Back to Journals » Drug Design, Development and Therapy » Volume 16
Predicting Outcome in Combination Treatment of TACE and Camrelizumab for Advanced Hepatocellular carcinoma: Tumor Hypervascularity and Reactive Cutaneous Capillary Endothelial Proliferation
Authors Yin L, Liu KC , Lv WF, Xu SB, Lu D, Zhou CZ, Cheng DL , Gao ZG, Shi CS, Su MX
Received 7 May 2022
Accepted for publication 15 September 2022
Published 30 September 2022 Volume 2022:16 Pages 3421—3429
DOI https://doi.org/10.2147/DDDT.S372276
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Georgios Panos
Liang Yin,1,* Kai-Cai Liu,2,* Wei-Fu Lv,1 Shao-Bao Xu,2 Dong Lu,1 Chun-Ze Zhou,1 De-Lei Cheng,1 Zong-Gen Gao,2 Chang-Sheng Shi,2 Ming-Xue Su2
1Department of Radiology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230022, People’s Republic of China; 2Infection Hospital, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei-Fu Lv, Department of Radiology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, 230000, People’s Republic of China, Email [email protected]
Objective: To validate the robust predictive values of tumor vascularity and reactive cutaneous capillary endothelial proliferation (RCCEP) in combination treatment of transarterial chemoembolization (TACE) and camrelizumab for patients with advanced hepatocellular carcinoma (HCC) and then select the potential candidates who would survive best from such treatment.
Methods: The clinical data of 113 patients with advanced HCC treated with TACE and camrelizumab from January 2019 to December 2021 were analyzed retrospectively. Mann Whitney U-test was used to evaluate the correlation between vascular distribution and RCCEP and tumor response; Kaplan Meier technique was used to evaluate time to progress (TTP) and overall survival (OS), and log rank test was used for comparison; multivariate Cox regression analysis was used to evaluate the related influencing factors.
Results: The TTP and OS of TACE combined with carrelizumab in patients with advanced HCC were 7.1 and 14.3 months. Hypervascularity and development of RCCEP were good predictors of TTP (HR 2.561, P < 0.001; HR 1.486, P = 0.032) and OS (HR 2.854, P < 0.001; HR 1.634, P = 0.011). The median TTP and OS of patients with hypervascularity and RCCEP were 10.6 and 19.3 months, which were better than those with only hypervascularity (6.8 months, P = 0.016; 11.6 months, P = 0.003) and only RCCEP (6.2 months, P = 0.039; 13.5 months, P = 0.042), as well as those with neither (3.8 months, P < 0.001; 7.4 months, P < 0.001).
Conclusion: Tumor hypervascularity and development of RCCEP were favorable predictive factors for the combination treatment of TACE and carrelizumab, with both of which the patients survived longest and might be the potential candidates.
Keywords: hepatocellular carcinoma, transarterial chemoembolization, camrelizumab, vascularity, RCCEP
Introduction
The morbidity and mortality of HCC ranked sixth and third, respectively, in the world’s malignant tumors.1 At present, the clinical treatment methods for HCC are diversified. The more commonly used ones are radical resection, TACE, microwave ablation, etc. For patients with surgical indications, radical resection should be the first choice. TACE is mainly used for the treatment of unresectable intermediate and advanced HCC.2 Although the curative effect of TACE has been confirmed,3,4 Generally, the tumor burden of middle-advanced HCC is relatively large, with abundant collateral circulation and abnormal blood supply, often accompanied by portal vein tumor thrombus and arteriovenous leakage, which often requires multiple TACE embolization in a short time. Moreover, TACE embolization may stimulate blood supply proliferation and reduce tumor reactivity, resulting in unsatisfactory long-term results of TACE.5 At present, TACE-based combination therapy is a research hotspot.6–9
In recent years, immunotherapy for HCC has made great progress, which has significantly improved the prognosis of patients with HCC. Tumor immune escape is an important mechanism for the occurrence and development of malignant tumors. Programmed death-1 (PD-1) is one of the most studied immunosuppressive molecule. Many studies at home and abroad have confirmed that the interaction between PD-1 and its receptor ligands plays an important role in tumor immune escape.10,11 PD-1 inhibitor can block the binding of PD-1 and its receptor ligand, thus reactivating T lymphocytes, producing sustained antitumor effect and inhibiting tumor growth. Carrelizumab is a new PD-1 inhibitor, which has been included in the recommended drug for the treatment of HCC.12 Therefore, the combination of TACE and Carrelizumab may be an effective intervention to make up for the shortage and side effects of TACE alone.13
