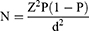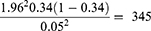Back to Journals » Journal of Blood Medicine » Volume 14
Prevalence and Associated Factors of Anemia Among Hospital Admitted Patients in Eastern Ethiopia
Authors Yusuf MU, Abdurahman N, Asmerom H , Atsbaha T, Alemu A, Weldegebreal F
Received 17 July 2023
Accepted for publication 6 November 2023
Published 15 November 2023 Volume 2023:14 Pages 575—588
DOI https://doi.org/10.2147/JBM.S431047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
Mohammed Umer Yusuf,1 Nuredin Abdurahman,1 Haftu Asmerom,2 Tesfaye Atsbaha,1 Adisu Alemu,3 Fitsum Weldegebreal2
1Department of Internal Medicine, School of Medicine, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 3Department of Pathology, School of Medicine, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
Correspondence: Haftu Asmerom, School of Medical Laboratory Sciences, College of Health and Medical Sciences, Haramaya University, PO BOX 235, Harar, Ethiopia, Tel +251910089878, Email [email protected]
Background: Anemia is one of the most common comorbidities frequently seen in admitted patients. However, there is a scarcity of evidence regarding anemia among hospital admitted patients in Ethiopia, particularly in the Harari Region. Therefore, this study aimed to assess the prevalence and associated factors of anemia among hospital admitted patients in Eastern Ethiopia.
Methods: A hospital-based cross-sectional study was conducted from October 25 to December 30, 2022. Four milliliters of venous blood were collected and complete blood count was done using the DxH 800 (Beckman Coulter, Inc, Miami, FL) hematology analyzer. The data were entered in Epi-data version 4 and exported to SPSS version 26 for statistical analysis. Bivariable and multivariable logistic regression models were fitted. The level of significance was declared at a p-value of < 0.05.
Results: Of the 381 hospital admitted patients, 64.8% (95% CI = 60.01, 69.65) of the participants were anemic. Admitted patients who drank standard alcohol daily (AOR = 3.78, 95% CI = 1.71, 8.30), underweight (AOR = 9.39, 95% CI = 2.90, 30.46), and undernourished patients (AOR = 2.59, 95% CI = 1.15, 5.84), patients admitted with chronic kidney disease (AOR = 11.16, 95% CI = 4.06, 30.64), chronic liver disease (AOR = 3.20, 95% CI = 1.21, 8.47), deep vein thrombosis (AOR = 6.22, 95% CI = 1.98, 19.52), infectious disease (AOR = 9.71, 95% CI = 2.77, 34.02), and chronic non-communicable disease (AOR = 7.01, 95% CI = 1.90, 25.99) were all significantly associated with anemia.
Conclusion: Anemia was common among hospital admitted patients and should prompt the focus on admission diagnoses that are likely to play leading roles in etiology. This information indicates a need for routine screening of anemia for all admitted patients to improve their health.
Keywords: anemia, prevalence, associated factors, admitted patients, Ethiopia
Introduction
The majority of hospital admitted patients are adults and geriatrics (≥15 years old) with chronic diseases and several comorbidities, which cause frequent hospitalizations.1 Anemia is one of the more prevalent comorbidities.2–6 It is a condition in which the number of red blood cells (RBC) or hemoglobin (Hgb) concentration within them is lower than normal.7 Accordingly, it is diagnosed in non-pregnant women ≥ 15 years old Hgb < 12.0 g/dl; men ≥ 15 years old Hgb < 13.0g/dl.8 Factors that contribute to the onset of anemia in admitted patients include nutritional deficiencies (iron, folate, and vitamin B12), acute or chronic blood loss, chronic diseases, hemoglobinopathies, infectious diseases (malaria, tuberculosis, the Human Immune Virus (HIV), and parasitic infections), and even other unknown causes.9
Compared to the general population, admitted patients have a higher prevalence of anemia.10 It is thought to affect ranging from 30% to 90% of newly admitted patients over the duration of their illnesses.2–6,11,12 Its prevalence is higher in female subjects, from 15 years of age to adulthood, and in older (> 80 years old) patients of both genders, affecting 42% of subjects.13 The most typical cause of anemia worldwide is iron deficiency anemia (IDA).14 However, multifactorial anemia includes anemia of chronic diseases (ACD), chronic kidney disease (CKD), dietary deficiencies, hemorrhages, use of antithrombotic medications, inflammation of iron metabolism,15,16 and anemia of chronic diseases (ACD), which have a higher prevalence of anemia.13 Recent research has shown that ACD and IDA, both of which have low iron stores, have altered iron metabolism.17 Hepcidin, a hormone produced by the liver during chronic inflammation, rises significantly when ferroportin activity is inhibited by hepcidin, which reduce iron absorption in the intestine while simultaneously causing iron sequestration in macrophages and enterocytes.18
Data from previous studies conducted in Ethiopia showed that the prevalence of anemia was higher in hospitalized patients.19,20 Despite the high prevalence of anemia in admitted patients, very little research has been conducted in our setup. Therefore, the purpose of this study was to determine the prevalence of anemia and its contributing factors among hospital admitted patients in Eastern Ethiopia.
Materials and Methods
Study Design, Area, and Period
A hospital-based prospective cross-sectional study was conducted in Harar Town, one of the Federal Democratic Republic of Ethiopia’s regional states in the country’s east. Harari Region is one of Ethiopia’s nine regional states, with Harar as its capital city. It is located 526 kilometers east of Addis Ababa, Ethiopia’s capital. There are four public hospitals, two private hospitals, and five health centers in the region. Among these hospitals, the study was conducted at two public hospitals in the region (Hiwot Fana Comprehensive Specialized University Hospital (HFCSUH) and Jugol General Hospital) and one private hospital (Harar General Hospital). These hospitals provide curative, rehabilitative, and preventive health care services. The study was conducted at these three selected hospitals from October 25 to December 30, 2022.
