50 IU/kg and following repeated administration every 72 h, stratified by VWD type, are shown in Figure 2A and B. Summary measures of FVIII exposure were computed from the simulated profiles in terms of area under the activity–time curve during the dosing interval (AUC72) and maximum activity (Cmax) after a single dose and at steady state, and their relationship with VWF:RCo clearance is shown in Figure 2C and D. The simulations at steady state indicated that the median FVIII:C in patients with type 3 VWD was between 40 and 100 IU/dL, and that at least 80% and 97.5% of the treated population would achieve maximal FVIII plasma levels below 150 and 250 IU/dL, respectively.
PK Model for pdVWF/FVIII
The starting point for the population PK model of pdVWF/FVIII was the PK model developed for rVWF; detailed results of the PK modeling for pdVWF/FVIII are provided in the Supplementary Results. Goodness-of-fit plots demonstrated that the model for pdVWF/FVIII adequately predicted the observed VWF:RCo (Figure 3A), and FVIII:C (Figure 3B), respectively. Subsequently, there is no indication of a marked model misspecification in these plots.
The primary PK parameter estimates for the pdVWF/FVIII model are presented alongside estimates for the rVWF model in Table 4. There was a marked difference in clearance between the rVWF model and pdVWF/FVIII model (2.10 dL/h vs 4.14 dL/h, respectively), which led to a 1.76 longer mean residence time (MRT, which represents the persistence of VWF:RCo activity in the body) and 1.74 times longer half-life (t½) for rVWF than for pdVWF/FVIII. The VWF:RCo AUC0-inf was estimated to be 1.97 times greater following rVWF administration than after pdVWF/FVIII.
 |
Table 4 Parameter Estimates of the Population PK (VWF:RCo) and PK/PD (FVIII) Models |
The estimated interindividual variability (IIV) in VWF:RCo clearance following pdVWF/FVIII administration was 23.5% versus 40.1% following rVWF administration. There was considerable uncertainty in the estimated IIV for the pdVWF/FVIII model, with a relative standard error of 38.3% versus 9.9% for the rVWF model.
PK/PD Model for pdVWF/FVIII
The starting point for the population PK/PD model of pdVWF/FVIII was the indirect response model developed for rVWF; additional details are provided in the Supplementary Results. Parameter estimates of the final PK/PD model are shown in Table 4. Estimated maximum inhibition (Imax) values were similar in both the rVWF and pdVWF/FVIII models, but activity level at half maximum inhibition (IC50) was lower in the pdVWF/FVIII model than in the rVWF model (0.0577 IU/dL vs 0.0658 IU/dL). A volume of distribution term for FVIII (FVIII V) was added to account for the distribution of the pdFVIII dose. The FVIII V was estimated to be 32.9 dL, which is in line with a previous report (32.8 dL).24
PK and PK/PD Model Simulations
PK/PD model simulations of the typical VWF:RCo and FVIII:C profiles following repeated administration of rVWF 50 IU/kg or pdVWF/FVIII 50 IU/kg every 72 h are shown in Figure 4A. There was a modest accumulation of VWF:RCo and FVIII:C with both treatments. The simulations suggest that a FVIII:C above 40 IU/dL can be maintained for the full 72 h dosing interval for a typical patient at this dose of rVWF, in line with recommendations from the European Medicines Agency.25
Additional simulations with an irregular dosing interval of 72 h and 96 h are shown in Figure 4B, reflecting how prophylactic rVWF treatment of patients with VWD could be administered in clinical practice. FVIII activity–time profiles for rVWF followed a similar trend as that for regular dosing (interval of 72 h), and as expected, only trough levels in the 96 h interval were below 40 IU/dL (unlike in hemophilia A,26 FVIII trough levels are not well established in VWD). The ratio of the elimination FVIII t½ for rVWF treatment to the FVIII t½ for pdVWF/FVIII ranged from 2.4 to 3.8 during a dosing interval of 72 h, demonstrating a marked difference in the impact on FVIII:C between the two products (Figure 5A and B).
