Back to Journals » Vascular Health and Risk Management » Volume 20
Postoperative Insulin Dose for Cardiac Artery Bypass Graft and Other Cardiac Surgeries in Patients with Type 2 Diabetes: A Retrospective Study
Authors Fukuda Y , Ushigome E, Yamazaki M, Fukui M
Received 28 October 2023
Accepted for publication 30 January 2024
Published 23 February 2024 Volume 2024:20 Pages 59—68
DOI https://doi.org/10.2147/VHRM.S447077
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Yukiko Fukuda, Emi Ushigome, Masahiro Yamazaki, Michiaki Fukui
Department of Endocrinology and Metabolism, Kyoto Prefectural University of Medicine, Graduate School of Medical Science, Kyoto, Japan
Correspondence: Emi Ushigome, Department of Endocrinology and Metabolism, Kyoto Prefectural University of Medicine, Graduate School of Medical Science, 465 Kajii-cho, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto, 602-8566, Japan, Tel +81-75-251-5506, Fax +81-75-252-3721, Email [email protected]
Purpose: Recommendations on perioperative glycemic control in cardiac surgery are based on coronary artery bypass graft surgery (CABG), though coronary artery disease and valvular disease are pathologically distinct. We aimed to compare the postoperative insulin requirement between CABG and other cardiac surgeries in type 2 diabetic patients and identify predictive factors for the maximum postoperative insulin dose.
Patients and Methods: We retrospectively included 60 Japanese patients with diabetes/glucose intolerance (HbA1c > 37 mmol/mol [5.6%]) who were hospitalized for cardiovascular surgery between April 2017 and March 2019. We categorized the subjects into the CABG and non-CABG groups, and performed subgroup analysis on patients who received postoperative insulin therapy.
Results: The CABG group required a significantly higher insulin dose on postoperative days 2, 5, 6, and 7, and a significantly higher maximum postoperative insulin dose (24.6 U vs 9.7 U, P < 0.001) than the non-CABG group. Multivariate linear regression analyses showed that the independent determinants of the maximum postoperative insulin dose were HbA1c and duration of diabetes in the non-CABG group, and HbA1c in the CABG group.
Conclusion: CABG had a higher postoperative insulin requirement than other cardiovascular surgeries; early aggressive insulin therapy is indicated, especially for patients with higher HbA1c levels/longer duration of diabetes.
Keywords: cardiac surgery, coronary artery bypass grafting, insulin dose, postoperative glycemic control, type 2 diabetes
Introduction
Perioperative hyperglycemia is associated with a higher risk of mortality and morbidity, such as infections, acute renal failure, neurological damage, and myocardial infarction.1–3 Several studies have shown the usefulness of glycemic control in improving prognosis and reducing complications during the perioperative period.4
Postoperative hyperglycemia has been investigated in many trials. A recent study reported that acute hyperglycemia in critical illness and hyperglycemia due to chronic diabetes are two distinct pathophysiological conditions.4 Diabetes mellitus increases morbidity and mortality in patients undergoing cardiac surgeries.5,6 The current recommendations for glycemic control in patients undergoing cardiac surgery are primarily based on patients undergoing coronary artery bypass surgery (CABG).3,7 However, various types of cardiovascular surgeries, such as CABG and valve replacement, may not be affected by the same degree of hyperglycemia. This is because some reports have suggested that diabetes is more likely to develop after CABG.8 However, few studies have reported on glycemic control post CABG compared with that after other cardiac surgeries performed in patients with diabetes.9
This study investigated the differences in postoperative glycemic control associated with CABG and other cardiac surgeries. We focused on the required insulin dose for achieving target glucose levels in the postoperative period and aimed to compare the postoperative insulin doses for CABG and other cardiac surgeries in patients with type 2 diabetes.