Population, Inclusion, and Exclusion Criteria
The study population included all individuals over the age of 15 who were hospitalized at the selected hospitals and agreed to participate in the study. The admission diagnosis of the patients was obtained by reviewing the patient’s card or chart. On the other hand, pregnant women and individuals who were unable to communicate were excluded from the study.
Sample Size Determination and Sampling Technique
The sample size of study participants was determined using a single population proportion formula by considering the 34% prevalence of anemia among hospital-admitted patients from a previous study conducted in Ethiopia.19
where n represents the minimum sample size required; z is 1.96, which is the standard normal value at the level of confidence desired (for a 95% confidence interval); d is 0.05, the acceptable error willing to be committed; and p, which is 0.34, is the estimated proportion of anemia prevalence in hospital admitted patients.
Based on the above assumption, the sample size was calculated as follows:
After adding 10% non-respondent rates, a total of 381 study participants were recruited using a simple random sampling technique. Sampling frames were created and the number of patients to be included in the study was determined for each of the Hospitals by probability proportion allocation to size sampling, followed by simple random sampling (Figure 1).
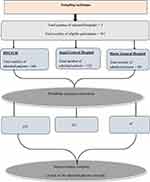 |
Figure 1 Schematic presentation of sampling procedure among hospital admitted patients in Eastern Ethiopia, 2022. |
Data Collection and Measurement
A Structured questionnaire was prepared from different literature.3,4,11,21,22 A total of nine data collectors (one senior clinical nurse, one senior medical resident, and one senior medical laboratory professional) from each of the selected hospitals collected socio-demographic characteristics, lifestyle, and history and clinical conditions (age at admission, cause of hospital admission, and laboratory tests) of each patient’s data. The study participants’ weight and height were measured using a digital weight scale with a connected height scale (Adult Scale ASTOR). Every day, the scale was reset to zero and calibrated. The heights of the participants were measured to the closest 0.1cm. The body mass index (BMI) of the subjects was calculated by dividing their weight (in kilograms) by their height (in meters squared), and they were classified as underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (≥ 30 kg/m2).23
The middle upper arm circumference (MUAC) was used to measure nutritional status. With no clothing on the arm, the left arm circumference was measured in triplicate using a non-stretchable standard MUAC tape to the nearest 0.1 cm. Three measurements were obtained and the average was calculated. A standard scale was used to quantify the MUAC at the midpoint between the acromion and the shoulder. The mean of the two nearest recorded measures was utilized when the variation surpassed the average values of the three measures. Patients admitted with MUACs of < 22 cm were considered undernourished, whereas MUACs of ≥ 22 cm were regarded as normal.24 One standard alcohol drink was defined as a drink with a net alcohol content of approximately 10g of ethanol (for example, one standard bottle of regular beer (285 mL), one single measure of spirits (30 mL), and one medium-sized glass of wine (120 mL)). Cigarette smoking was defined as admitted patients who had smoked at least one stick of cigarettes per day in the month (30 days) preceding data collection. Khat chewing was defined as admitted patients who had chewed khat in the month (30 days) before data collection.
Sample Collection and Laboratory Analysis
Four mL of venous blood was drawn using venipuncture according to World Health Organization (WHO) protocol25 under aseptic conditions from each admitted patient. The blood samples were then put into an ethylene diamine tetraacetic acid (EDTA) tube and gently mixed by inverting the tube several times to avoid clotting. An automated hematology analyzer, UniCel DxH 800 Coulter (Beckman Coulter, Inc, Miami, FL) was used for the determination of Hgb, mean cell volume (MCV), mean cell hemoglobin (MCH), and mean cell hemoglobin concentration (MCHC).
Diagnosis of Anemia
Anemia was defined according to WHO criteria,26 with a Hgb value of < 13g/dl for males and < 12g/dl for females. Anemia severity was also classified as mild (males: 11–12.9 g/dl; females: 11–11.9 g/dl), moderate (Hgb between 7 and 10.9 g/dl in both males and females), and severe (Hgb levels ≤ 7 g/dl in both males and females). Anemia was also classified into different morphology classifications based on MCV and MCHC values: microcytic hypochromic anemia (MCV < 80 fl and MCHC < 32%), macrocytic normochromic anemia (MCV >100 fl and MCHC between 32% and 36%), and normocytic normochromic anemia (MCV between 80 fl and 100 fl and MCHC between 32% and 36%).27
Data Processing and Analysis
Following data collection, the data were edited and cleaned, and each questionnaire was coded and validated for completeness. Epi-Data version 4.6 was used to enter data into the computer, and the statistical program Statistical Package for Social Sciences (SPSS) version 26 was used for analysis. Frequency, percentages, tables, and figures were used to describe categorical variables. The Kolmogorov -Smirnov test was used to ensure that continuous data had a normal distribution. Bivariable logistic regression analysis was employed, and the Crude Odds Ratio (COR) with 95% CI was computed to analyze the relationship between each independent variable and the outcome variable. Variables with p-values less than 0.25 were included for multivariable logistic regression analysis. Model goodness-of-fit was checked by the Hosmer and Lemeshow test, and the final model was well-fitted with the included independent variables (p-value = 0.81). The final model was performed to control the confounding variables and identify the associated factors by estimating the Adjusted Odds Ratio (AOR) with a 95% CI. Statistical significance was declared at a p-value of < 0.05.