Discussion
A personalized approach to the management of VWD has several benefits, as previously discussed.27,28 A population PK/PD model in which individual PK data can be more easily considered when making dosage recommendations in VWD is important to facilitate this approach. The population PK and PK/PD models for rVWF developed in this study predict VWF:RCo and FVIII activity over time in patients with severe VWD type 1, 2 and 3 treated with rVWF. Patients with VWD type 3 have a total or near-total absence of VWF in the circulation, while patients with severe VWD types 1 and 2 have reduced VWF in the circulation.1,29 The population PK and PK/PD models developed for rVWF were refined to fit pdVWF/FVIII (VWF:RCo/FVIII:C 2.4:1) data for a similar VWD type 3 patient population. The population PK for VWF:RCo following a single dose of rVWF or pdVWF/FVIII was adequately described by a two-compartment disposition model with first-order elimination from the central compartment (Supplementary Figure 1). This type of modeling is often used in PK analyses to represent the distribution of a drug in the body occurring between two compartments: a central compartment (commonly representing blood and well-perfused organs), and a peripheral compartment (commonly representing poorly perfused tissues).30 Our findings demonstrated a slower clearance of VWF:RCo following rVWF compared with pdVWF/FVIII as indicated by longer MRT and t½ values and a larger VWF:RCo AUC0-inf with rVWF; this results in a prolonged effect on FVIII turnover with rVWF compared with pdVWF/FVIII.
Several published studies have investigated the population PK of pdVWF.17,24,31 However, these studies did not quantify the PK/PD relationship in a formal model. The current model-based analysis is in good agreement with the findings of an exploratory noncompartmental analysis,32 which was based on the observed VWF:RCo exposure data from patients with severe VWD who were administered rVWF in phase 1 (NCT00816660)19 and phase 3 on-demand (NCT01410227)6 studies. That exploratory noncompartmental analysis also showed longer MRT and t½ values and a larger AUC0-inf for rVWF versus pdVWF/FVIII with respect to VWF:RCo.32 A longer t½ and larger AUC0-inf of VWF:RCo following rVWF could potentially allow for lower doses and fewer infusions. In a recent multicenter, retrospective study conducted in France in patients with VWD who underwent surgery, a low number of infusions and low doses of rVWF (median [range] total dose: major surgery, 108 [22–340] rVWF IU/kg; minor surgery 37 [12–288] rVWF IU/kg) provided effective prevention of bleeding in major and minor surgeries.33 Our results also suggest that rVWF can be administered without FVIII in most patients receiving surgery. In some cases where the FVIII:C level is <40 IU/dL, it may be necessary to administer a rFVIII product to achieve a hemostatic plasma level of FVIII:C.5,8
The present results suggest a greater stabilization of endogenous FVIII due to slower elimination of VWF:RCo following rVWF administration compared with pdVWF/FVIII. This is in line with VWF acting as a chaperone for FVIII and protecting it from rapid clearance.1 The longer FVIII t½ following rVWF administration compared with pdVWF/FVIII might relate to differences in the VWF multimer profile of the two products. rVWF has a nonproteolytically degraded VWF multimer structure at administration and includes the most hemostatically active ultra-large multimers, which are not present in pdVWF/FVIII concentrates due to exposure to ADAMTS13 during manufacture.34 pdVWF products contain a number of plasma-derived extraneous proteins, some including human albumin, that might lower their specific activity.34
Because patients with type 3 VWD have a total or near-total absence of VWF, they are at an increased risk of bleeding, which can be life-threatening or lead to long-term complications.1,29 International management guidelines for VWD conditionally recommend long-term prophylaxis in patients with VWD and a history of severe and frequent bleeds.29 We investigated VWF:RCo and FVIII:C profiles relevant to a prophylactic dosing regimen (ie, every 72 or 96 h). Simulations showed that following repeated administration of rVWF 50 IU/kg, FVIII:C >40 IU/dL can be maintained for the full 72 h between dosing, consistent with recommendations from the EMA.25 Also based on the simulations in our study, a dosing schedule with an interval of 72 or 72/96 h could be a suitable option for prophylaxis in line with previous studies that demonstrate the potential for prophylaxis to help reduce bleed rates.35–38
Several studies have demonstrated that repeated dosing of pdVWF/FVIII concentrates with a VWF:RCo/FVIII:C ratio of >1, results in less FVIII accumulation if VWF concentrate dosing is based only on VWF levels.39,40 However, FVIII accumulation has been observed after perioperative treatment with pdVWF/FVIII complex (human, VWF:RCo/FVIII:C 2.4:1).41 In this work, simulations of the VWF:RCo and FVIII:C profiles following repeated administration of rVWF 50 IU/kg or pdVWF/FVIII 50 IU/kg (2.4:1 VWF/FVIII ratio) with either regular or irregular dosing intervals did not indicate marked FVIII accumulation. The aim of the simulations was to mimic VWF prophylaxis treatment regimens of approximately twice-weekly administration. More frequent dosing, such as during surgery, might increase the risk of factor VIII accumulation.42
A study limitation was that the PK/PD comparison of rVWF and pdVWF/FVIII was based on a single pdVWF/FVIII (Humate-P, VWF:RCo/FVIII:C 2.4:1), so the study findings may not be applicable to other pdVWF concentrates with FVIII or to Wilfactin/Willfact (LFB, Les Ulis, France), which contains very little FVIII.43 In addition, an in silico analysis of PK and PK/PD cannot fully reproduce in vivo interactions and due to the small number of patients in the current study, a formal covariate analysis was not possible for the pdVWF/FVIII PK model. However, given the mechanistic plausibility of the covariates identified for the rVWF model (body weight and hematocrit), these were applied also for the pdVWF/FVIII model.