Materials and Methods
Study Design
We retrospectively evaluated 71 consecutive patients who were hospitalized for cardiovascular surgery and referred from the Department of Cardiovascular Surgery to Diabetology at Kyoto Prefecture University of Medicine between April 1, 2017 and March 31, 2019. The following patients were referred: patients diagnosed with and treated for diabetes, patients with suspected glucose intolerance with HbA1c > 37mmol/mol (5.6%)10,11 and patients who were previously treated with anti-diabetic drugs (as per information from patient medical records). The exclusion criteria were as follows: patients who did not undergo surgery, patients with glutamic acid decarboxylase (GAD) antibody and patients who could not continue diet therapy. To compare the effects of different types of surgery on insulin levels, patients with postoperative complications such as using mechanical auxiliary devices or infection, were excluded. All procedures were approved by the Kyoto Prefectural University of Medicine Medical Ethics Committee and were conducted in accordance with the Declaration of Helsinki. Informed consent was obtained by giving patients the choice to opt out on the website (https://www.h.kpu-m.ac.jp/doc/aboutus/clinical-research.html). Those who opted out were excluded. Patients underwent the following procedures: CABG alone (n = 20) or with valve surgery (n = 5), valve surgery alone (n = 24), arterial bypass (n = 8), Bentall surgery (n = 1), removal of left atrial myxoma (n = 1), and surgical closure of an atrial septal defect (n = 1). We categorized all subjects into the CABG group or non-CABG group (non-coronary artery bypass graft surgery). We performed a subgroup analysis among patients who required insulin therapy in the postoperative period to compare the dose of insulin required for achieving target glucose levels in the two groups.
Data Collection
We collected demographic data, including sex, age, height, weight, smoking history, duration of diabetes, preoperative diabetic therapy and operation time. We also collected laboratory data pertaining to hemoglobin A1c (HbA1c), creatinine (Cr), fasting blood glucose (FBG), GAD antibody, fasting C-peptide immunoreactivity (F-CPR), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and urinary albumin excretion (UAE) using MODULAR ANA-LYTICS (Hitachi High-Technologies Corp. Ltd., Tokyo, Japan). Hypertension was defined as systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg or requiring treatment with specific antihypertensive medications. Dyslipidemia was defined as TG > 1.7 mmol/L, LDL-C > 3.6 mmol/L, HDL-C < 1.0 mmol/L, or requiring treatment with specific agents for hyperlipidemia. Smoking status was categorized into three groups (never smoker, ex-smoker, and current smoker). Body mass index (BMI) was calculated as body weight in kilograms divided by the square of the participant’s height in meters. Preoperative diabetic therapy was categorized into four groups (only diet therapy, only oral medication, a glucagon-like peptide 1(GLP-1) analog, and insulin therapy). We calculated the C-peptide immunoreactivity (CPR) index to assess insulin secretion. The CPR index was calculated using the following formula:
CPR index = FBG/ fasting CPR ×100
We calculated the C-peptide immunoreactivity-insulin resistance (CPR-IR) to assess insulin resistance.12 The CPR-IR was calculated using the following formula:
CPR-IR = FBG × (F-CPR - 1.32)/ 78.6.
Blood Glucose (BG) Monitoring and Management
After surgery, serum BG was measured every 2 – 4 h in patients in the intensive care unit (ICU). It was aimed to be maintained at > 3.9 mmol/L and < 10.0 mmol/L using a single sliding scale with continuous intravenous insulin infusion. After transfer from the ICU to the general ward, capillary BG was measured four times per day, before every meal and before going to bed. The mean BG was defined as the mean value of the BG measurements on each day. The BG was maintained by the subcutaneous injection of insulin aspart and insulin glargine as part of a basal plus correction insulin regimen, followed by basal insulin plus premeal insulin (basal-bolus insulin therapy). Insulin levels were recorded daily from admission to discharge. The maximum postoperative insulin level was defined as the highest insulin dose recorded between the first postoperative day to the day of discharge. After surgery, an infusion of extracellular fluid was administered for approximately for one week. There was no instance of the use of high-calorie infusions. For extracellular fluid solutions containing glucose concentration > 5%, one unit of insulin was added to extra cellular fluid solution containing 8 – 10 g of glucose. All the patients fasted on the first day after surgery. Oral intake was initiated for the patients after approval from the attending physician. We checked the percentage of intake of staple food on each day from postoperative days 2 to 7. After surgery, food was resumed, and after confirming that the patient was able to consume food, the hypoglycemic medications administered before the surgery were resumed in sequence.
