Back to Journals » Clinical Interventions in Aging » Volume 19
Predictive Value of Lysophosphatidylcholine for Determining the Disease Severity and Prognosis of Elderly Patients with Community-Acquired Pneumonia
Authors Gu M , Lv S, Song Y, Wang H, Zhang X, Liu J, Liu D, Han X , Liu X
Received 10 December 2023
Accepted for publication 13 March 2024
Published 19 March 2024 Volume 2024:19 Pages 517—527
DOI https://doi.org/10.2147/CIA.S454239
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Minghao Gu,1,2 SenSen Lv,1 Yihui Song,3 Hong Wang,4 Xingyu Zhang,5 Jing Liu,1 Deshun Liu,1 Xiudi Han,1 Xuedong Liu1
1Department of Respiratory and Critical Care Medicine, Qingdao Municipal Hospital, Qingdao, 266011, People’s Republic of China; 2School of Medicine, Qingdao University, Qingdao, 266071, People’s Republic of China; 3Department of Neurology, Weihai Municipal Hospital, Weihai, 264200, People’s Republic of China; 4Hospital-Acquired Infection Control Department, Qingdao Municipal Hospital, Qingdao, 266011, People’s Republic of China; 5Human Resources Department, Qingdao Municipal Hospital, Qingdao, 266011, People’s Republic of China
Correspondence: Xuedong Liu; Xiudi Han, Department of Respiratory and Critical Care Medicine, Qingdao Municipal Hospital, Qingdao, 266011, People’s Republic of China, Email [email protected]; [email protected]
Purpose: To investigate the clinical value of serum lysophosphatidylcholine (LPC) as a predictive biomarker for determining disease severity and mortality risk in hospitalized elderly patients with community-acquired pneumonia (CAP).
Methods: This prospective, single-center study enrolled 208 elderly patients, including 67 patients with severe CAP (SCAP) and 141 with non-SCAP between November 1st, 2020, and November 30th, 2021 at the Qingdao Municipal Hospital, Shandong Province, China. The demographic and clinical parameters were recorded for all the included patients. Serum LPC levels were measured on day 1 and 6 after admission using ELISA. Propensity score matching (PSM) was used to balance the baseline variables between SCAP and non-SCAP patient groups. Receiver operative characteristic (ROC) curve analysis was used to compare the predictive performances of LPC and other clinical parameters in discriminating between SCAP and non-SCAP patients and determining the 30-day mortality risk of the hospitalized CAP patients. Univariate and multivariate logistic regression analyses were performed to identify the independent risk factors associated with SCAP. Cox proportional hazard regression analysis was used to determine if serum LPC was an independent risk factor for the 30-day mortality of CAP patients.
Results: The serum LPC levels at admission were significantly higher in the non-SCAP patients than in the SCAP patients (P = 0.011). Serum LPC level < 24.36 ng/mL, and PSI score were independent risk factors for the 30-day mortality in the elderly patients with CAP. The risk of 30-day mortality in the elderly CAP patients with low serum LPC levels (< 24.36ng/mL) was > 5-fold higher than in the patients with high serum LPC levels (≥ 24.36ng/mL).
Conclusion: Low serum LPC levels were associated with significantly higher disease severity and 30-day mortality in the elderly patients with CAP. Therefore, serum LPC is a promising predictive biomarker for the early identification of elderly CAP patients with poor prognosis.