Data Quality Control
A questionnaire was created in English and then translated into the local languages (Afan Oromo and Amharic) and then returned to English to maintain its consistency. One week before the actual data collection, 5% of the questionnaires were pre-tested among patients admitted at Dilchora General Hospital, which is about 52 kilometers away from the study region. The investigators attentively monitored the data collectors. The investigators evaluated the completeness of each questionnaire daily.
Ethical Consideration
The study protocol was approved by the Institutional Health Research Ethics Review Committee (IHRERC) of Haramaya University’s College of Health and Medical Sciences, with reference number (IHRERC-175-2022). We conducted the investigations in accordance with the Helsinki Declaration’s principles. The head of each hospital and participant above the age of 18 years provided informed, voluntary, written, and signed consent. In addition, written informed consent was obtained from the child’s parent/legal guardian and oral assent was obtained from children under 18 years of age after explaining the benefits and risks of the study. Personal identifiers were not included during the data collection to ensure anonymity.
Results
Socio-Demographic Characteristics
A total of 381 admitted patients took part in the study. One hundred ninety-eight (52.0%) participants were female. The ages of the study participants ranged from 15 to 90 years old, with a mean age of 40.98 (SD = 17.3). The majority of 210 (55.1%) lived in rural areas. Regarding educational status, 82 (21.5%) admitted patients were in secondary school, and 70 (18.4%) were in college or above (Table 1).
 |
Table 1 Sociodemographic Characteristics of Hospital Admitted Patients in Eastern Ethiopia, 2022 |
Nutritional and Behavioral-Related Factors
One hundred fourteen (29.9%) of the 381 admitted patients were underweight (BMI < 18.5 kg/m2). In contrast, 59 (15.5%) of the patients who were admitted were obese (BMI ≥ 30 kg/m2). One hundred seventy admitted patients (44.6%) had an undernourished condition (MUAC 22 cm). According to the study, 245 (64.3%) of the participants were cigarette smokers, with 125 (32.8%) of them smoking ≥ 5 cigarette sticks each day. Additionally, 223 (58.5%) of the study participants were current alcohol users, with 133 (34.9%) of them consuming at least one standard alcoholic drink per day. Furthermore, 208 (54.6%) of the study participants were current khat chewers, with 119 (31.2%) of them chewing khat < 300 g per day (Table 2).
 |
Table 2 Nutritional and Behavioral Related Factors of Hospital Admitted Patients in Eastern Ethiopia, 2022 |
Admission Diagnosis of the Patients
Of 381 patients admitted to the selected hospitals, 90 (23.6%) were admitted with CKD, followed by chronic liver disease (CLD), 59 (15.5%), and chronic obstructive pulmonary disease (COPD) (13.9%) (Figure 2).
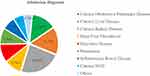 |
Figure 2 Admission diagnosis of hospital admitted patients in Eastern Ethiopia, 2022. Abbreviation: NCD, Non-Communicable Disease. |
Prevalence of Anemia Among Admitted Patients
The mean hemoglobin level of the study participants was 10.85 g/dl ± 3.5 and ranged from 4.0 to 18.0 g/dl. The overall prevalence of anemia among admitted patients was 64.8% (CI: 60.01, 69.65). Out of this prevalence, the most common causes of anemia were CKD (31.2%), followed by CLD (14.6%) and infectious disease (13.4%). The least common cause of anemia in the current study was pneumonia, with a prevalence of 2.8% (Figure 3).
 |
Figure 3 Prevalence of anemia among admission diagnosis of hospital admitted patients in Eastern Ethiopia, 2022. Abbreviation: NCD, Non-Communicable Disease. |
The Severity of Anemia Among Admitted Patients
Out of 247 (64.8%) anemic patients, 54 (14.2%) had mild anemia, 132 (34.6%) had moderate anemia, and 61 (16.0%) had severe anemia. In terms of anemia severity, the highest number of mild anemias was observed in participants admitted with CKD (31.5%), followed by CLD (18.4%). On the other hand, subjects admitted with chronic NCD (3.7%) showed the least mild anemia. In terms of moderate anemia, patients admitted with CKD had the highest percentage of cases (35.8%), followed by infectious diseases and deep vein thrombosis (15.2% of each). However, those admitted with inflammatory bowel disease (IBD) (1.5%) had the least amount of moderate anemia. In terms of severe anemia, patients admitted with CKD and CLD (23.0% of each) had the greatest percentages of severe anemia. However, admitted patients with pneumonia did not exhibit severe anemia (Figure 4).
 |
Figure 4 Severity of anemia among admission diagnosis of hospital admitted patients in Eastern Ethiopia, 2022. Abbreviation: NCD, Non-Communicable Disease |
Morphological Types of Anemia Among Admitted Patients
Based on MCV and MCHC, normocytic normochromic anemia constituted the majority of anemia (33.2%), followed by microcytic hypochromic anemia (27.9%). However, macrocytic normochromic anemia had the lowest percentage of anemia (7.8%) (Figure 5).
 |
Figure 5 Morphological types of anemia among hospital admitted patients in Eastern Ethiopia, 2022. |
Factors Associated with Anemia
In the bi-variable logistic regression analysis, educational status, alcohol consumption, BMI, MUAC, and admission diagnosis were significantly associated at a p - value of < 0.25 and considered potential candidates for multivariable logistic regression analysis. In multivariable logistic regression, alcohol consumption, BMI, MUAC, and admission diagnosis were independently associated with greater odds for the presence of anemia at a p - value of < 0.05. Admitted patients who drank standard alcohol for 1– 4 days per week were 2.88 times more likely (AOR = 2.88, 95% CI = 1.45, 5.70) to develop anemia than those who did not drink standard alcohol. Moreover, admitted patients who drank standard alcohol daily were 3.8 times more likely (AOR = 3.78, 95% CI = 1.71, 8.30) to develop anemia than those who did not drink standard alcohol.