Statistical Analysis
We divided the participants into the CABG and non-CABG groups based on baseline examination. Continuous variables were expressed as means (± standard deviations) and analyzed using the Student’s t-test. Categorical variables were expressed as numbers and analyzed using the Pearson’s chi-squared test.
Subgroup analysis was performed to investigate the total daily insulin dose and mean glucose level. The Student’s t-test was used to compare the two groups on each day from the first postoperative day to the seventh postoperative day. Student’s t-test was also used to compare the maximum postoperative insulin levels. Because the UAE showed a skewed distribution, a logarithmic transformation was carried out before performing statistical analysis. Pearson’s correlation was used to determine the relationship between the maximum postoperative insulin level and the duration of diabetes, BMI, HbA1c, FBG, logarithm, UAE (log UAE), HDL and operation time. Multivariate linear regression analysis was used to investigate the relationship between maximum postoperative insulin level and the factors that were statistically significant in the univariate analysis and operation time. Missing data were excluded from the analysis. Statistical analyses were performed using JMP version 12.0 software (SAS Institute Inc., Cary, North Carolina), and a P-value < 0.05 was considered statistically significant.
Results
The baseline characteristics of the study population are shown in Table 1. Of the 71 patients, 1 patient did not undergo surgery, 2 patients had GAD antibody, 7 patients developed postoperative infection affecting glycemic control, and 1 patient could not continue diet therapy. They were therefore excluded, and the remaining 60 patients were included in this study (Figure 1). The perioperative mean HbA1c level in both groups (CABG and non-CABG groups) was 50 mmol/mol (6.8%) (P= 0.90). There were no significant differences in age, sex, BMI, FBG, or CPR index between the groups. The operation time was significantly longer in the CABG group than in the non-CABG group. The number of patients in the CABG group who used a cardiopulmonary bypass was 8 (32 %), compared to 26 (74%) in the non-CABG group (P < 0.01). The longer pump time, the higher the insulin requirement (P= 0.02). However, there was no difference in duration of pump use between the CABG and non-CABG groups (82.2 ± 126.5 minutes vs 126 ± 90.3 minutes, P= 0.13).
 |
Table 1 Characteristics of the Study Population |
 |
Figure 1 Inclusion and exclusion flow chart. |
The mean glucose level and insulin dose on each day from postoperative day 1 to postoperative day 7 are shown in Figures 2 and 3. On days 5, 6, and 7, the mean glucose level was significantly higher in the CABG group than in the non-CABG group (Figure 2). Hypoglycemia (BG < 3.9 mmol/L) or symptoms of suspected hypoglycemia were not observed in either of the groups. Patient numbers 10, 11, 12, 15, 15, 14, and 13 (out of 25) in the CABG group and patient numbers 17, 12, 16, 15, 15, 15, and 16 (out of 35) in the non-CABG group required postoperative insulin therapy on postoperative day 1, 2, 3, 4, 5, 6, and 7, respectively (Figure 3). On days 2, 5, 6, and 7, the CABG group required a significantly higher insulin dose than the non-CABG group. The postoperative insulin dose reached its maximum around postoperative day 7 in the CABG group and day 5 in the non-CABG group. The maximum postoperative insulin dose was significantly higher in the CABG group than in the non-CABG group (24.6 U vs 9.7 U, P < 0.001). There was no difference in the mean postoperative CRP values between the two groups (CABG group: 11.2 ± 3.3 mg/dL vs non-CABG group: 9.6 ± 4.0 mg/dL, P= 0.11). The patients were stable postoperatively, and most of them were discharged from the ICU within 24 hours after surgery. Oral intake was started on the second postoperative day if the patient’s general condition was stable So, all patients in the analysis began eating on the postoperative day 2. There was no difference in the percentage of staple food consumed each day from postoperative days 2 to 7 between the two groups. There was no difference in oral medications between the two groups before and after operation (Table 2).
