Back to Journals » Research and Reports in Neonatology » Volume 14
Prevalence, Prevention and Management of Bronchopulmonary Dysplasia
Authors Gilfillan MA, Mejia MJ , Bhandari V
Received 1 September 2023
Accepted for publication 8 December 2023
Published 4 January 2024 Volume 2024:14 Pages 1—33
DOI https://doi.org/10.2147/RRN.S405985
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
Margaret A Gilfillan,1 Michelle J Mejia,1 Vineet Bhandari2
1Department of Pediatrics, St. Christopher’s Hospital for Children/Drexel University College of Medicine, Philadelphia, PA, USA; 2Department of Pediatrics, The Children’s Regional Hospital at Cooper/Cooper Medical School of Rowan University, Camden, NJ, USA
Correspondence: Vineet Bhandari, Division of Neonatology, The Children’s Regional Hospital at Cooper, One Cooper Plaza, Camden, NJ, 08103, USA, Tel +1-856-342-2000 Extn 1006156, Fax +1-856-342-8007, Email [email protected]
Abstract: Bronchopulmonary dysplasia (BPD) is a complex pulmonary condition that arises in preterm infants secondary to a constellation of adverse exposures contributing to impaired lung development and repair. In many developed countries, the incidence of BPD continues to rise despite the increasing use of evidence-based therapies and efforts to minimize adverse post-natal exposures. Refinement of perinatal care practices that reduce mortality in the most immature infants is likely contributing to this trend; however, a growing population of survivors with respiratory morbidity significantly burdens individuals and health-care systems. Racial and socioeconomic disparities also result in unequal distribution of morbidity within society. In this review, we will outline trends in the incidence of BPD over the last decade and review important risk factors for adverse pulmonary outcomes. The consequences of BPD for long-term health will be described, followed by a comprehensive summary of evidence-based therapies and potential future treatments that can be applied to reduce the incidence, and the prevalence of BPD.
Keywords: chronic lung disease, prematurity, invasive mechanical ventilation, non-invasive respiratory support, surfactant, steroids, oxygen, inflammation
Introduction
Bronchopulmonary dysplasia (BPD) was first described over 50 years ago as a predominantly fibrocystic lung disease occurring in late preterm infants requiring invasive mechanical ventilation (IMV) and high concentrations of O2 for management of respiratory distress syndrome (RDS).1,2 Subsequent advances such as surfactant replacement and antenatal steroid administration have altered the disease phenotype3 and allowed for increases in long-term survival of extremely preterm infants.4,5 Although our understanding of the patho-mechanisms of BPD has increased rapidly in the last two decades, there remains a paucity of therapeutic interventions proven to reduce the incidence of both short- and long-term respiratory dysfunction.6,7 This situation is reflected by reports indicating that the incidence of BPD is increasing in several high-income countries,4,5,8 often despite increased adoption of evidence-based practices. As BPD is associated with significant long-term morbidity9–11 and increased health-care costs,12 the lack of significant progress in preventing this most common complication of prematurity has serious implications for individual and population health.
Prevalence of BPD-related morbidity in pediatric and adult populations is challenging to estimate, as individuals born preterm without BPD may also demonstrate differences in lung function13 and increased respiratory morbidity.14,15 The term post-prematurity respiratory disease (PPRD) can be used to describe ongoing morbidity in survivors of preterm birth. While BPD remains an important risk factor for PPRD, other factors such as lower gestational age (GA)16 and birth weight (BW),14 respiratory infections early in life17 and maternal smoking14,18 are also important. The first part of this review will address current trends in the incidence of BPD and describe the impact of PPRD on individuals and health-care systems. The second part summarizes evidence-based interventions and approaches used to prevent and manage early, evolving and established BPD.
Prevalence of BPD
Trends in the Incidence of BPD and Associated Risk Factors
Over the last 5–10 years, increases in survival continue to be reported in extremely low BW (ELBW) infants,4,5,8,19 including those born between 22 and 23 weeks GA.4,5,20,21 Although reductions in mortality have been accompanied by decreases in other neonatal morbidities such as necrotizing enterocolitis (NEC)4 and severe intraventricular hemorrhage (IVH),4 increasing trends in the incidence of BPD have been recognized in several populations.4,5,8 The most recent report on care practices and outcomes of extremely preterm infants cared for in hospitals that are part of the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) described an overall increase in BPD from 44.7% in 2008–2012 to 49.8% in 2013–2018 (adjusted difference 4.3%, 95% CI: 2.8–5.8%).4 Outcomes reported by the NRN of Japan that included 19,370 infants born between 22 and 27 weeks GA demonstrated a significant increase in BPD from 41.4% (95% confidence interval or CI: 36.4–46.4%) in 2003 to 52% (95% CI: 48.2–55.9%) in 2016.5 The composite outcome of BPD and mortality prior to 36 weeks post-menstrual age (PMA) also increased from 52.5% (99% CI: 48–57%) in 2003 to 55.9% (99% CI: 52.2–59.5%) in 2016, indicating that improvements in survival rates may not be sufficient to explain the increase in pulmonary morbidity.5 Of concern, a lack of improvement in BPD rates has been noted despite more frequent use of practices that have been linked to reductions in respiratory morbidity. These practices include decreased prophylactic administration of surfactant,22 increased use of post-natal steroids,23 decreased exposure to IMV5 and increased use of non-invasive respiratory support (NRS).24,25 It appears that the relative weight of different risk factors and injurious exposures need to be further explored with ongoing refinement of treatment strategies.