Keywords: lysophosphatidylcholine, community-acquired pneumonia, biomarker, severity, mortality, propensity score matching
Introduction
Community-acquired pneumonia (CAP) is a common infectious respiratory disease that is associated with high morbidity and mortality rates, especially in patients with severe co-morbidities.1 In patients with severe community-acquired pneumonia (SCAP), the mortality rate is >35% for those with septic shock and requiring mechanical ventilation; mortality rate is >30% for those who develop acute respiratory distress syndrome.2 The advanced age and underlying disease in the elderly CAP patients is associated with higher mortality rates, the need of intensive care, and increased disease burden.3,4
Currently, the early clinical diagnosis and assessment of CAP severity is based on the pneumonia severity index (PSI) score5 and the CURB-65 score.6 However, these methods are not effective in predicting the morbidity and mortality outcomes of the elderly SCAP patients.4,7,8 Biomarkers such as C-reactive protein (CRP),9 procalcitonin (PCT),10 and interleukin-6 (IL-6)11 can be measured with high accuracy in the blood samples and are widely used to assess the severity of adverse outcomes. Several studies have demonstrated that CRP, PCT, and IL-6 are potential prognostic risk factors in CAP patients.12,13
Lysophosphatidylcholine (LPC) is a core component of the cellular membranes and is the most abundant lysophospholipid in human blood. LPC activates immune cells such as monocytes, macrophages, T-lymphocytes, and neutrophils by binding to the G protein-coupled receptors (GPCRs)14 and toll-like receptors.15 Furthermore, LPC activates several signaling pathways that regulate oxidative stress and inflammatory responses.16,17 In patients with pneumonia, low serum levels of LPC are associated with poor prognosis18 and acute phase of the disease.19,20 The association between serum LPC levels and the severity of COVID-19 is controversial. Metabolomics and lipidomics data demonstrated that low serum LPC levels were associated with increased disease severity in patients with COVID-19.21,22 However, Barberis et al and Song et al reported that serum LPC levels were elevated in patients with COVID-19.23,24 The reasons for these contradictory findings are not clear. However, these data suggested that lipids and metabolic dysfunction were significantly associated with the disease severity in patients with COVID-19. Serum LPC levels are altered in patients with cardiovascular disease25 and diabetes26 as well as elderly subjects,27 and even in gender,28 all of which are risk factors for assessing the severity of pneumonia. Therefore, we hypothesized that serum LPC levels may be used to accurately predict the disease severity in elderly patients with CAP. In this study, we investigated the predictive value of serum LPC levels in accurately estimating the disease severity and prognosis of hospitalized elderly patients with CAP.
Materials and Methods
Institutional Review Board Statement
This study involving human participants was reviewed and approved by the Ethics Committee of the Qingdao Municipal Hospital (Approval No. 2020CXJJ001-052). The written informed consent was obtained from the patients or the patient’s guardian. This study was performed in accordance with the Declaration of Helsinki guidelines.
Study Design
This prospective study was conducted between November 2020 and November 2021 at the Department of Pulmonary and Critical Care Medicine, the Intensive Care Unit (ICU), and the Emergency room of the Qingdao Municipal Hospital, Shandong Province, China. The diagnostic criteria for all the included subjects were strictly according to the Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Chinese Adults (2016 revision).29 Patients were diagnosed with CAP if they met criterion A, criterion C, and any one of the conditions for criterion B. Criterion A was defined as the onset of pneumonia in a community setting. Criterion B included the following clinical manifestations of pneumonia: (1) new onset of cough or expectoration, or aggravation of existing symptoms of respiratory tract diseases with or without purulent sputum, chest pain, dyspnea, or hemoptysis; (2) fever; (3) signs of pulmonary consolidation and/or moist rales; and (4) peripheral white blood cell (WBC) counts were >10 × 109/L or <4 × 109/L with or without a left shift. Criterion C was defined by chest radiograph findings showing new patchy infiltrates, lobar or segmental consolidation, ground-glass opacities or interstitial changes with or without pleural effusion. Patients were diagnosed with severe CAP30 if they met any one of the major criteria or ≥3 of the minor criteria for severe pneumonia. Sepsis was diagnosed according to the revised sepsis 3 definition (infection + an increase ≥ e r2 in the Sequential Organ Failure Assessment (SOFA) score).31 The exclusion criteria were as follows: (1) patients undergoing anti-infection treatment for more than 3 days prior to hospitalization; (2) pregnant women; (3) immunocompromised patients, including those with an history of taking glucocorticoids for more than 4 weeks, history of immunosuppressive therapy, human immunodeficiency virus infection, solid tumor or hematologic malignancy; (4) patients hospitalized during the past one month; or (5) patients participating in other clinical trials.