At present, there are few studies on TACE combined with carrelizumab in the treatment of HCC, and its clinical effect still lacks a large amount of data to support. At the same time, its predictive value for TACE combined with Carrelizumab treatment has never been reported before. Vascular distribution can be used as a predictive marker for TACE treatment of HCC.14,15 Carrelizhu-related adverse events (AES) are widely considered as predictive markers for disease control and survival of patients with advanced HCC, especially RCCEP, which has been found in previous studies.16,17 In this study, we tried to investigate the efficacy of TACE combined with carrelizumab in the treatment of advanced HCC, so as to determine the utility of vascular distribution and RCCEP as robust predictors, so as to select the potential benefit patients of TACE combined with carrelizumab according to these factors.
Materials and Methods
The study was conducted in accordance with the principles set out in the 1964 Helsinki declaration. With the approval of the ethics committee of the First Affiliated Hospital of University of science and technology of China, the ethics committee of the First Affiliated Hospital of University of science and technology of China officially abandoned the informed consent form through retrospective research design and analysis of clinical data. All patient information is confidential.
Clinical Data
A total of 113 patients with advanced HCC diagnosed in our hospital from January 2019 to December 2021 were selected, including 87 males and 26 females, aged from 25 to 80 years, with an average age of (58.63 ± 12.01) years. Inclusion criteria: 1) HCC diagnosed by pathological results and/or imaging examination; 2) BCLC stage is stage B or C; 3) Child-Pugh total score <9; 4) ECoG score ≤2; 5) Unable to tolerate or refuse surgery, radiotherapy or ablation. Exclusion criteria: 1) immune deficiency disease or history of organ transplantation; 2) Severe allergy to carrelizumab; 3) Severe liver and kidney dysfunction and cardiopulmonary disease; 4) Severe coagulation insufficiency; 5) Received systemic chemoradiotherapy and targeted therapy.
Tace
The right femoral artery was punctured with improved Seldinger technique, and hepatic arteriography was performed to observe the size, location and blood supply of the tumor. A 2.7F microcatheter (Progreat™, Terumo, Tokyo, Japan) was inserted into the tumor blood supply artery. Oxaliplatin (100–200 mg) and/or fluorouracil (500–1000 mg) were injected; then, epirubicin (30–60 mg) and 5–25 mL iodized oil mixture were injected under fluoroscopic monitoring, and finally 150–350um gelatin sponge particles were used to enhance embolization. The number of TACE treatments is based on imaging evaluation and performed as needed.
Immunotherapy
Camrelizumab for injection of 200 mg was reconstituted in 5 mL of sterile water, and after reconstitution, injected into 100 mL of 0.9% NS or 5% GS injection for intravenous drip. It was administered once every 3 weeks until intolerable toxicity or disease progression occurred. The allowable interruption time shall not exceed 12 weeks.
Observation Index
The distribution of tumor blood vessels was observed. Definition of hypervascular lesions on DSA (one of the following signs): obvious tumor staining, vasodilation and tortuosity, venous siltation, “ball holding” sign, and clear lesion boundary. Otherwise, it is considered to be a lesion with insufficient vascular volume. The DSA features were confirmed by two independent radiologists. According to the modified response evaluation criteria in solid tumors (mRecist): the disease remission rate (ORR) = complete remission (CR) + partial remission (PR), disease control rate (DCR) = complete remission (CR) + partial remission (PR) + stable (SD) were analyzed. (2) Adverse reactions refer to the common adverse reaction rating standard 3.0 of the National Cancer Institute to describe the occurrence of adverse reactions. (3) Treatment effect is measured by overall survival rate (OS) and time to progression (TTP). OS and TTP are defined as the time from the first combined treatment to death or the last follow-up and the time from the first treatment to tumor progression or death, respectively.