Several recent studies have identified important risk factors associated with adverse respiratory outcomes (Table 1). Demographic characteristics such as low GA, low BW and male sex continue to be strongly associated with increased risk for BPD.4,5,26–29 Duration of exposure to IMV continues to be a strong predictor of adverse outcomes.27–31 Specifically, IMV exposure in the delivery room (DR)24 and first 3–7 days of life27,28,32 has been linked with increased risk for BPD. These observations indicate that this time-period is of particular importance in determining long-term pulmonary outcomes.33 Adverse consequences of early exposure to inflammation are reflected in associations between chorioamnionitis,5 elevated C-reactive protein (CRP)30 and presence of Gram-negative organisms30 or Ureaplasma species (spp.)34 in respiratory cultures with BPD. Placental insufficiency is linked to the development of BPD and BPD associated pulmonary hypertension (BPD-PH).35 Gestational diabetes mellitus has also been associated with increased risk for BPD30 and BPD-PH.36
 |
Table 1 Summary of Studies Evaluating Trends in BPD Incidence and/or Relationships with Associated Risk Factors |
The presence of risk factors can be used to identify infants with a high likelihood of developing BPD32 and specific adverse pulmonary phenotypes such as BPD-PH. Retrospective studies can only demonstrate associations between exposures and outcomes and not causation. Parallel efforts in pre-clinical research to identify the specific molecular pathways driving these observations are also critical for the development of novel treatments.6
Limitations of Current Clinical Definitions of BPD
As BPD continues to be defined by clinician-prescribed support at 36 weeks37–39,43 or 40 weeks41 PMA, differences in the use of respiratory support modalities or changes in criteria may result in alterations in reported incidence.44,45 The NRN 2019 definition developed by Jensen et al37 has been reported to be better than previously used criteria38,39,43 in predicting long-term respiratory37,44,46 and neurodevelopmental37,47 morbidity, although this has not been consistently seen across all studies.48–50 Grading of BPD severity using NRN 2019 definition was found to correlate more closely with health-care costs such as increased length of stay, costs related to the initial admission and hospital charges sustained in the first year of life45 than either the NICHD 201843 or the Canadian Neonatal Network (CNN) definitions.51 Severity of BPD by NRN 2019 definition does not, however, constitute the only risk factor for PPRD. When the ratio of peripheral oxygen saturation and fraction of inspired O2 (FiO2) was measured in 1005 preterm infants at 36 weeks PMA, lower values were significantly associated with a greater degree of prematurity in infants without BPD.16 These findings indicate that a greater degree of respiratory immaturity at birth could have a significant impact on ongoing pulmonary function. Respiratory viral and bacterial infections sustained early in life can increase risk for pulmonary morbidity,52 while socioeconomic disparities have also been linked to increased frequency of symptoms and health-care utilization in those born preterm.53–55 Non-Hispanic black infants and those receiving public insurance were found to have increased odds of tracheostomy placement for BPD relative to non-Hispanic white infants or infants with private coverage (adjusted odds ratio (aOR)1.25, 95% CI: 1.09–1.43) and (aOR 1.25, 95% CI 1.09–1.43), respectively.56 Active efforts to both further characterize and address factors driving interracial and socioeconomic disparities in pulmonary outcomes are urgently needed.
Infants who remain on respiratory support at 36 weeks PMA have been shown to vary widely in terms of their clinical presentation and response to therapy.57 Specific BPD phenotypes characterized by central airway malacia and PH are disproportionately associated with morbidity and mortality.58,59 There remains a continued need for an objective consensus definition for BPD that allows for more accurate prediction of long-term outcomes and benchmarking between institutions.33
Impact of BPD on Individuals and Healthcare Costs
Analysis of data from a US insurance commercial claims database revealed that 29% of infants born ≤28 weeks GA required one of the following in the first year after discharge: pulmonary medications for ≥30 days, supplemental O2, hospital readmission or emergency room (ER) visit for respiratory indications.15 BPD was present in 86.7% of these infants and was associated with markedly elevated health-care costs.15 Extrapolation of this data to the total US population of infants born at ≤28 weeks in 2015 suggests that nearly 8000 extremely preterm infants would be expected to sustain respiratory complications each year.