Patient Data Collection
A case report form was used to collect the data from patients for parameters such as age, sex, comorbidities, other demographic parameters, and clinical signs and symptoms. The following laboratory parameters were measured for all the patients within 24 hours of hospital admission: routine blood parameters, biochemical indices, C-reactive protein (CRP), procalcitonin (PCT), and other clinical parameters. We also extracted data for chest computed tomography, length of hospital stays, and death. We calculated the CURB-65 and PSI scores for all the patients. We used structured telephone interviews on day 30 after enrollment to assess the outcomes (mortality or survival).
Measurement of Serum LPC Levels
Peripheral blood was collected in a sterile pro-coagulation tube on days 1 and 6 after hospital admission and centrifuged within 3 hours at 3000 rpm for 10 minutes. The serum was separated and stored at −80°C for further analysis. Serum LPC levels were measured using a specific ELISA kit (Cat. no.MM-50888H1; Jiangsu Meimian Industrial Co., Ltd, Jiangsu, China) according to the manufacturer’s instructions.
Propensity Score Matching Analysis
Propensity score matching (PSM) analysis was used to match non-SCAP and SCAP patients to balance the potential baseline confounding factors.32 “Matchit” package in R studio (http://www.r-project.org) was used to match the propensity scores between the cohorts. The matching approach was set as the nearest neighbor algorithm with a matching ratio of 1:1 and a caliper value of 0.02. PSM was used to balance variables such as age, cardiovascular disease, chronic lung diseases, and diabetes.
Statistical Analysis
We grouped the patients according to disease severity (non-severe CAP group vs severe CAP group) and described the following characteristics of the groups separately: demographics; underlying conditions; clinical characteristics; laboratory results; and case fatality rate (survivors v. non-survivors). The categorical variables were reported as numbers or percentages. The categorical variables between distinct groups were compared using the Fisher’s exact test or the chi-squared test. The distributions of continuous variables were analyzed using the Kolmogorov–Smirnov test. The continuous variables with a normal distribution were expressed as means ± SD. The continuous variables with a non-normal distribution were reported as median and interquartile range (IQR). The inter-group differences for continuous variables with a non-normal distribution were analyzed using the Wilcoxon signed-rank test for 2 paired samples, the Kruskal–Wallis H-test for 2 or more groups that were independent of each other, and the Mann–Whitney U-test or Wilcoxon signed-rank test for 2 independent samples. Univariate and multivariate logistic regression analyses were performed to determine the association between LPC levels and severity of the disease. The results were presented as odds ratios (ORs) and 95% CI. P-values <0.10 (two-sided) were considered as statistically significant when evaluating the logistic regression models. Stepwise regression analysis was used to screen the indicators and obtain the probability prediction model for combined prediction indicators. The prediction accuracy was evaluated using the receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) values were calculated for each parameter. The Kaplan–Meier survival curves were used to determine the 30-day mortality rates for distinct groups of patients, and the log–rank test was used to compare the mortality rates. The data was entered in pairs using the epidata (v3.1) software and was uniformly quality controlled. Statistical analyses were performed using the SPSS software v26.0 (IBM, Armonk, NY, USA) and GraphPad Prism software v9.3.1 (GraphPad Software, San Diego, CA, USA).
Results
Study Design and Patients
This study enrolled 208 elderly patients (age ≥ 65 years) with CAP, including 67 (32.2%) patients with SCAP and 141 (67.8%) patients with non-SCAP (Figure 1).
 |
Figure 1 Flow diagram of patient selection and grouping in this study. |
Basic Characteristics of the Included Patients
In the unmatched cohort, the proportion of male and elderly patients in the SCAP group were significantly higher than in the non-SCAP group (both P < 0.05); moreover, the proportion of patients with cardiovascular diseases were significantly higher in the SCAP group than in the non-SCAP group (P = 0.042) (Table 1). Furthermore, the indicators of inflammatory response, including WBC counts, neutrophil (NEU) counts, neutrophil-to-lymphocyte ratio (NLR), and the serum levels of procalcitonin (PCT) and interleukin-8 (IL-8) were significantly higher in the SCAP group than in the non-SCAP group (all P < 0.001) (Table 1). Our data also showed that 18 (8.6%) CAP patients died within 30 days of hospitalization and one patient in the non-SCAP group died because of acute myocardial infarction.
 |
Table 1 Clinical Characteristics of Non-SCAP and SCAP Patients Before and After Propensity Score Matching Analysis |
Propensity Score Matching
Considering that shows LPC levels are significantly lower in the SCAP patients compared with the non-SCAP patients.