Underweight admitted patients were 9.39 times more likely (AOR = 9.39, 95% CI = 2.90, 30.46) to develop anemia than patients who had normal weight. Additionally, obese admitted patients were 2.30 times more likely (AOR = 2.30, 95% CI = 1.00, 5.27) to develop anemia than normal-weight admitted patients. Undernourished admitted patients had 2.59 times higher odds (AOR = 2.59, 95% CI = 1.15, 5.84) of developing anemia than normal admitted patients. Patients admitted with CLD had 3.20 times higher odds (AOR = 3.20, 95% CI = 1.21, 8.47), CKD had 11.2 times higher odds (AOR = 11.16, 95% CI = 4.06, 30.64), deep vein thrombosis had 6.22 times higher odds (AOR = 6.22, 95% CI = 1.98, 19.52), infectious disease had 9.71 times higher odds (AOR = 9.71, 95% CI = 2.77, 34.02), and chronic NCD had 7.01 times higher odds (AOR = 7.01, 95% CI = 1.90, 25.99) to develop anemia as compared to patients admitted with COPD (Table 3).
 |
Table 3 Bivariable and Multivariable Logistic Regression Analysis for the Factors Associated with Anemia Among Hospital Admitted Patients in Eastern Ethiopia, 2022 |
Discussion
In this study, the overall prevalence of anemia among hospital admitted patients was 64.80%. Of these, 14.2% had mild anemia, 34.6% had moderate anemia, and 16.0% had severe anemia. This study was concordant with the studies conducted in Germany 60%,28 Benin 61.8%,4 and Uganda 64.3%.5 However, Results from the present study revealed a lower prevalence of anemia than the previous studies conducted in Pakistan 71%,3 Bahrain 72.2%,2 and Tanzania 79.5%,11 and a higher prevalence than other similar studies done in Italy 48%,6 Germany 54.2%,29 Pakistan 55.5%,30 and Tanzania 44%.12 This inconsistency might be attributed to variations in the study design, sampling techniques and sample size, socio-demographic, behavioral characteristics, type of admission diagnosis, nutritional variation, and the age of the study subjects.
Normocytic normochromic (33.2%) blood picture was the most common morphological type of anemia, followed by microcytic hypochromic (27.9%) anemia. Whereas the least morphological type of anemia was macrocytic normochromic (2.8%) anemia found in this study. It is not surprising to see a normocytic normochromic blood picture of anemia in this study, as various previous studies conducted in India,31 Pakistan,32 Malaysia,33 Saudi Arabia,22 and Benin4 also revealed this situation. Since the majority of participants in this study were admitted patients with CKD, chronic NCD, and ACD, which are expected to be normocytic and normochromic in morphology at the early stage of the disease.33–35
Admitted patients who drank standard alcohol for 1– 4 days per week and daily were 2.88 (AOR = 2.88, 95% CI =1.45, 5.70) and 3.78 (AOR = 3.78, 95% CI =1.71, 8.30) times more likely to develop anemia than those who did not drink standard alcohol, respectively. The result was in agreement with the studies conducted in Taiwan,36 Finland,37 and Japan,38 as heavy drinkers were more likely to develop anemia than non-drinkers. Anemia in alcoholics has a complicated and multifaceted etiology, with causes ranging from poor nutrition to chronic inflammation, blood loss, liver failure, and inefficient erythropoiesis.39,40
Underweight admitted patients had 9.39 (AOR = 9.39, 95% CI = 2.90, 30.46) times higher odds of developing anemia than patients who had normal weight. The result was consistent with the studies conducted in Japan,41 Israel,42 and Tanzania.43 The possible reasons for the underweight might be due to disease-specific factors such as loss of appetite, inflammation, swallowing difficulties, hypercatabolism, and treatment-related factors such as fasting episodes, treatment side effects, and psychological factors (anxiety, depression, and loneliness).44,45
Additionally, obese admitted patients had 2.30 (AOR = 2.30, 95% CI = 1.00, 5.27) times higher odds of developing anemia than patients who had normal weight. The result was in agreement with the studies conducted in the United States of America,46 Pakistan,47 Iran,48 and Salzburg, Austria.49 The principal mechanism that links obesity and anemia is low-grade systemic inflammation, which is observed in people with obesity.50 In patients with obesity, serum hepcidin, and serum interleukin-6 are significantly higher, which results in reduced iron absorption and sequestration of iron within splenic and hepatic macrophages, which leads to a reduction in iron use in the obese.51
Undernourished admitted patients had 2.59 times higher odds (AOR = 2.59, 95% CI = 1.15, 5.84) of developing anemia than normal admitted patients. The result was in line with the studies conducted in the Netherlands,44 and India.52 Malnutrition is associated with poor tolerance to treatment, decreased quality of life, increased health care costs, social factors (such as poverty), and clinical conditions like malabsorption syndrome, intestinal obstruction, and gastric atony.53
Patients admitted with CLD were 3.20 (AOR = 3.20, 95% CI = 1.21, 8.47) times more likely to develop anemia than COPD patients. This finding was supported by researchs undertaken in the United States of America,54 India,55 and Bangladesh.56 This could be due to the following factors: Hemorrhage, particularly in the gastrointestinal tract, is a prominent cause of anemia in people with chronic liver disease and cirrhosis.56 Anemia owing to bleeding can arise as a consequence of chronic liver illness due to a deficiency of one or more blood clotting factors produced by the liver, thrombocytopenia, and/or impaired platelet activity.57 Furthermore, iron insufficiency is common in late CLD patients because the liver secretes hepcidin, the major regulator of iron homeostasis.58