Within the population of infants meeting criteria for BPD, there exist several, often overlapping, clinical phenotypes that are differentially associated with adverse outcomes.58,60,61 Impairment of alveolar development that leads to a paucity of abnormally distended airspaces typically presents with impairment of gas exchange and prolonged dependency on supplemental O2.60,61 BPD-PH affects up to 25% of infants with moderate-to-severe BPD62 and is associated with both increased morbidity and mortality.58,62 Prolonged exposure to invasive positive pressure can disrupt normal central airway development, leading to varying degrees of tracheomalacia, bronchomalacia and tracheomegaly.63 In one multi-center study population, tracheobronchomalacia was associated with longer duration of IMV, greater likelihood of tracheostomy placement and increased incidence of co-morbidities such as gastroesophageal reflux and pneumonia.59 Findings from a single-center study also identified central airway disease along with BPD-PH as phenotypes associated with higher rates of tracheostomy placement, use of pulmonary vasodilator therapies and mortality prior to discharge.58 Other clinical phenotypes that have been described include interstitial lung disease and peripheral airway involvement that is associated with increased airway resistance and wheezing in later life.57,60,61
Although the prevalence of symptomatic PPRD decreases after the first 3 years of life, studies continue to demonstrate that children and adults with BPD have reduced exercise tolerance,64 lower quality of life scores,65,66 increased airflow obstruction67,68 and earlier decline in pulmonary function tests (PFTs).67,69 An obstructive pattern of lung disease, characterized by a reduced functional expiratory volume in 1 sec (FEV1) or a reduced ratio of FEV1 to forced vital capacity (FVC) is the phenotype most frequently identified in pediatric and adult survivors of prematurity.52 Clinically, increased airway resistance likely contributes to the elevated frequency of asthmatic symptoms noted in preterm-born children,15,70,71 which in turn, contribute to increased usage of medications such as bronchodilators,71 inhaled and systemic steroids72 and antibiotics,70 in addition to more frequent hospitalizations for respiratory indications.15,70,72
Continued alveolar development in early childhood typically results in improvement in symptoms and weaning from respiratory support;33 however, differences in lung function remain persistent throughout adolescence and early adulthood.73,74 A recent study that compared the exercise capacity, lung function and habits of children with BPD to term-born controls revealed that while oxygen consumption parameters by body weight were comparable between groups, children with BPD demonstrated a greater degree of airflow limitation.75 Children with BPD achieved lower levels of workload on cardiopulmonary exercise test and were over twice as likely to report difficulties in physical exercise and symptoms such as wheezing and shortness of breath on exertion than term-born controls.75 These findings indicate that while alveolar growth may result in improvements in gas exchange, the involvement of peripheral airways may have lasting consequences for future cardiopulmonary and metabolic health. Patterns of early decline in FEV1/FVC in adolescents and young adults with either a history of BPD or extreme prematurity raise concern that PPRD may be a precursor to early development of chronic obstructive pulmonary disease (COPD) in some patients with severe parenchymal involvement.74,76 Severe BPD is linked with neurodevelopmental impairment47,77 which places an additional burden on patients and caregivers. Recent studies continue to report associations between BPD and neurodevelopmental disabilities in infants,47,78 toddlers,78 school-age children79 and adolescents.80
Both respiratory and neurological morbidities associated with BPD contribute to marked increases in health-care costs.12,79 A retrospective study utilizing data obtained from infants born in California between 2008 and 2011 calculated median hospitalization costs during the first year of life for a <30 week GA infant with BPD to be $377,871 compared with $175,836 for an infant without BPD.81 Severe BPD was associated with a median total incremental admission cost of $35,773 (95% CI: $32,018 - $39,528) making this second most expensive complication of prematurity after surgical NEC.82 A microsimulation model based on mortality and morbidity data noted in populations of preterm infants with and without BPD estimated that patients with BPD would incur an average lifetime healthcare cost in Canadian Dollars (CAD) of $716,912 (95% CI: CAD $416,000 - $1,275,000).9 Coordinated efforts to prevent BPD both through optimization of existing treatment strategies and through the development of new therapies therefore have strong potential to conserve healthcare spending.