LPC is a low-density lipoprotein and its level is affected by statins.33 Therefore, we excluded 20 patients prescribed with statins for a prolonged period. Subsequently, to accurately evaluate the clinical value of LPC levels, we performed a 1:1 PSM for age, cardiovascular disease, and diabetes. PSM analysis showed that the LPC levels were significantly lower in patients from the SCAP group than those in the non-SCAP group (P = 0.007) (Figure 2a). Patients in the non-survivor group showed lower LPC levels compared to the survivor group, but the differences were not statistically significant (P = 0.104); furthermore, LPC levels before and after treatment did not show statistically significant differences between patients in the survivor and non-survivor groups (P = 0.079 and 0.843, respectively) (Figures 2b and c).
Predictive Value of Serum LPC Levels in Discriminating SCAP Patients from Non-SCAP Patients
Multivariate logistic regression analysis showed that CURB-65 score (OR: 4.712, 95% CI: 1.292–17.191, P = 0.019) and PSI (OR: 1.052, 95% CI: 1.010–1.097, P = 0.016) were independent risk factors of SCAP (Table 2).
 |
Table 2 Binary Logistic Regression Analysis of SCAP Incidence |
ROC curve analysis was performed to determine the predictive performance of serum LPC levels in discriminating between SCAP and non-SCAP patients. The AUC value for the serum LPC levels was 0.698 (95% CI: 0.566–0.831, P = 0.007) with a sensitivity of 51.6% and specificity of 87.1%. The AUC values for CURB-65 and PSI scores were 0.893 (0.813–0.974) and 0.914 (0.842–0.986), respectively. Furthermore, predictive performance of the serum LPC levels in combination combining the PSI score (AUC: 0.914 to 0.926) or the CURB65 score (AUC: 0.893 to 0.924) was higher than the serum LPC levels alone. Subsequently, we performed the DeLong test to compare the ROC curves for the SCAP and non-SCAP patients and did not find any significant differences in the predictive performance of the serum LPC levels before or after combination with the PSI or CURB-65 scores (P = 0.43 and 0.08, respectively) (Figure 3).
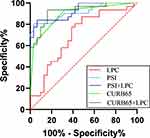 |
Figure 3 ROC curve analysis shows the prediction performances of LPC, PSI score, CURB-65 score, LPC + PSI score, and LPC + CURB-65 score in discriminating between SCAP and non-SCAP patients. |
Predictive Value of Serum LPC Levels in Predicting 30-Day Mortality in the Elderly Patients with CAP
We compared the clinical performances of serum LPC, IL-6, and IL-8 levels as well as CURB-65 and PSI scores in predicting the 30-day mortality of patients with CAP. ROC curve analysis showed that the predictive performance of the serum LPC levels (AUC = 0.692; sensitivity = 86.0%, specificity = 58.3%, P = 0.040) were lower than the performance of the serum IL-8 levels (AUC = 0.707, 95% CI: 0.539–0.874, P = 0.027), CURB-65 score (AUC = 0.768, 95% CI: 0.643–0. 892, P = 0.004), and PSI score (AUC=0.906, 95% CI: 0.827–0.985, P < 0.001), and higher than the serum IL-6 levels (AUC=0.578, 95% CI:0.402–0.754, P = 0.402) (Table 3).