Conclusion
The population PK and PK/PD models reported herein allowed for the in silico comparison of rVWF and pdVWF/FVIII with respect to the relationship between VWF:RCo and FVIII:C following administration of either product. The slower elimination of VWF:RCo following rVWF administration results in a prolonged effect on FVIII turnover compared with pdVWF/FVIII. The improved understanding of the PK and PK/PD of these products should help with the individualized treatment of patients with VWD based on PK/PD-guided dosing strategies.
Abbreviations
AUC72, area under the activity–time curve during the dosing interval; Cmax, maximum activity; FVIII, factor VIII; FVIII:C, FVIII activity; FVIII V, volume of distribution for FVIII; IC50, activity level at half maximum inhibition; Imax, maximum inhibition; IIV, interindividual variability; LLOQ, lower limit of quantification; MRT, mean residence time; PD, pharmacodynamic; pdFVIII, plasma-derived FVIII; pdVWF, plasma-derived VWF; PK, pharmacokinetic; RCo, ristocetin cofactor; rFVIII, recombinant FVIII; rVWF, recombinant VWF; t½, half-life; VWD, von Willebrand disease; VWF, von Willebrand factor.
Data Sharing Statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization. Requests should be sent to the corresponding author, Alexander Bauer.
Ethics Approval and Informed Consent
All trials contributing data for the research reported herein were approved by the respective institutional review boards or independent ethics committees at all participating sites (Supplementary Table 2), and patients provided written informed consent. The trials were conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors acknowledge Bettina Ploder from Baxalta Innovations GmbH, a Takeda company, Vienna, Austria, who contributed to the conception and design of the study, and to the data collection and analysis of the data. The authors also acknowledge Björn Mellgard from Takeda Development Center Americas Inc., Cambridge, MA, USA, who contributed to the interpretation of the data, and Zhaoyang Li and Indranil Bhattacharya from Takeda Development Center Americas, Cambridge, USA, who provided expert review of the rVWF model data during the development of the manuscript. Medical writing support was provided by Nasser Malik, PhD, employee of Excel Medical Affairs (Fairfield, CT, USA), and was funded by Takeda Development Center Americas, Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was funded by Baxalta Innovations GmbH, a Takeda company, Vienna, Austria, and Baxalta US Inc., a Takeda company, Lexington, MA, USA.
Disclosure
AB and MW are employees of Baxalta Innovations GmbH, a Takeda company, and are Takeda shareholders. SF-H is a co-owner and employee of Pharmetheus AB and GS is an employee of Pharmetheus AB. Both SF-H and GS have received funding from Baxalta Innovations GmbH and Baxalta US Inc for the conduct of this research. The authors report no other conflicts of interest in this work.
References
1. Leebeek FW, Eikenboom JC. Von Willebrand’s disease. N Engl J Med. 2016;375(21):2067–2080. doi:10.1056/NEJMra1601561
2. Mannucci PM. Treatment of von Willebrand’s disease. N Engl J Med. 2004;351(7):683–694. doi:10.1056/NEJMra040403
3. Budde U, Metzner HJ, Müller HG. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost. 2006;32(6):626–635. doi:10.1055/s-2006-949668
4. Reininger AJ. The function of ultra-large von Willebrand factor multimers in high shear flow controlled by ADAMTS13 [Die Funktion von ultragroßen von-Willebrand-Faktor-Multimeren und ihre Regulation durch ADAMTS 13 unter Flussbedingungen mit sehr hohen Scherraten]. Hamostaseologie. 2015;35(3):225–233. doi:10.5482/HAMO-14-12-0077
5. Baxalta US Inc. VONVENDI (von Willebrand factor [recombinant]) lyophilized powder for solution for intravenous injection. Available from: https://www.fda.gov/media/94863/download.