Statistical Analysis
Statistical analysis was performed using SPSS version 19.0 (IBM Corporation, Armonk, New York, USA). The Mann–Whitney U-test was used to compare ordinal and categorical variables. The survival curve was constructed by Kaplan Meier method, and the log rank test was performed to detect the significant difference. Univariate and multivariate Cox proportional hazards regression analysis was used to determine the risk factors affecting survival. To determine the predictive value of vascular distribution and RCCEP. Two-sided p-values <0.05 were considered significant.
Result
Baseline Patient Characteristics
The baseline demographic and clinical characteristics of patients are shown in Table 1. Of the 113 eligible patients, 87 (76.9%) were male; the average age was 58.63 ± 12.01 years; Hepatitis B virus (HBV) is the most common underlying cause of HCC (76.9%). Among them, 45 cases (39.82%) were diagnosed with BCLC-B stage, and 81 cases (71.68%) had ECOG status ≤1. Eighty-eight patients (77.88%) belonged to Child-Pugh A, and 46 patients (40.71%) had a small amount of ascites. None of the 25 patients belonging to Child-Pugh B class had contraindications to TACE. The median diameter of the largest measurable lesion was 7.93 cm, and 51 cases (45.13%) had a single lesion. The median number of TACE treatments was 3 times. All patients received carrelizumab after the first TACE, and the time interval was less than 15 days. For carilizumab treatment, the median administration time was 13.18 months. Fifteen (13.3%) patients had temporary dose interruptions due to adverse events (2 patients due to myocarditis, 8 patients due to general condition or hepatic impairment, 2 patients due to immune pneumonitis, and 3 patients due to other non-disease-related reasons). Finally, 45 patients (39.82%) stopped the continuous treatment of carrelizumab due to disease progression (n = 21), deterioration of liver function (n = 19) or other reasons (n = 5). All included patients did not permanently stop carrelizumab treatment due to adverse events.
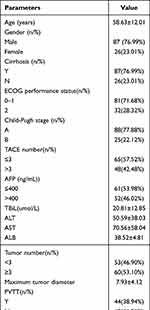 |
Table 1 Baseline Characteristics |
Efficacy Analysis
A total of 113 patients underwent an imaging evaluation 8 weeks after the first TACE. The imaging findings showed complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD) were 24 (21.2%), 41 (36.3%), 37 (32.7%) and 11 (9.7%), respectively. The objective remission rate (ORR) and disease control rate (DCR) were 57.5% and 90.3%, respectively. Subsequent follow-up showed that the median TTP was 7.1 months (range 1.0–24.6 months) (Figure 1A). The median OS was 14.3 months (range 2.5–34.9 months) (Figure 1B).
 |
Figure 1 The time to radiologic progression (A) and survival (B) after combination therapy of TACE and camrelizumab. |
Hypervascularity as a Predictor for TTP and OS
In the whole cohort, 62 patients (54.87%) had obvious tumor staining in DSA. Most (51,82.26%) of the tumor blood supply vessels were dilated and tortuous. Thirty-two cases (51.61%) and 36 cases (58.06%) had venous stasis and “ball holding” sign. The tumor boundary of 60 patients (96.77%) was clear. The analysis showed that the objective remission rate of tumor response in the combination of TACE and carrelizumab in the treatment of hypervascular lesions was better than that of hypovascular lesions (69.35% vs 46.7%, P = 0.009). In addition, patients with hypervascular tumors had greater benefit than those with hypovascular in TTP (9.5 months vs 4.1 months, P = 0.001) and OS (18.1 months vs 10.3 months, P < 0.001) (Figure 2A and B).