 |
Table 3 Area Under the Curve Values and Thresholds of Different Parameters for Predicting the 30-Day Mortality in CAP Patients |
Serum LPC Level <24.36 Ng/mL is an Independent Predictor of the Risk of Mortality in the Elderly CAP Patients
CAP patients were categorized into two groups, high LPC group (≥ 24.36 ng/mL) and low LPC group (< 24.36 ng/mL) based on the optimal cut-off value for the serum LPC levels (24.36 ng/mL), which was as calculated by the ROC curve analysis. Cox proportional regression analysis demonstrated that the serum LPC levels <24.36 ng/mL (OR:6.212; 95% CI:1.155–33.413) and PSI score (OR:0.960; 95% CI:0.937–0.985) were independent risk factors for predicting the 30-day mortality in elderly CAP patients (Table 4).
 |
Table 4 Cox Proportional Hazard Regression Analysis of Multiple Variables for Predicting the 30-Day Mortality of Elderly Patients with CAP |
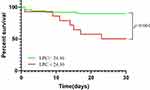
Furthermore, Kaplan-Meier survival curve analysis showed that the 30-day mortality rates were significantly higher for patients in the low-LPC group compared to the patients in the high-LPC group (log-rank χ2 =10.76, P = 0.001); the hazard ratio (HR) was 5.459 (1.341–22.220) (Figure 4).
Discussion
This is the first study to investigate the clinical value of serum LPC levels in elderly patients with CAP. Our data showed that the serum LPC level within 24 hours of hospital admission was significantly lower in patients with SCAP compared to patients with non-SCAP (p = 0.011). Furthermore, ROC curve analysis showed that the AUC value for serum LPC levels in predicting the 30-day mortality in CAP patients was 0.692 (95% CI: 0.518–0.865, P = 0.040) with a sensitivity of 86.0% and specificity of 58.3%. Cox proportional hazards regression analysis results showed that serum LPC level <24.36 ng/mL and PSI score were independent risk factors predicting the 30-day mortality in patients with CAP. K-M survival curve analysis results showed that the mortality rate of elderly CAP patients with low serum LPC levels (< 24.36 ng/mL) was significantly higher than those with high serum LPC levels (> 24.36 ng/mL), with a hazard ratio of 5.459 (1.341–22.220).
LPC is the most abundant lysophosphatidylcholine in human blood and accounts for 8–12% of the blood plasma; its level is dynamically regulated by the activities of phospholipase A2 (PLA2) and lysophosphatidylcholine acyltransferase (LPCAT) through the Lands cycle.34 Furthermore, activation of the autotaxin/lysophosphatidic acid (ATX/LPA) axis at the onset of pneumonia decreased LPC levels.35 Phospholipids such as phosphatidylcholine (PC) play an important role in the Lands cycle.36 PC and LPC are important components of cell membranes and function as lung surfactants. During respiratory infection or lung injury, LPC metabolism and levels are significantly affected because of alveolar epithelial cell damage and dysfunction.
Previous studies have demonstrated that the blood LPC levels are candidate biomarkers for inflammatory diseases. Banoei et al reported that lower LPC levels were significantly associated with mortality in bacterial CAP; therefore, LPC levels are useful biomarkers for predicting in-hospital mortality, the need for ICU admission, and the severity of bacterial CAP.37 Cho et al performed a single-center prospective study that included 56 patients with CAP and showed that low LPC levels were associated with poor hospital outcomes, the need for mechanical ventilation, vascular compressors, and admission to the intensive care unit, and hospital mortality.18 In this study, the mean age of the patients was 64 years. Therefore, our data reflects the role of LPC in older patients. Park et al investigated the predictive value of LPC in sepsis patients and reported that there were no significant differences between the survivor and non-survivor groups at admission; however, on the 7th day of hospitalization, LPC levels of patients in the survivor group were significantly higher than those in the non-survivor group; therefore, serial measurement of LPC levels were beneficial in predicting the 28-day mortality rate in hospitalized patients with severe sepsis or septic shock.38 The average age of patients in this study was 69 years old and most of the sepsis cases were pulmonary in origin, thereby further supporting our conclusion. Nan et al used metabolomics to identify potential biomarkers for assessing the disease severity and potential therapeutic targets of CAP disease and reported significant differences in LPC levels between the acute phase and the remission phase; moreover, serum LPC levels negatively correlated with disease severity; among the various LPC subgroups, LPC 14:0 showed the best diagnostic performance.39 Based on numerous studies, serum LPC levels show significant association with disease severity and show great promise for clinical application as a prognostic prediction biomarker. Ma et al21 reported that the serum LPC levels were significantly lower in patients with SCAP than in patients with non-SCAP. Furthermore, the AUC value for LPC was 0.708 (P = 0.0044) for predicting SCAP and 0.789 for predicting 30-day death in CAP patients (P < 0.0001). These data are consistent with our results. In our study, the clinical performance of LPC for predicting the 30-day mortality was weak in the elderly CAP patients but improved significantly after stratification at 24.36 ng/mL. Therefore, our data suggested that stratification of the elderly CAP patients based on serum LPC levels would be better for prognostic prediction.