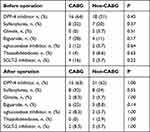 |
Table 2 Oral Medications Before and After Surgery |
In the univariate analysis, preoperative HbA1c and FBG levels were associated with the maximum insulin dose in the CABG group. The duration of diabetes and HbA1c were associated with the maximum insulin dose in the non-CABG group (Table 3). Multivariate linear regression analyses showed that HbA1c was an independent determinant of the maximum postoperative insulin dose in the CABG group, while HbA1c and duration of diabetes were independent determinants of the maximum postoperative insulin dose in the non-CABG group (Table 4). The preoperative CPR-IR was 1.4 in the CABG group and 1.2 in the non-CABG group (P= 0.71). CPR-IR around postoperative day 7 was 2.5 and 1.5 for the CABG and non-CABG groups, respectively (P= 0.42). The increments in CPR-IR after surgery were 1.13 in the CABG group and − 0.23 in the non-CABG group (P= 0.09).
 |
Table 3 Univariate Analyses of Factors Affecting Max Postoperative Insulin |
 |
Table 4 Multivariate Linear Regression Analyses of Factors Affecting Max Postoperative Insulin |
We compared the number of different hypoglycemic medications between the two groups of patients taking oral hypoglycemic medications. The mean number of different hypoglycemic drug medications was 2.13 ± 0.99 in the CABG group and 1.62 ± 0.74 in the non-CABG group, which was not significantly different (P= 0.08). We performed a multivariate logistic regression analysis to determine whether the number of different drug compounds was related to postoperative insulin use. The type of surgery, duration of diabetes and HbA1c levels were used as covariates. While there was an association between the number of hypoglycemic agents and postoperative insulin use, this association was not statistically significant (OR 5.26, 95% CI: 0.95–28.9, P= 0.06).
Discussion
This study showed that patients who underwent CABG required a higher dose of postoperative insulin than those who underwent other cardiac artery surgeries. The postoperative insulin dose was significantly associated with the HbA1c level and duration of diabetes.
Operation is associated with an upregulation of catecholamines, cortisol, and inflammatory cytokines, which subsequently increase insulin resistance and secretion of glucagon and growth hormone.12 These stress hormones lead to increase hepatic glucose production and reduce glucose uptake in peripheral tissues and can result in hyperglycemia3 Then, to manage blood glucose level require increase insulin requirement.
Some studies have reported an association between HbA1c and postoperative glycemic variability13 and major adverse outcomes after cardiac surgery.14 Conversely, a prospective cohort study reported that HbA1c and postoperative glycemic variability were not associated with major adverse events after isolated cardiac valvular surgery.10 The authors of the above mentioned study focused on the difference in the pathophysiology of valvular diseases and coronary artery disease. They emphasized the importance of the etiology of surgical disease and its interaction with diabetes. Another study reported that acute hyperglycemia in critical illness and hyperglycemia due to chronic diabetes are pathologically distinct.5 Pathophysiological differences may influence postoperative insulin metabolism. The pathophysiology of coronary artery disease and other cardiac diseases partly accounts for the difference in results between the previous study and ours.
CABG is performed for coronary artery atherosclerosis. Atherosclerosis of the coronary arteries causes ischemia of the myocardium, leading to chronic inflammation and oxidative stress.15,16 Some of the common pathways include the elevation of inflammatory cytokines such as monocyte chemoattractant protein-1 and interleukin-6 as well as high levels of oxidized low-density lipoprotein in diabetes mellitus, which may in turn induce the secretion of several proinflammatory cytokines from macrophages in atherosclerotic tissues.17 Chronic inflammation is reported to induce insulin resistance and adversely affect glucose metabolism.18 Moreover, oxidative stress leads to impaired glucose uptake in muscle and fat cells and decreases insulin secretion from beta cells.19 Induced insulin resistance and decrease insulin secretion increase insulin requirements for good glycemic control. On the other hand, other cardiac surgeries are performed for diseases less related to coronary atherosclerosis, such as valvular calcification, degenerative valvular disease, congenital defect, and rheumatic heart disease. These dissimilarities can result in the differences in glucose metabolism between CABG and other cardiac surgeries. In this study, the increase in postoperative insulin dose was significantly greater in the CABG group than in the non-CABG group.