RCCEP-Response as a Predictor for TTP and OS
Overall, 104 patients (92.03%) experienced at least one adverse event during this period. The most common camrelizumab-related adverse events were RCCEP (81,71.68%), hypothyroidism (13,11.51%) and impaired cardiac function (3,2.65%). However, most of them were mild (61.53%) or moderate (20.19%). Sixty-eight patients developed RCCEP, all of which were grades II–IV (classified as I–V according to severity) and were considered to be clinically significant RCCEP. The median survival time of its occurrence was 2.3 months, and 45 patients had no RCCEP response. In addition, TTP patients with RCCEP response (9.5 months vs 4.8 months, P = 0.009) and OS patients (16.9 months vs 10.4 months, P = 0.007) were better than patients without RCCEP response (Figure 3A and B).
Combing Vascularity and RCCEP for the Prediction of Outcomes
The combination of vascular and RCCEP response were good predictors of combination therapy. Patients were divided into subgroups: group A included patients with hypervascular and RCCEP response (38 patients); Group B represents patients with hypervascular but no response to RCCEP (30 cases); Group C included patients with hypovascular but RCCEP response (24 cases); Group D included patients with hypovascular and no response to RCCEP (21 cases). The median TTP of groups A, B, C, and D were 10.6, 7.1, 6.2, and 3.8 months, respectively, and the median OS was 19.3, 11.6, 13.5, and 7.4 months, respectively. The TTP and OS of patients in group A were better than the other groups (p = 0.016, p = 0.039, p < 0.001) (p = 0.003, p = 0.042, p < 0.001), and the TTP and OS of patients in group B and C were similar (p = 0.683 and p = 0.491), but TTP and OS in group B and group C were better than those in group D (P = 0.044, P = 0.021), (P = 0.030, P = 0.018) (Figure 4A and B).
Validation and Adjustment in Multivariate Analysis
Although vascular distribution and RCCEP response are important predictors of TTP and OS in TACE combined with carrelizumab in the treatment of advanced HCC, they have not been adjusted by other prognostic factors in multivariate analysis. Therefore, vascular distribution and RCCEP response were included in multivariate analysis of a variety of different TTP and OS. Univariate analysis showed that the prognostic factors of TTP were AFP ≤400/>400 (HR1.425, P = 0.012), tumor size ≤5/>5cm (HR1.318, P = 0.003), vascular distribution (HR2.456, P < 0.001) and RCCEP response (HR1.623, P = 0.021). Multivariate analysis showed that only tumor size ≤5/>5cm (HR1.326, P = 0.006), vascular distribution (HR2.561, P < 0.001) and RCCEP response (HR1.486, P = 0.032). See Table 2. OS multivariate analysis showed that AFP ≤400/>400 (HR1.133, P = 0.002), PVTT (HR1.542, P = 0.017), tumor size ≤5/>5cm (HR1.326, P = 0.004), vascular distribution (HR2.854, P < 0.001) and RCCEP response (HR1.634, P = 0.011) were independent risk factors (Table 3).
 |
Table 2 Prognostic Factors for Time to Radiologic Progression in HCC Patients Treated with Combination Treatment |
 |
Table 3 Prognostic Factors for Overall Survival in HCC Patients Treated with Combination Treatment |
Discussion
TACE has become the best treatment for unresectable advanced HCC. It blocks the blood supply of the tumor and accumulates high concentrations of chemotherapeutic drugs in the tumor to kill tumor cells to the greatest extent.18,19 However, TACE can lead to ischemia and hypoxia of embolic tissue cells, stimulate the expression of VEGF in the residual lesions, thus promoting tumor angiogenesis and causing tumor recurrence and metastasis. At the same time, the long-term chronic inflammatory response of the tumor-bearing liver can upregulate PD-1 receptors and promote the spontaneous apoptosis of immune cells. Therefore, TACE combined with immunotherapy is considered as a new direction of HCC treatment. Previous studies have proved that TACE combined with carrelizumab has certain clinical efficacy in the treatment of advanced HCC.20,21 In this study, the median TTP and OS of the whole cohort were 7.3 months and 14.3 months, respectively, which was better than previous studies using TACE or camrelizumab alone.22–24
Subgroup analysis showed that for HCC patients with hypervascularity and RCCEP response, the median TTP and OS were 10.6 months and 19.3 months, respectively, which was superior to other groups of patients in the cohort study. Relevant studies have proved that the tumor blood supply of HCC is a key factor affecting the efficacy of TACE.14 The richer the tumor blood supply, the more conducive it is to the perfusion of chemotherapy drugs and the filling and deposition of lipiodol emulsion. On the contrary, due to insufficient perfusion of chemotherapy and poor deposition of lipiodol in HCC patients with hypovascularity, TACE treatment cannot fully inactivate tumor tissue and reduce the efficacy of TACE. This study further confirmed that hypervascularity is a predictor of better prognosis in patients with advanced HCC treated with TACE and carrelizumab.