Muller et al19 continuously monitored LPC levels in 33 patients with CAP and reported that LPC levels significantly decreased during the acute phase of pneumonia (within 48 hours) and gradually returned to normal levels (60 days) after antimicrobial treatment. In our study, we did not observe significant differences in the LPC levels between the survivor and non-survivor groups before and after treatment. We speculate that this may be caused by the underlying diseases in the elderly patients. Furthermore, the status of the patients in the survival group was poor. This may have resulted in a lag in the recovery of the serum LPC levels over time. However, further research is needed to confirm these findings. Although the blood lipid levels of the elderly subjects are influenced by age and the underlying diseases, significant changes are observed during SCAP. Therefore, it may be more valuable and convincing to compare the changes in LPC levels of the patient with his/her own baseline lipid levels.
There are several limitations in this study. Firstly, the sample size of this study was small, especially in the matched group. Secondly, we did not select healthy controls matched for age and underlying disease. Finally, this study was limited to older patients and may not be applicable to younger CAP patients.
Conclusion
In conclusion, this study showed that serum LPC was a promising biomarker for assessing disease severity and predicting the in-hospital mortality of elderly patients with CAP. Our data suggested that early monitoring of serum LPC levels may help the clinicians to adjust the treatment strategy and improve the survival rates of elderly patients with CAP. Therefore, monitoring the serum LPC levels can help in the early management of elderly patients with CAP.
Abbreviations
AUC, Area under the ROC curve; CAP, Community-acquired pneumonia; CRP, C-reactive protein; HR, Hazard ratio; ICU, Intensive Care Unit; NLR, Neutrophil-to-lymphocyte ratio; OR, Odds ratios; PSI, Pneumonia severity index; PSM, Propensity score matching; ROC, Receiver operative characteristic; SOFA, Sequential Organ Failure Assessment; WBC, White blood cell.
Data Sharing Statement
The datasets generated and analyzed during the current study are not publicly available due to health privacy concerns but are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The protocol of this study was approved by the Ethics Committee of the Qingdao Municipal Hospital (Approval No. 2020CXJJ001-052). This study was in compliance with the Declaration of Helsinki and informed consent was obtained from all patients or their families before this study.