Admitted patients with CKD were 11.2 times (AOR = 11.16, 95% CI = 4.06, 30.64) more likely to develop anemia than COPD patients. Studies conducted in the United States,59 Korea,60 Indonesia,61 Peshawar, Pakistan,62 and Ghana63 all corroborated this finding. This could be because people with CKD are more likely to develop anemia due to decreased erythropoietin production by failing kidneys. This might be since patients with CKD have a higher risk of developing anemia associated with decreased production of erythropoietin by failing kidneys.64 Chronic kidney disease causes anemia by reducing erythropoietin production by the kidney’s peritubular fibroblasts resulting in normocytic normochromic anemia, which was found in nearly half of CKD patients.65
Admitted deep vein thrombosis patients were 6.22 times (AOR = 6.22, 95% CI = 1.98, 19.52) more likely to develop anemia than COPD patients. This finding was reinforced by researchs undertaken across different corners of China,66–68 France,69 and Senegal.70 The erythropoietin reaction to changes in Hgb levels is linearly logarithmic, which means that the lower the Hgb level, the higher the erythropoietin response.71 Erythropoietin can promote hypercoagulability and raise the risk of thrombosis by increasing platelet and RBC levels as well as blood viscosity.72
Admitted patients with infectious illness were 9.7 times (AOR = 9.71, 95% CI = 2.77, 34.02) more likely to develop anemia than patients admitted with COPD. This finding was supported by researchs from Iran,73 Cameroon,74 and Ethiopia.75 The primary factor in the development of malaria-related anemia is increased hemolysis of parasitized RBCs and increased destruction of non-parasitized RBCs.76 This could be because hepcidin is elevated during infections, which also contributes to anemia.77 Anemia prevalence in HIV-positive individuals increases with the progression of the disease and results in chronic acute phase response, elevated hepcidin, anemia of inflammation, and altered iron metabolism.78,79 Anemia among pulmonary TB patients is thought to result from anemia of inflammation, as well as increased blood loss from hemoptysis (blood in sputum), decreased RBC production, and poor appetite and food intake, leading to poor nutrient status.80
Admitted patients with chronic NCDs were found to be 7.01 times (AOR = 7.01, 95% CI = 1.90, 25.99) more likely to be anemic than patients admitted with COPD. This result was in line with studies conducted in Malaysia,81 India,82 Pakistan,83 and Ghana.21 The pathogenesis of anemia in hematological malignant patients is largely speculative. In solid cancers, the most likely cause may be RBC fragmentation in small vessels of cancerous tissue, particularly in bone marrow, the lung, or other organs. Tumor cell cytokine production may also play a role in tumor necrosis factor (TNF)-mediated red cell damage84 that activates the coagulation cascade and leads to RBC consumption via thrombotic microangiopathies.82
Conclusion
In this study, 64.80% of hospital admitted patients had anemia, indicating a need for routine screening for all admitted patients.
Abbreviations
ACD, Anemia of Chronic Disease; BMI, Body Mass Index; CKD, Chronic kidney disease; CLD, Chronic Liver Disease; COPD, Chronic Obstructive Pulmonary Disease; HIV, Human Immune Virus; Hgb, Hemoglobin; HFCSUH, Hiwot Fana Comprehensive Specialized University Hospital; IBD, Inflammatory Bowel Disease; IDA, Iron Deficiency Anemia; MCH, Mean Cell Hemoglobin; MCHC, Mean cell Hemoglobin concentration; MCV, Mean Cell Volume; MUAC, Middle-Upper Arm Circumference; NCD, Non-Communicable Disease; RBC, Red Blood Cell; WHO, World Health Organization.
Acknowledgments
The authors would like to thank Haramaya University, all hospital staff members of the hospitals, data collectors, supervisors, study participants, and questionnaire translators for their assistance.
Author Contributions
All authors contributed significantly to the work reported in the conceptualization, study design, execution, acquisition, analysis, and interpretation of data, as well as took part in drafting, revising, and critically assessing the paper. All authors have agreed to approve the final paper for publication in the current journal and to be held accountable for all elements of the work.
Funding
This study got no specific financing from government, commercial, or non-profit organizations.
Disclosure
The authors declare that they have no competing interests.
References
1. Fonseca C, Araujo M, Moniz P, et al. Prevalence and prognostic impact of anemia and iron deficiency in patients hospitalized in an internal medicine ward: the Pro‐Iron study. Eur J Haematol. 2017;99(6):505–513. doi:10.1111/ejh.12963
2. Alsaeed M, Ahmed S, Seyadi K, Ahmed A, Alawi S, Abulsaad K. The prevalence and impact of anemia in hospitalized older adults: a single center experience from Bahrain. J Taibah Univer Med Sci. 2022;17(4):587–595. doi:10.1016/j.jtumed.2022.02.003
3. Bashir F, Nageen A, Kidwai S, Zulfikar S, Shiraz S, Ara J. Anemia in hospitalized patient: prevalence, etiology and risk factors. J Liaq Univer Med Health Sci. 2017;16:2.
4. Dovonou C, Alassani A, Attinsounon C, et al. Epidemiology of anemia at the internal medicine department in Borgou Departmental Hospital Center (DHC) in Parakou (Benin). Open J Inter Med. 2018;8(2):123–130. doi:10.4236/ojim.2018.82013
5. Kuule J, Seremba E, Freers J. Anaemia among patients with congestive cardiac failure in Uganda–its impact on treatment outcomes. South Af Med J. 2009;99(12):876–880.