In addition to common rashes and itchiness, RCCEP was one of the most common adverse drug reactions associated with camrelizumab.25,26 In the Phase 2 clinical study of carrelizumab monotherapy for advanced HCC,16 the incidence of RCCEP was 66.8% (145/217), respectively, and all events were grade 1 or 2, which did not lead to the interruption of treatment. It was observed that the incidence of RCCEP was significantly associated with higher objective response rate and prolongation of PFS and OS. Camrelizumab-related RCCEP as a predictor of efficacy in patients with advanced HCC treated with camrelizumab has been confirmed in prospective studies. In this study, we confirmed that the occurrence of RCCEP is not only an adverse reaction of carrelizumab in the treatment of HCC but also an important predictor of better prognosis of TACE and carrelizumab in the treatment of advanced HCC. Previous studies focused on patients treated with carrelizumab alone rather than in combination with TACE; however, from another perspective, the prognostic value of RCCEP in combination therapy validates and further extends the findings of previous studies. In addition, although the occurrence of RCCEP means more survival benefits, the absence of RCCEP does not mean no survival benefits due to the lack of studies comparing these patients with untreated patients.
Our study has some limitations. First, our study is retrospective, and selection bias may exist; Secondly, the sample size of patients is small, and they are single-center patients. The nature of patients may limit its representativeness. In addition, this paper only focuses on the two factors of tumor vascular distribution and immune-related RCCEP. There is no comprehensive analysis on the influencing factors of HCC patients treated with TACE combined with carrelizumab, which needs further prospective research and extended analysis, or more variables need to be included.
In conclusion, TACE combined with carrelizumab has good curative effect in the treatment of advanced HCC. The occurrence of hypervascularity and RCCEP are good predictors of combined treatment. Patients with liver cancer with these two characteristics may be the best candidates. It is helpful for clinical decision-making for the treatment of patients with HCC.
Funding
This work was supported by the Natural Science Project of Anhui Province of China (No. 1808085MH254).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
2. Medical administration of the State Health Commission of the people’s Republic of China. Standard for diagnosis and treatment of primary liver cancer (2019 Edition). Elect J Compr Tumor Ther. 2020;6(02):55–85. Chinese.
3. Korean Liver Cancer Association; National Cancer Center. 2018 Korean liver cancer association-national cancer center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019;13:227–299. doi:10.5009/gnl19024
4. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
5. Liu KC, Hao YH, Lv WF, et al. Transarterial chemoembolization combined with sorafenib in patients with BCLC stage C hepatocellular carcinoma. Drug Des Devel Ther. 2020;14:3461–3468. doi:10.2147/DDDT.S248850
6. Liu J, Zhen L, Zhang W, et al. Comprehensive treatment of transarterial chemoembolization plus lenvatinib followed by camrelizumabfor advanced hepatocellular carcinoma patients. Front Pharmacol. 2021;18(12):709060. doi:10.3389/fphar.2021.709060
7. Qiu Z, Shen L, Jiang Y, et al. Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study. Ann Transl Med. 2021;9(4):283. doi:10.21037/atm-20-5360
8. Wang W, Shen J, Wang C, et al. Safety and feasibility of helical I-125 seed implants combined with transcatheter arterial chemoembolization in hepatocellular carcinomas with main portal vein tumor thrombus. Cardiovasc Intervent Radiol. 2019;42(10):1420–1428. doi:10.1007/s00270-019-02256-z
9. Yoon SM, Ryoo BY, Lee SJ, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4(5). doi:10.1001/jamaoncol.2017.5847
10. Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
11. Fang W, Yang Y, Ma Y. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, Phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi:10.1016/S1470-2045(18)30495-9
12. Wang S, Wu Q, Li X, et al. Interpretation of standards for diagnosis and treatment of primary liver cancer. J Clin Hepatobil Dis. 2020;36:996–999. Chinese.