A prospective cohort study reported a negative correlation between the HbA1c level and intraoperative insulin sensitivity during cardiac surgery.20 We compared preoperative HbA1c and postoperative insulin requirements. There was a correlation between them (date not shown). In the preoperative stage, there was no difference HbA1c between the two groups (Table 1). The glucose clamp technique is the gold standard for assessing insulin resistance; however, it is a complicated and rare technique. Ohno et al reported that C-peptide immunoreactivity-insulin resistance (CPR-IR) is useful for assessing insulin resistance.21 They reported that CPR-IR could serve as a feasible standard for insulin resistance in patients with type 2 diabetes. In our study, the increments in CPR-IR after surgery tended to be higher in the CABG group around postoperative day 7 (1.13 vs - 0.23, P= 0.09). This suggests the involvement of insulin resistance. Greater changes in insulin resistance may lead to the difficulty of postoperative glycemic control in the CABG group.
In the CABG group, there was a trend toward more oral hypoglycemic medications, although the difference was not statistically significant. The number of oral hypoglycemic drugs may be associated with postoperative insulin use. Moreover, in the CABG group, the disease duration tended to be longer, although there was no significant difference between the two groups. As mentioned earlier, there may be an increase in insulin resistance and a decrease in insulin secretion, which requires a variety of therapeutic agents. These findings suggest that preoperative conditions may also play a role in the need for postoperative insulin therapy. However, in our study, the number of patients who received preoperative insulin therapy was 4 (16.7%) in the CABG group and 5 (14.3%) in the non-CABG group (P= 0.80). The mean preoperative insulin dose was 14.5 U in the CABG group and 10.2 U in the non-CABG group (P= 0.42). Neither group had a correlation between the preoperative insulin dose and postoperative maximum insulin dose (CABG group, P= 0.16; non-CABG group, P= 0.15). In contrast, the increase in the insulin dose from before surgery to after surgery was significantly greater in the CABG group than in the non-CABG group (14.5 U vs - 2 U, P= 0.02). Thus, we considered the possibility that the CABG intervention would require more insulin. In this study, CABG was associated with higher insulin levels, although the reasons for this are unclear. We believe this could be due to either the invasiveness of the CABG procedure or the patients themselves undergoing CABG.
The relationship between blood glycemic variability, such as the amplitude of glycemic excursion (MAGE) and time in range (TIR) and prognosis, has also been reported;22–24 however, in this study, we used HbA1c as an indicator of glycemic control. Several studies have reported that preoperative HbA1c is associated with mortality and adverse cardiac events.25,26 We recommend HbA1c, which is easy to assess, for predicting increased postoperative insulin requirements.
The duration of diabetes tended to be longer in the CABG group, although there was no significant difference between the two groups. This means that insulin resistance may be higher in the CABG group. However, in our study, the number of patients who received preoperative insulin therapy was 4 (16.7%) in the CABG group and 5 (14.3%) in the non-CABG group (P= 0.80). The mean preoperative insulin dose was 14.5 U in the CABG group and 10.2 U in the non-CABG group (P= 0.42). There was no correlation between preoperative insulin dose and postoperative maximum insulin dose in either group (CABG group; P= 0.16, non-CABG group; P= 0.15). On the other hand, the increase in the insulin dose from before surgery to after surgery was significantly greater in the CABG group than in the non-CABG group (14.5 U vs - 2 U, P= 0.02). Thus, we consider the possibility that the CABG intervention would require more insulin.