6. Migone De Amicis M, Poggiali E, Motta I, et al. Anemia in elderly hospitalized patients: prevalence and clinical impact. Intern Emerg Med. 2015;10:581–586. doi:10.1007/s11739-015-1197-5
7. World Health Organization. Nutritional Anaemias: Tools for Effective Prevention and Control. World Health Organization; 2017.
8. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In: Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2011.
9. Halawi R, Moukhadder H, Taher A. Anemia in the elderly: a consequence of aging? Expert Rev Hematol. 2017;10(4):327–335. doi:10.1080/17474086.2017.1285695
10. Kurniali P, Curry S, Brennan K, et al. A retrospective study investigating the incidence and predisposing factors of hospital-acquired anemia. Anemia. 2014;2014:1–6. doi:10.1155/2014/634582
11. Chamba C, Nasser A, Mawalla W, et al. Anaemia in the hospitalized elderly in Tanzania: prevalence, Severity, and micronutrient deficiency status. Anemia. 2021;2021:1–6. doi:10.1155/2021/9523836
12. Munenguni E, Huda A, Jaffer T, Zimbeiya A, Henke O. Anemia in hospitalized patients in A referral hospital in Northern Tanzania. East Afr Med J. 2021;98:5.
13. Kassebaum N, Collaborators GA. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):247–308. doi:10.1016/j.hoc.2015.11.002
14. Randi M, Bertozzi I, Santarossa C, et al. Prevalence and causes of anemia in hospitalized patients: impact on diseases outcome. J Clin Med. 2020;9(4):950. doi:10.3390/jcm9040950
15. Girelli D, Marchi G, Camaschella C. Anemia in the elderly. HemaSphere. 2018;2:e40. doi:10.1097/HS9.0000000000000040
16. Weiss G, Goodnough LT. Anemia of chronic disease. New England J Med. 2005;352(10):1011–1023. doi:10.1056/NEJMra041809
17. Busti F, Marchi G, Ugolini S, Castagna A, Girelli D. Anemia and iron deficiency in cancer patients: role of iron replacement therapy. Pharmaceuticals. 2018;11(4):94. doi:10.3390/ph11040094
18. Camaschella C. Iron-deficiency anemia. New England J Med. 2015;372(19):1832–1843. doi:10.1056/NEJMra1401038
19. Bekele A, Teji K, Egata G, Gebremichael B. Anemia and associated factors among type-2 diabetes mellitus patients attending public hospitals in Harari Region, Eastern Ethiopia. PLoS One. 2019;14(12):e0225725. doi:10.1371/journal.pone.0225725
20. Wassie M, Aemro A, Fentie B, Khatami F. Prevalence and associated factors of baseline anemia among cervical cancer patients in Tikur Anbesa Specialized Hospital, Ethiopia. BMC Women’s Health. 2021;21(1):1–8. doi:10.1186/s12905-020-01152-w
21. Anderson A. Prevalence of anemia, overweight/obesity, and undiagnosed hypertension and diabetes among residents of selected communities in Ghana. Int J Chronic Dis. 2017;2017:1–7. doi:10.1155/2017/7836019
22. Elsayid M, Al-Qahtani A, Alanazi A, Qureshi S. Determination of the most common morphological patterns of anemia among Saudi anemic patients attending King Abdul-aziz Medical City-Riyadh. Intern J Med Public Health. 2015;5:4.
23. Pus A, Moriyama M, Uno M, Rahman MM. Identifying factors of obesity in Papua New Guinea: a descriptive study. Health. 2016;8(14):1616–1629. doi:10.4236/health.2016.814158
24. Benítez Brito N, Suárez Llanos J, Fuentes Ferrer M, et al. Relationship between mid-upper arm circumference and body mass index in inpatients. PLoS One. 2016;11(8):e0160480. doi:10.1371/journal.pone.0160480
25. World Health Organization. Guidelines on Drawing Blood: Best Practices in Phlebotomy. World Health Organization; 2010.
26. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. In: Vitamin and Mineral Nutrition Information System. Document Reference World Health Organization; 2011.
27. Ahmed K, Danial K, Khurram A, Wasey M, Ahmed M, Jangda Z. To evaluate the renal function deterioration along with other anemia predictors in patients with diabetes mellitus type 2 in Karachi, Pakistan. Pak J Surg. 2017;33(2):135–139.
28. Geisel T, Martin J, Schulze B, et al. An etiologic profile of anemia in 405 geriatric patients. Anemia. 2014;2014:1–7. doi:10.1155/2014/932486
29. Röhrig G, Klossok W, Becker I, Benzing T, Schulz R. Prevalence of anemia among elderly patients in an emergency room setting. Eur Geriatr Med. 2014;5(1):3–7. doi:10.1016/j.eurger.2013.10.008
30. Awan M, Fazal I, Lodhi M, Azhar R, Anwar A, Tariq M. Frequency of iron deficiency anemia in patients of inflammatory bowel disease at Pak Emirates Military Hospital, Rawalpindi. Pakistan Arme Forces Med J. 2022;72(SUPPL–2):S168–71. doi:10.51253/pafmj.v72iSUPPL-2.2785
31. Aithal K, Meti K, Jain S. A study of pattern and causes of anaemia in elderly patients admitted at tertiary centre. Sch J Appl Med Sci. 2017;5(4D):1483–1486.
32. Ashraf A. Frequency of anaemia in patients presenting to a tertiary care hospital in Lahore, Pakistan. Medicine. 2018;4(19):12.
33. Idris I, Tohid H, Muhammad N, et al. Anaemia among primary care patients with type 2 diabetes mellitus (T2DM) and chronic kidney disease (CKD): a multicentred cross-sectional study. BMJ open. 2018;8(12):e025125. doi:10.1136/bmjopen-2018-025125
34. Abdulqadir A, Polus R. Prevalence of anemia of chronic disease and iron deficiency anemia among adult diabetic patients in Erbil City. Zanco J Med Sci. 2014;18:1.