Acknowledgments
The authors thank all the patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by the Qingdao Municipal Hospital Clinical Diagnosis and Treatment Technology Innovation Fund (Grant No. CXJJ-034). The funding agency did not play any role in the design of the study, collection, analyses, or interpretation of data, writing of the manuscript, and the decision regarding publishing the results.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet. 2015;386(9998):1097–1108. doi:10.1016/S0140-6736(15)60733-4
2. Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Resp Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
3. Furman CD, Leinenbach A, Usher R, Elikkottil J, Arnold FW. Pneumonia in older adults. Curr Opin Infect Dis. 2021;34(2):135–141. doi:10.1097/QCO.0000000000000718
4. Baek MS, Park S, Choi JH, Kim CH, Hyun IG. Mortality and prognostic prediction in very elderly patients with severe pneumonia. J Intensive Care Med. 2020;35(12):1405–1410. doi:10.1177/0885066619826045
5. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi:10.1056/NEJM199701233360402
6. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi:10.1136/thorax.58.5.377
7. Zhang ZX, Yong Y, Tan WC, Shen L, Ng HS, Fong KY. Prognostic factors for mortality due to pneumonia among adults from different age groups in Singapore and mortality predictions based on PSI and CURB-65. Singapore Med J. 2018;59(4):190–198. doi:10.11622/smedj.2017079
8. Lv CX, Chen Y, Shi W, Pan T, Deng JH, Xu JY. Comparison of different scoring systems for prediction of mortality and ICU admission in elderly CAP population. Clin Interv Aging. 2021;16:1917–1929. doi:10.2147/CIA.S335315
9. Travlos A, Bakakos A, Vlachos KF, Rovina N, Koulouris N, Bakakos P. C-reactive protein as a predictor of survival and length of hospital stay in community-acquired pneumonia. J Pers Med. 2022;12(10):1710. doi:10.3390/jpm12101710
10. Gupta S, Jaswani P, Sharma RK, et al. Procalcitonin as a diagnostic biomarker of sepsis: a tertiary care centre experience. J Infect Public Health. 2019;12(3):323–329. doi:10.1016/j.jiph.2018.11.004
11. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295–a016295. doi:10.1101/cshperspect.a016295
12. Szabo M, Kardos Z, Olah C, et al. Severity and prognostic factors of SARS-CoV-2-induced pneumonia: the value of clinical and laboratory biomarkers and the A-DROP score. Front Med. 2022;9:920016. doi:10.3389/fmed.2022.920016
13. Martin-Loeches I, Valles X, Menendez R, et al. Predicting treatment failure in patients with community acquired pneumonia: a case-control study. Respir Res. 2014;15(1):75. doi:10.1186/1465-9921-15-75
14. Drzazga A, Okulus M, Rychlicka M, Biegala L, Gliszczynska A, Gendaszewska-Darmach E. Lysophosphatidylcholine containing anisic acid is able to stimulate insulin secretion targeting G protein coupled receptors. Nutrients. 2020;12(4):1173. doi:10.3390/nu12041173
15. Magalhaes KG, Luna-Gomes T, Mesquita-Santos F, et al. Schistosomal lipids activate human eosinophils via toll-like receptor 2 and PGD2 receptors: 15-LO role in cytokine secretion. Front Immunol. 2018;9:3161. doi:10.3389/fimmu.2018.03161
16. da Silva JF, Alves JV, Silva-Neto JA, et al. Lysophosphatidylcholine induces oxidative stress in human endothelial cells via NOX5 activation - implications in atherosclerosis. Clin Sci. 2021;135(15):1845–1858. doi:10.1042/CS20210468
17. Lee HJ, Ko HJ, Song DK, Jung YJ. Lysophosphatidylcholine promotes phagosome maturation and regulates inflammatory mediator production through the protein kinase A-phosphatidylinositol 3 kinase-p38 mitogen-activated protein kinase signaling pathway during mycobacterium tuberculosis infection in mouse macrophages. Front Immunol. 2018;9:920. doi:10.3389/fimmu.2018.00920
18. Cho WH, Yeo HJ, Yoon SH, et al. Lysophosphatidylcholine as a prognostic marker in community-acquired pneumonia requiring hospitalization: a pilot study. Eur J Clin Microbiol. 2015;34(2):309–315. doi:10.1007/s10096-014-2234-4
19. Muller DC, Kauppi A, Edin A, Gylfe A, Sjostedt AB, Johansson A. Phospholipid levels in blood during community-acquired pneumonia. PLoS One. 2019;14(5):e0216379. doi:10.1371/journal.pone.0216379