6. Gill JC, Castaman G, Windyga J, et al. Hemostatic efficacy, safety, and pharmacokinetics of a recombinant von Willebrand factor in severe von Willebrand disease. Blood. 2015;126(17):2038–2046. doi:10.1182/blood-2015-02-629873
7. Peyvandi F, Mamaev A, Wang JD, et al. Phase 3 study of recombinant von Willebrand factor in patients with severe von Willebrand disease who are undergoing elective surgery. J Thromb Haemost. 2019;17(1):52–62. doi:10.1111/jth.14313
8. Baxalta Innovations GmbH. VEYVONDI (vonicog alfa). Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/veyvondi-epar-product-information_en.pdf.
9. Leebeek FWG, Peyvandi F, Escobar MA, et al. Recombinant von Willebrand factor prophylaxis in patients with severe von Willebrand disease: phase 3 study results. Blood. 2022;140(2):89–98. doi:10.1182/blood.2021014810
10. Bello IF, Yuste VJ, Molina MQ, Navarro FH. Fanhdi, efficacy and safety in von Willebrand’s disease: prospective international study results. Haemophilia. 2007;13(Suppl 5):25–32. doi:10.1111/j.1365-2516.2007.01570.x
11. Rivard GE, Aledort L. Alphanate surgical investigators. Efficacy of factor VIII/von Willebrand factor concentrate Alphanate in preventing excessive bleeding during surgery in subjects with von Willebrand disease. Haemophilia. 2008;14(2):271–275. doi:10.1111/j.1365-2516.2007.01616.x
12. Thompson AR, Gill JC, Ewenstein BM, Mueller-Velten G, Schwartz BA; Humate-P Study Group. Successful treatment for patients with von Willebrand disease undergoing urgent surgery using factor VIII/VWF concentrate (Humate-P). Haemophilia. 2004;10(1):42–51. doi:10.1046/j.1351-8216.2003.00809.x
13. Federici AB, Castaman G, Franchini M, et al. Clinical use of Haemate P in inherited von Willebrand’s disease: a cohort study on 100 Italian patients. Haematologica. 2007;92(7):944–951. doi:10.3324/haematol.11124
14. Franchini M, Rossetti G, Tagliaferri A, et al. Efficacy and safety of factor VIII/von Willebrand’s factor concentrate (Haemate-P) in preventing bleeding during surgery or invasive procedures in patients with von Willebrand disease. Haematologica. 2003;88(11):1279–1283.
15. Castaman G, Linari S. Vonicog alfa for the treatment of von Willebrand disease. Expert Opin Orphan Drugs. 2016;4(5):549–554. doi:10.1517/21678707.2016.1171138
16. Castaman G. Treatment of von Willebrand disease with FVIII/VWF concentrates. Blood Transfus. 2011;9(Suppl 2):s9–s13. doi:10.2450/2011.003S
17. Hazendonk HC, Heijdra JM, de Jager NCB, et al. Analysis of current perioperative management with Haemate® P/Humate P® in von Willebrand disease: identifying the need for personalized treatment. Haemophilia. 2018;24(03):460–470. doi:10.1111/hae.13451
18. Lethagen S, Kyrle PA, Castaman G, Haertel S, Mannucci PM; HAEMATE P Surgical Study Group. von Willebrand factor/factor VIII concentrate (Haemate P) dosing based on pharmacokinetics: a prospective multicenter trial in elective surgery. J Thromb Haemost. 2007;5(7):1420–1430. doi:10.1111/j.1538-7836.2007.02588.x
19. Mannucci PM, Kempton C, Millar C, et al. Pharmacokinetics and safety of a novel recombinant human von Willebrand factor manufactured with a plasma-free method: a prospective clinical trial. Blood. 2013;122(5):648–657. doi:10.1182/blood-2013-01-479527
20. European Medicines Agency. Guideline on reporting the results of population pharmacokinetic analyses. CHMP/EWP/185990/06. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-reporting-results-population-pharmacokinetic-analyses_en.pdf.
21. US Food and Drug Administration. Population pharmacokinetics. Guidance for industry. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics.
22. Beal SL, Sheiner LB, Boeckmann AJ, Bauer RJ. NONMEM Users’ Guides (1989–2014). Icon Development Solutions; 2014.
23. R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2019.
24. de Jager NCB, Bukkems LH, Heijdra JM, et al. One piece of the puzzle: population pharmacokinetics of FVIII during perioperative Haemate P® /Humate P® treatment in von Willebrand disease patients. J Thromb Haemost. 2020;18(2):295–305. doi:10.1111/jth.14652
25. European Medicines Agency. Guideline on the core SPC for human plasma derived von Willebrand factor (CPMP/BPWG/278/02). Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003423.pdf.
26. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia. Haemophilia. 2020:26(Suppl 6);1–158. doi:10.1111/hae.14046
27. Favaloro EJ. Towards personalised therapy for von Willebrand disease: a future role for recombinant products. Blood Transfus. 2016;14(2):262–276. doi:10.2450/2016.0258-15
28. Phua CW, Berntorp E. A personalized approach to the management of VWD. Transfus Apher Sci. 2019;58(5):590–595. doi:10.1016/j.transci.2019.08.009
29. Connell NT, Flood VH, Brignardello-Petersen R, et al. ASH ISTH NHF WFH 2021 guidelines on the management of von Willebrand disease. Blood Adv. 2021;5(1):301–325. doi:10.1182/bloodadvances.2020003264
30. Mansoor A, Mahabadi N. Volume of Distribution. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK545280/.
31. Bukkems LH, Heijdra JM, de Jager NCB, et al. Population pharmacokinetics of the von Willebrand factor-factor VIII interaction in patients with von Willebrand disease. Blood Adv. 2021;5(5):1513–1522. doi:10.1182/bloodadvances.2020003891
32. European Medicines Agency. Veyvondi, summary of product characteristics and public assessment report. Available from: https://www.ema.europa.eu/en/documents/assessment-report/veyvondi-epar-public-assessment-report_en.pdf.
33. Desprez D, Drillaud N, Flaujac C, et al. Efficacy and safety of a recombinant von Willebrand factor treatment in patients with inherited von Willebrand disease requiring surgical procedures. Haemophilia. 2021;27(2):270–276. doi:10.1111/hae.14242
34. Turecek PL, Mitterer A, Matthiessen HP, et al. Development of a plasma- and albumin-free recombinant von Willebrand factor. Hamostaseologie. 2009;29(Suppl 1):S32–S38. doi:10.1055/s-0037-1617202
35. Abshire T, Cox-Gill J, Kempton CL, et al. Prophylaxis escalation in severe von Willebrand disease: a prospective study from the von Willebrand disease prophylaxis network. J Thromb Haemost. 2015;13(9):1585–1589. doi:10.1111/jth.12995
36. Abshire TC, Federici AB, Alvárez MT, et al. Prophylaxis in severe forms of von Willebrand’s disease: results from the von Willebrand Disease Prophylaxis Network (VWD PN). Haemophilia. 2013;19(1):76–81. doi:10.1111/j.1365-2516.2012.02916.x
37. Berntorp E, Petrini P. Long-term prophylaxis in von Willebrand disease. Blood Coagul Fibrinolysis. 2005;16(Suppl 1):S23–S26. doi:10.1097/01.mbc.0000167659.23262.18
38. Holm E, Abshire TC, Bowen J, et al. Changes in bleeding patterns in von Willebrand disease after institution of long-term replacement therapy: results from the von Willebrand Disease Prophylaxis Network. Blood Coagul Fibrinolysis. 2015;26(4):383–388. doi:10.1097/MBC.0000000000000257
39. Raquet E, Stockschläder M, Dickneite G. Repeated infusions of VWF/FVIII concentrate: impact of VWF:FVIII ratio on FVIII trough and peak levels in a rabbit model. Haemophilia. 2011;17(5):808–814. doi:10.1111/j.1365-2516.2011.02603.x
40. Srivastava A, Serban M, Werner S, Schwartz BA, Kessler CM. Wonders Study Investigators. Efficacy and safety of a VWF/FVIII concentrate (wilate®) in inherited von Willebrand disease patients undergoing surgical procedures. Haemophilia. 2017;23(2):264–272. doi:10.1111/hae.13106
41. Gill JC, Shapiro A, Valentino LA, et al. von Willebrand factor/factor VIII concentrate (Humate-P) for management of elective surgery in adults and children with von Willebrand disease. Haemophilia. 2011;17(6):895–905. doi:10.1111/j.1365-2516.2011.02534.x
42. Peyvandi F, Kouides P, Turecek PL, Dow E, Berntorp E. Evolution of replacement therapy for von Willebrand disease: from plasma fraction to recombinant von Willebrand factor. Blood Rev. 2019;38:100572. doi:10.1016/j.blre.2019.04.001
43. Castaman G, Linari S. Human von Willebrand factor/factor VIII concentrates in the management of pediatric patients with von Willebrand disease/hemophilia A. Ther Clin Risk Manag. 2016;12:1029–1037. doi:10.2147/TCRM.S87543
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.