35. Madu AJ, Ughasoro MD. Anaemia of chronic disease: an in-depth review. Med Princ Pract. 2017;26(1):1–9. doi:10.1159/000452104
36. Paramastri R, Hsu C, Lee H, Lin L, Kurniawan A, Chao J. Association between dietary pattern, lifestyle, anthropometric status, and anemia-related biomarkers among adults: a population-based study from 2001 to 2015. Int J Environ Res Public Health. 2021;18(7):3438. doi:10.3390/ijerph18073438
37. Latvala J, Parkkila S, Niemelä O. Excess alcohol consumption is common in patients with cytopenia: studies in blood and bone marrow cells. Alcohol Clin Exp Res. 2004;28(4):619–624. doi:10.1097/01.ALC.0000122766.54544.3B
38. Yokoyama A, Yokoyama T, Brooks P, et al. Macrocytosis, macrocytic anemia, and genetic polymorphisms of alcohol dehydrogenase‐1 b and aldehyde dehydrogenase‐2 in J apanese Alcoholic Men. Alcohol Clin Exp Res. 2014;38(5):1237–1246. doi:10.1111/acer.12372
39. Colman N, Herbert V. Hematologic complications of alcoholism: overview. Semin Hematol. 1980;17(3):164–176.
40. Lewis G, Wise M, Poynton C, Godkin A. A case of persistent anemia and alcohol abuse. Nat Clin Pract Gastroenterol Hepatol. 2007;4(9):521–526. doi:10.1038/ncpgasthep0922
41. Kubo S, Hosomi N, Hara N, et al. Ischemic stroke mortality is more strongly associated with anemia on admission than with underweight status. J Stroke Cerebrov Dis. 2017;26(6):1369–1374. doi:10.1016/j.jstrokecerebrovasdis.2017.02.016
42. Aronson D, Nassar M, Goldberg T, Kapeliovich M, Hammerman H, Azzam Z. The impact of body mass index on clinical outcomes after acute myocardial infarction. Int J Cardiol. 2010;145(3):476–480. doi:10.1016/j.ijcard.2009.12.029
43. Cox S, Makani J, Fulford A, et al. Nutritional status, hospitalization and mortality among patients with sickle cell anemia in Tanzania. haematologica. 2011;96(7):948. doi:10.3324/haematol.2010.028167
44. Loh K, Vriens M, Gerritsen A, et al. Unintentional weight loss is the most important indicator of malnutrition among surgical cancer patients. Neth J Med. 2012;70(8):365–369.
45. Mariette C, De Botton M-L, Piessen G. Surgery in esophageal and gastric cancer patients: what is the role for nutrition support in your daily practice? Ann Surg Oncol. 2012;19:2128–2134. doi:10.1245/s10434-012-2225-6
46. Yanoff L, Menzie C, Denkinger B, et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int J Obes. 2007;31(9):1412–1419. doi:10.1038/sj.ijo.0803625
47. Khan A, Khan W, Ayub M, Humayun M, Haroon M. Ferritin is a marker of inflammation rather than iron deficiency in overweight and obese people. J Obes. 2016;2016:1–7. doi:10.1155/2016/1937320
48. Arshad M, Jaberian S, Pazouki A, Riazi S, Rangraz M, Mokhber S. Iron deficiency anemia and megaloblastic anemia in obese patients. Rom J Intern Med. 2017;55(1):3–7. doi:10.1515/rjim-2016-0046
49. Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients. 2014;6(9):3587–3600. doi:10.3390/nu6093587
50. Sal E, Yenicesu I, Celik N, et al. Relationship between obesity and iron deficiency anemia: is there a role of hepcidin? Hematology. 2018;23(8):542–548. doi:10.1080/10245332.2018.1423671
51. Saad R, Qutob H. The relationship between anemia and obesity. Expert Rev Hematol. 2022;15(10):911–926. doi:10.1080/17474086.2022.2131521
52. Thakur N, Chandra J, Pemde H, Singh V. Anemia in severe acute malnutrition. Nutrition. 2014;30(4):440–442. doi:10.1016/j.nut.2013.09.011
53. van Bokhorst-de van der Schueren M. Nutritional support strategies for malnourished cancer patients. Europ J Oncol Nurs. 2005;9:S74–S83.
54. Tseng F, Gou G, Wang S, Shyu J, Pan R. Chronic liver disease and cirrhosis increase morbidity in geriatric patients treated surgically for Hip fractures: analysis of the US Nationwide Inpatient Sample. BMC Geriatr. 2022;22(1):150. doi:10.1186/s12877-022-02832-y
55. Rauf A, Gupta V, Bansal R, et al. Anemia profile in patients of chronic liver disease from North Indian tertiary hospital. J Clin Exp Hepatol. 2014;4:S41–S2.
56. Nuruddin A, Sultana A, Akter F, Ahmed E, Dutta B. Prevalence and pattern of anemia among the hospitalized patients of chronic liver disease in a tertiary care hospital. J Chittagong Med Coll Teach Assoc. 2017;28(2):111–115. doi:10.3329/jcmcta.v28i2.62433
57. Farrell G, Larter C. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(S1):S99–S112. doi:10.1002/hep.20973
58. Gkamprela E, Deutsch M, Pectasides D. Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment. Anna Gastroenterol. 2017;30(4):405. doi:10.20524/aog.2017.0152
59. Stauffer M, Fan T. Prevalence of anemia in chronic kidney disease in the United States. PLoS One. 2014;9(1). doi:10.1371/journal.pone.0084943
60. Lee Y, Chang Y, Kang J, et al. Risk factors for incident anemia of chronic diseases: a cohort study. PLoS One. 2019;14:5.