In the present study, the duration of diabetes was associated with the maximum postoperative insulin dose. Few studies have reported an association between the duration of diabetes and postoperative glucose variability or morbidity after surgery. A recent study reported that in the CABG group, the risk of stroke was higher in patients with a diabetes duration of at least 5 years.27
Anderson R.E.et al showed that cardiopulmonary bypass had a negative effect on glycemic control28 because of steroid and catecholamine use, which induced insulin resistance. In our study, none of the patients received steroids during the intraoperative and postoperative period. Moreover, previous studies have shown that cardiopulmonary bypass increases postoperative blood glucose and insulin consumption.29,30 We compared pump time and insulin requirement in cases with pump. The longer pump time, the higher the insulin requirement. However, there was no difference in duration of pump use between the CABG and non-CABG groups. Rather, pump was used more frequently in the non-CABG group than in the CABG group.
Recent studies have reported hyperglycemia induced with severe illnesses such as acute kidney injury or acute respiratory failure. Some studies have reported an association between acute kidney injury and hyperglycemia.31,32 In our study, there was no difference in postoperative insulin requirement between patients who met the definition of postoperative acute kidney injury33 and other patients (data not shown). Other studies have reported an association between ventilators and hyperglycemia.34 In this study, on postoperative day 3, all patients in the analysis returned to the general ward, weaned from the ventilator. Patients with poor respiratory conditions due to complications, such as pneumonia (n = 2), were not included in the analysis because they met the exclusion criteria for infection.
In this study, the operative time was significantly longer in the CABG group than in the non-CABG group. This may be because the CABG group included CABG with valve replacement and CABG alone.
It is difficult to investigate the effects of inflammation-induced insulin resistance on glycemic metabolism among surgeries, since the location, operation time, and other factors vary between surgeries. In our study, although the postoperative insulin dose was higher in the CABG group, the mean glucose level was also higher in the CABG group than in the non-CABG group. This shows that maintaining sufficient glucose level requires more insulin. Uncontrolled hyperglycemia with glucotoxicity can make it difficult to achieve target glucose levels. Perioperative hyperglycemia impairs neutrophil phagocytosis, increases the likelihood of infection, and leads to delayed wound healing.35 Hyperglycemia also induces endothelial dysfunction, affecting microvascular function, and is critical for wound healing after surgery. Endothelial dysfunction in patients with diabetes may lead to relative tissue ischemia, and reduced microvascular function.36 These factors, along with persistent postoperative hyperglycemia, increase postoperative mortality and morbidity. Several studies have also shown that hormonal and inflammation-induced abnormalities associated with stress hyperglycemia return to normal levels after treatment with insulin and resolution of hyperglycemia.37 Thus, maintaining good glycemic control in the perioperative period improves vascular function, prevents infection and aids wound healing.
In the perioperative period, insulin doses are often adjusted based on blood glucose levels. This can lead to hyperglycemia because the insulin dose increases after the blood glucose level increases. In contrast, aggressive insulin therapy increases the risk of hypoglycemia, leading to increased postoperative morbidity and long term allcause mortality.38 Therefore, we need to individualize our approach according to patient’s background and surgical technique. The ability to predict insulin requirements pre- and postoperatively may be one solution to these problems. Therefore, based on this study, we recommend aggressive insulin therapy in patients undergoing CABG, especially those with high HbA1c levels and long diabetes duration.
This study had several limitations. First, this was a retrospective observational study with small sample size. Hence, the unknown patient selection process may have caused bias. Second, the small sample size limits the statistical power. However, we could detect a statistically significant relationship between HbA1c and/or duration of diabetes and the maximum postoperative insulin dose. Moreover, based on the results of this study, the sample size was 60 patients, the standard deviation of insulin dose for each group was 14.3 U, and the difference between groups was 8.9 U. The two-tailed significance level was set at 0.05, and power was calculated to be 0.92 by paired t-test. Third, we did not measure the levels of stress hormones such as cortisol and catecholamines, or inflammatory cytokines such as IL-6 that affect perioperative glycemic metabolism. Fourth, we were unable to collect the data on adherence to the diet therapy. This may affect glycemic control after hospitalization.
Conclusion
In conclusion, our study showed that patients with diabetes who underwent CABG required a higher dose of postoperative insulin than those who underwent other cardiovascular surgeries. Early aggressive insulin therapy should be undertaken, especially in patients with a higher HbA1c level or longer duration of diabetes.