20. Arshad H, Alfonso JCL, Franke R, et al. Decreased plasma phospholipid concentrations and increased acid sphingomyelinase activity are accurate biomarkers for community-acquired pneumonia. J Transl Med. 2019;17(1):365. doi:10.1186/s12967-019-2112-z
21. Ma X, Chen L, He Y, et al. Targeted lipidomics reveals phospholipids and lysophospholipids as biomarkers for evaluating community-acquired pneumonia. Ann Transl Med. 2022;10(7):395. doi:10.21037/atm-21-4008
22. Ciccarelli M, Merciai F, Carrizzo A, et al. Untargeted lipidomics reveals specific lipid profiles in COVID-19 patients with different severity from Campania region (Italy). J Pharm Biomed Anal. 2022;217:114827. doi:10.1016/j.jpba.2022.114827
23. Barberis E, Timo S, Amede E, et al. Large-scale plasma analysis revealed new mechanisms and molecules associated with the host response to SARS-CoV-2. Int J Mol Sci. 2020;21(22):8623. doi:10.3390/ijms21228623
24. Song JW, Lam SM, Fan X, et al. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 2020;32(2):188–202. doi:10.1016/j.cmet.2020.06.016
25. Zakiev ER, Sukhorukov VN, Melnichenko AA, Sobenin IA, Ivanova EA, Orekhov AN. Lipid composition of circulating multiple-modified low-density lipoprotein. Lipids Health Dis. 2016;15(1):134. doi:10.1186/s12944-016-0308-2
26. Morze J, Wittenbecher C, Schwingshackl L, et al. Metabolomics and type 2 diabetes risk: an updated systematic review and meta-analysis of prospective cohort studies. Diabetes Care. 2022;45(4):1013–1024. doi:10.2337/dc21-1705
27. Johnson AA, Stolzing A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell. 2019;18(6):e13048. doi:10.1111/acel.13048
28. Honarvar NM, Zarezadeh M, Molsberry SA, Ascherio A. Changes in plasma phospholipids and sphingomyelins with aging in men and women: a comprehensive systematic review of longitudinal cohort studies. Ageing Res Rev. 2021;68:101340. doi:10.1016/j.arr.2021.101340
29. Cao B, Huang Y, She DY, et al. Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association. Clin Resp J. 2018;12(4):1320–1360. doi:10.1111/crj.12674
30. Salih W, Schembri S, Chalmers JD. Simplification of the IDSA/ATS criteria for severe CAP using meta-analysis and observational data. Eur Resp J. 2014;43(3):842–851. doi:10.1183/09031936.00089513
31. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
32. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
33. Weber C, Erl W, Weber KS, Weber PC. Effects of oxidized low-density lipoprotein, lipid mediators and statins on vascular cell interactions. Clin Chem Lab Med. 1999;37(3):243–251. doi:10.1515/CCLM.1999.043
34. Law SH, Chan ML, Marathe GK, Parveen F, Chen CH, Ke LY. An updated review of lysophosphatidylcholine metabolism in human diseases. Int J Mol Sci. 2019;20(5):1149. doi:10.3390/ijms20051149
35. Nikitopoulou I, Fanidis D, Ntatsoulis K, et al. Increased autotaxin levels in severe COVID-19, correlating with IL-6 levels, endothelial dysfunction biomarkers, and impaired functions of dendritic cells. Int J Mol Sci. 2021;22(18):10006. doi:10.3390/ijms221810006
36. Lands WE. Metabolism of glycerolipides: a comparison of lecithin and triglyceride synthesis. J Biol Chem. 1958;231(2):883–888. doi:10.1016/S0021-9258(18)70453-5
37. Banoei MM, Vogel HJ, Weljie AM, Yende S, Angus DC, Winston BW. Plasma lipid profiling for the prognosis of 90-day mortality, in-hospital mortality, ICU admission, and severity in bacterial community-acquired pneumonia (CAP). Crit Care. 2020;24(1):461. doi:10.1186/s13054-020-03147-3
38. Park DW, Kwak DS, Park YY, et al. Impact of serial measurements of lysophosphatidylcholine on 28-day mortality prediction in patients admitted to the intensive care unit with severe sepsis or septic shock. J Crit Care. 2014;29(5):882.e5–882.e11. doi:10.1016/j.jcrc.2014.05.003
39. Nan W, Xiong F, Zheng H, et al. Myristoyl lysophosphatidylcholine is a biomarker and potential therapeutic target for community-acquired pneumonia. Redox Biol. 2022;58:102556. doi:10.1016/j.redox.2022.102556
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.