61. Suega K, Bakta M, Dharmayudha TG, Lukman J, Suwitra K. Profile of anemia in chronic renal failure patients: comparison between predialyzed and dialyzed patients at the division of nephrology, department of internal medicine, Sanglah Hospital, Denpasar, Bali, Indonesia. Dep Intern Med Sanglah Hosp Denpasar Bali Indonesia Inflam. 2005;1:6–9.
62. Khan A, Afridi M, Ali G, Idrees M, Waqas M. Prevalence of anemia in chronic kidney disease patients in Lady Reading Hospital, Peshawar. Khyber J Med Sci. 2018;11:439–442.
63. Amoako Y, Laryea D, Bedu-Addo G, Andoh H, Awuku Y. Clinical and demographic characteristics of chronic kidney disease patients in a tertiary facility in Ghana. Pan Afr Med J. 2014;2014:18.
64. Donnelly S. Why is erythropoietin made in the kidney? The kidney functions as a critmeter. Am J Kid Dis. 2001;38(2):415–425. doi:10.1053/ajkd.2001.26111
65. Ryu S, Park S, Jung J, et al. The prevalence and management of anemia in chronic kidney disease patients: result from the Korean cohort study for outcomes in patients with chronic kidney disease (KNOW-CKD). J Korean Med Sci. 2017;32(2):249–256. doi:10.3346/jkms.2017.32.2.249
66. Feng L, Xu L, Yuan W, Xu Z, Feng Z, Zhang H. Preoperative anemia and total hospitalization time are the independent factors of preoperative deep venous thromboembolism in Chinese elderly undergoing Hip surgery. BMC Anesthesiol. 2020;20:1–6. doi:10.1186/s12871-020-00983-2
67. Xiong X, Xu S, Li T, Cheng B. Correlation of the severity of anemia in patients undergoing total joint arthroplasty with preoperative deep vein thrombosis: a retrospective cohort study. J Orthop Surg Res. 2022;17(1):1–9. doi:10.1186/s13018-021-02689-8
68. Hung S-H, Lin H-C, Chung S-D. Association between venous thromboembolism and iron-deficiency anemia: a population-based study. Blood Coagul Fibrinol. 2015;26(4):368–372. doi:10.1097/MBC.0000000000000249
69. Lecouffe-Desprets M, Néel A, Graveleau J, et al. Venous thromboembolism related to warm autoimmune hemolytic anemia: a case–control study. Autoimmun Rev. 2015;14(11):1023–1028. doi:10.1016/j.autrev.2015.07.001
70. Fall A, Proulle V, Sail A, et al. Risk factors for thrombosis in an African population. Clin Med Insig. 2014;7:S13401.
71. Goodnough L, Price T, Parvin C, et al. Erythropoietin response to anaemia is not altered by surgery or recombinant human erythropoietin therapy. Br J Haematol. 1994;87(4):695–699. doi:10.1111/j.1365-2141.1994.tb06725.x
72. Lippi G, Franchini M, Favaloro E. Thrombotic complications of erythropoiesis-stimulating agents. In: Seminars in Thrombosis and Hemostasis. © Thieme Medical Publishers; 2010.
73. Meidani M, Rezaei F, Maracy M, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17(2):138.
74. Bate A, Kimbi H, Lum E, et al. Malaria infection and anaemia in HIV-infected children in Mutengene, Southwest Cameroon: a cross sectional study. BMC Infect Dis. 2016;16(1):1–9. doi:10.1186/s12879-016-1853-z
75. Sahle T, Yemane T, Gedefaw L. Effect of malaria infection on hematological profiles of people living with human immunodeficiency virus in Gambella, southwest Ethiopia. BMC Hematol. 2017;17:1–8. doi:10.1186/s12878-017-0072-1
76. White N. Anaemia and malaria. Malar J. 2018;17(1):1–17.
77. Spottiswoode N, Duffy P, Drakesmith H. Iron, anemia and hepcidin in malaria. Front Pharmacol. 2014;5:125. doi:10.3389/fphar.2014.00125
78. Minchella P, Armitage A, Darboe B, et al. Elevated hepcidin is part of a complex relation that links mortality with iron homeostasis and anemia in men and women with HIV infection. J Nutr. 2015;145(6):1194–1201. doi:10.3945/jn.114.203158
79. Redig A, Berliner N. Pathogenesis and clinical implications of HIV-related anemia in 2013. Hematology. 2013;2013(1):377–381. doi:10.1182/asheducation-2013.1.377
80. Papathakis P, Piwoz E. Nutrition and Tuberculosis: a review of the literature and considerations for TB control programs. United States agency for international development. Africa’s Health. 2010;2008:1.
81. Krishnapillai A, Omar M, Ariaratnam S, et al. The prevalence of anemia and its associated factors among older persons: findings from the National Health and Morbidity Survey (NHMS) 2015. Int J Environ Res Public Health. 2022;19(9):4983. doi:10.3390/ijerph19094983
82. Gaspar B, Sharma P, Das R. Anemia in malignancies: pathogenetic and diagnostic considerations. Hematology. 2015;20(1):18–25. doi:10.1179/1607845414Y.0000000161
83. Burney S, Farooq M, Alvi A. Anemia in elderly hospitalized patients: frequency and association with comorbidities 1 2 3. J Islamic Inter Med Coll. 2018;13(4):194–199.
84. Lechner K, Obermeier H. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine. 2012;91(4):195–205. doi:10.1097/MD.0b013e3182603598
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.