Acknowledgments
We are grateful to Y Hashimoto for providing technical assistance with the data analysis. We would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vivien L, Kristal RT. Perioperative Management of Patients with Diabetes. Health Serv Insights. 2017;10:1178632917735075. doi:10.1177/1178632917735075
2. Carlos EM, Paul J, Roy OM, Barbara S. Hyperglycemia and Acute Kidney Injury During the Perioperative Period. Curr Diab Rep. 2016;16(1):10. doi:10.1007/s11892-015-0701-7
3. Galindo RJ, Fayfman M, Umpierrez GE. Perioperative Management of Hyperglycemia and Diabetes in Cardiac Surgery Patients. Endocrinol Metab Clin North Am. 2018;47(1):203–222. doi:10.1016/j.ecl.2017.10.005
4. Palermo NE, Garg R. Perioperative Management, of Diabetes Mellitus: novel Approaches. Curr Diab Rep. 2019;19(4):1–7.
5. Siegelaar SE, Devries JH, Hoekstra JB. Patients with diabetes in the intensive care unit; not served by treatment, yet protected? Crit Care. 2010;14(2):13–14.
6. Alexander K, Eilon R, Shany L, et al. Impact of type 2 diabetes mellitus on short- and long-term mortality after coronary artery bypass surgery. Cardiovasc Diabetol. 2018;17(1):151. doi:10.1186/s12933-018-0796-7
7. Marie EMD, Sara MA, Lynn WHLL. A primer for achieving glycemic control in the cardiac surgical patient. J Cardiac Surg. 2012;27(4):470–477.
8. Sailesh L, Krishna KS, Ajeet B, et al. Incidence of new diabetes following CABG surgery: analysis of a single centre registry data. Indian Heart J. 2018;70 Suppl 3(Suppl 3):S221–S223. doi:10.1016/j.ihj.2018.11.017
9. Bardia A, Khabbaz K, Mueller A, et al. The Association between Preoperative Hemoglobin A1C and Postoperative Glycemic Variability on 30-Day Major Adverse Outcomes Following Isolated Cardiac Valvular Surgery. Anesth Analg. 2017;124(1):16–22.
10. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256–261.
11. Hu Y, Liu W, Chen Y, et al. Combined use of fasting plasma glucose and glycated hemoglobin A1c in the screening of diabetes and impaired glucose tolerance. Acta Diabetol. 2010;47(3):231–236.
12. Sivakumar S, Salim RS. Guidelines for Perioperative Management of the Diabetic Patient. Surg Res Pract. 2015;2015:284063. doi:10.1155/2015/284063
13. Masla M, Gottschalk A, Durieux ME, et al. HbA1c and diabetes predict perioperative hyperglycemia and glycemic variability in on-pump coronary artery bypass graft patients. J Cardiothorac Vasc Anesth. 2011;25(5):799–803.
14. Zheng J, Cheng J, Wang T, et al. Does HbA1c Level Have Clinical Implications in Diabetic Patients Undergoing Coronary Artery Bypass Grafting? A Systematic Review and Meta-Analysis. Int J Endocrinol. 2017;2017:56.
15. Bochen Y, Ling-bing M, Meng-lei H, et al. Chronic stress: a critical risk factor for atherosclerosis. J Int Med Res. 2019;47(4):1429–1440. doi:10.1177/0300060519826820
16. Luc K, Schramm-Luc A, Guzik TJ, et al. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70(6):809–824.
17. Massimo F, Anna GB, Francesco B, et al. Stress and Inflammation in Coronary Artery Disease: a Review Psychoneuroendocrineimmunology-Based. Front Immunol. 2018;9:2031.
18. Chen L, Chen R, Wang H, et al. Mechanisms Linking Inflammation to Insulin Resistance. Int J Endocrinol. 2015;2015:57.
19. Maddux BA, See W, Lawrence JC, et al. Protection against oxidative stress-induced insulin resistance in rat l6 muscle cells by micromolar concentrations of α-lipoic acid. Diabetes. 2001;50(2):404–410.