13. Dufour JF. TACE with or without systemic therapy? J Hepatol. 2012;56(6):1224–1225. doi:10.1016/j.jhep.2012.02.011
14. Katyal S, Oliver JH, Peterson MS, Chang PJ, Baron RL, Carr BI. Prognostic significance of arterial phase CT for prediction of response to transcatheter arterial chemoembolization in unresectable hepatocellular carcinoma: a retrospective analysis. AJR Am J Roentgenol. 2000;175(6):1665–1672. doi:10.2214/ajr.175.6.1751665
15. Hu HT, Kim JH, Lee LS, et al. Chemoembolization for hepatocellular carcinoma: multivariate analysis of predicting factors for tumor response and survival in a 362- patient cohort. J Vasc Interv Radiol. 2011;22(7):917–923. doi:10.1016/j.jvir.2011.03.005
16. Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1). doi:10.1186/s13045-020-00886-2
17. Song G, Zhang -F-F, Cheng H-D. Thalidomide for prevention of camrelizumab-induced reactive cutaneous capillary endothelial proliferation. Australas J Dermatol. 2022;63(2):217–221. doi:10.1111/ajd.13812
18. Liu K-C, Wei-Fu L, Dong L, et al. Initial experience of drug-eluting bead-transcatheter arterial chemoembolization after lipiodol-based transcatheter arterial chemoembolization failure for patients with advanced hepatocellular carcinoma. Cancer Manag Res. 2021;19(13):7973–7980. doi:10.2147/CMAR.S332571
19. Shasha S, Fan X, HailinMa H, et al. Effect of prophylactic TACE on 5-year survival of patients with hepatocellular carcinoma after hepatectomy. Oncol Lett. 2019;18(2):1824–1830. doi:10.3892/ol.2019.10517
20. Guo Y, Ren Y, Chen L, et al. Transarterial chemoembolization combined with camrelizumab for recurrent hepatocellular carcinoma. BMC Cancer. 2022;22(1):270. doi:10.1186/s12885-022-09325-6
21. Zhang J-X, Chen P, Liu S, et al. Safety and efficacy of transarterial chemoembolization and immune checkpoint inhibition with camrelizumab for treatment of unresectable hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;31(9):265–272. doi:10.2147/JHC.S358658
22. Zhiyu Q, Shen L, Chen S, et al. Efficacy of apatinib in Transcatheter Arterial Chemoembolization (TACE) refractory intermediate and advanced-stage hepatocellular carcinoma: a propensity score matching analysis. Cancer Manag Res. 2019;11:9321–9330. doi:10.2147/CMAR.S223271
23. Shimose S, Tanaka M, Iwamoto H, et al. Prognostic impact of Transcatheter Arterial Chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: a comparison to TACE alone using decision-tree analysis after propensity score matching. Hepatol Res. 2019;49(8):919–928. doi:10.1111/hepr.13348
24. Psq A, Pzr B, Pzm C, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580.
25. Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100–2111. doi:10.1002/art.41068
26. Byrne MM, Lucas M, Pai L, et al. Immune-related adverse events in cancer patients being treated with immune checkpoint inhibitors. Eur J Haematol. 2021;107(6):650–665. doi:10.1111/ejh.13703
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