20. Sato H, Carvalho G, Sato T, et al. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95(9):4338–4344.
21. Ohno A, Ueki A, Saito Y, et al. Evaluation of Fasting Blood C Peptide as an Insulin-Resistance Indicator. J Japan Diab Soc. 2004;47(7):515–520.
22. Tomonori A, Daisuke S, Noriaki T, et al. Effects of the Mean Amplitude of Glycemic Excursions and Vascular Endothelial Dysfunction on Cardiovascular Events in Nondiabetic Patients With Coronary Artery Disease. J Am Heart Assoc. 2017;6(5):e004841. doi:10.1161/JAHA.116.004841
23. Jinbo L, Chunsheng C, Yituan X, Li Y. Acute glycemic variability and mortality of patients with acute stroke: a meta-analysis. Diabetol Metab Syndr. 2022;14(1):69. doi:10.1186/s13098-022-00826-9
24. Hiroki S, Michihiro H, Tomomi I, et al. Glucose Variability Based on Continuous Glucose Monitoring Assessment Is Associated with Postoperative Complications after Cardiovascular Surgery. Ann Thorac Cardiovasc Surg. 2017;23(5):239–247. doi:10.5761/atcs.oa.17-00045
25. Salil D, Varun S, Muhammad AS, et al. Preoperative glycaemic control and long-term survival in diabetic patients after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2021;60(5):1169–1177. doi:10.1093/ejcts/ezab180
26. Jia Z, Jing C, Tong W, et al. Does HbA1c Level Have Clinical Implications in Diabetic Patients Undergoing Coronary Artery Bypass Grafting? A Systematic Review and Meta-Analysis Int J Endocrinol. 2017:1537213. doi:10.1155/2017/1537213
27. Gao HY, Zhang EL, Liu QR, et al. Impact of diabetes duration on 3-year clinical outcomes following coronary revascularization. Coron Artery Dis. 2017;28(2):151–158.
28. Anderson RE, Brismar K, Barr G, et al. Effects of cardiopulmonary bypass on glucose homeostasis after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;28(3):425–430.
29. Piotr K, Paweł N, Ewa U, et al. Cardiopulmonary bypass increases postoperative glycemia and insulin consumption after coronary surgery. Ann Thorac Surg. 2009;87(6):1859–1865.
30. A RE, Kerstin B, Gunilla B, et al. Effects of cardiopulmonary bypass on glucose homeostasis after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;28(3):425–430.
31. Side G, Qingbo L, Hui C, et al. Predictive value of stress hyperglycemia ratio for the occurrence of acute kidney injury in acute myocardial infarction patients with diabetes. BMC Cardiovasc Disord. 2021;21(1):157.
32. Michaël PC, Dieter M, Miet RCS. Bench-to-bedside review: metabolism and nutrition. Crit Care. 2008;12(4):222.
33. Ravindra LM, John AK, Sudhir VS, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi:10.1186/cc5713
34. Fahimeh NB, Fatemeh H, Shahram A, et al. Effect of melatonin on stress-induced hyperglycemia and insulin resistance in critically-ill patients: a randomized double-blind, placebo-controlled clinical trial. Caspian J Intern Med. 2022;13(1):51–60.
35. Dandona P, Aljada A, Chaudhuri A, et al. Endothelial dysfunction, inflammation and diabetes. Rev Endocr Metab Disord. 2004;5(3):189–197.
36. Frankie BS, Guillermo EU, Ruben C, et al. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi:10.2337/diabetes.53.8.2079
37. Marie EM, Guillermo EU. Insulin Therapy for the Management of Hyperglycemia in Hospitalized Patients. Endocrinol Metab Clin North Am. 2012;41(1):175–201. doi:10.1016/j.ecl.2012.01.001
38. Elizabeth L, Kathleen S, Irena M, et al. Evaluation of Outcomes and Complications in Patients Who Experience Hypoglycemia After Cardiac Surgery. Endocr Pract. 2017;23(1):46–55. doi:10.4158/EP161427
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