Prevention of BPD
Recent updated evidence-based guidelines are included in Table 2, which is adapted from recommendations we have previously published.33 While individual interventions by themselves are important, it is critical that they are adopted in a systematic manner with multi-disciplinary team input and involvement. Quality improvement (QI) projects provide critical guidance on how approaches can be combined and implemented to improve local/institutional outcomes. Table 3 provides a summary of the results of several recently published QI projects that described success in improving respiratory outcomes.83–85 Each QI team placed an emphasis on optimization of DR management with standardized, GA specific protocols for surfactant replacement, initiation of caffeine, NRS and invasive ventilatory support.83–85 The results of these and other recent QI projects demonstrate the impact of systematic initiation of GA-specific approaches that begin at birth. Integration of evidence-based practices described in the following section using a QI-based framework is therefore strongly recommended.
 |
Table 2 A Summary of Evidence-Based Recommendations for Prevention and Management of BPD |
 |
Table 3 A Summary of the Strategies and Results of three Recent QI Projects That Successfully Reduced Adverse Pulmonary Outcome |
Prenatal Strategies
Exposure to antenatal steroids (ANS) is associated with reductions in multiple complications including RDS, IVH, NEC and mortality.126 These benefits have been shown to be relevant in populations born at <24 weeks GA.127 The early advantages gained by prenatal steroid exposure have not, however, been shown to result in improvement in long-term respiratory outcomes.128 Of note, receipt of repeated courses of ANS in the absence of preterm delivery has been linked to adverse respiratory and neurological outcomes in term infants.129 Current European perinatal guidelines advocate for routine administration of ANS at 24–33+6 weeks gestation in pregnant women deemed at high risk for preterm delivery in the next 7 days.130 ANS can be offered between 22 and 24 weeks GA when active management of the infant is anticipated.130
DR Strategies: Optimal Diagnosis and Management of RDS
Pooled meta-analysis data from four randomized clinical trials (RCTs) comparing early initiation of continuous positive airway pressure (CPAP) with prophylactic surfactant administration for infants 24–28 weeks GA,131–134 showed an association between early CPAP and reduced risk for BPD [relative risk (RR) 0.90, 95% CI: 0.83–0.98, risk difference (RD) −0.04, 95% CI: −0.08 −0.00), number needed to treat (NNT)=25].131,132,135 Given the strong link between early exposure to IMV and development of BPD,27 a greater advantage might be expected. Rates of CPAP failure approaching 50%131–134 were thought to explain the small effect size. Receipt of surfactant within 2 hr of birth is associated with significant reductions in the risk for BPD and the combined outcome of BPD and mortality.88 Delayed surfactant administration in patients who failed CPAP, may therefore, have contributed to lung injury.136 Factors predicting CPAP failure include lower GA, lower BW,90,91 lack of receipt of ANS,90 evidence of severe RDS on chest radiograph,90 and FiO2 ≥0.3.90 CPAP failure is associated with a significantly increased risk of pneumothorax, BPD and mortality90,91,137 suggesting that earlier interventions could be beneficial. The current European guidelines recommend surfactant administration for FiO2 ≥0.3 in infants supported by CPAP;138 however, more accurate predictive tools are needed. In USA, RDS-NExT workshop conducted by an expert panel of health-care providers has recently made specific recommendations for surfactant administration based on a clinical score, respiratory support status, FiO2 requirement and blood gas values.89 Lung ultrasound (US) performed at the bedside has been shown to be effective in diagnosing RDS and predicting need for surfactant treatment.139 While this approach shows promise, there is currently no direct evidence of any long-term impact in reducing BPD.
Techniques that allow for timely receipt of surfactant while minimizing exposure to IMV have been associated with reduced risk for BPD.51,140 The “Intubate, Surfactant, Extubate” (InSurE) strategy describes delivery of surfactant through an endotracheal tube (ETT) after which the tube is removed quickly and the infant placed back on NRS. Less invasive surfactant administration (LISA) involves delivery of surfactant into the airway via a thin flexible catheter during spontaneous breathing. Recent meta-analyses of RCTs comparing LISA to either InSurE or intubation with continued IMV reported both a decreased risk of BPD and a reduction in the combined outcome of BPD and mortality.140,141 Although pooled results from clinical trials suggest an advantage for LISA over InSurE, a direct comparison between these two methods in extremely preterm infants has not been made, as in many investigations a variable duration of IMV occurred following the procedure. There have also been concerns raised regarding the safety and effectiveness of LISA, after a recent RCT showed a trend towards increased mortality in infants 25–26 weeks GA who received minimally invasive surfactant therapy.142 These findings are in contrast with those of a large retrospective study of 6542 infants 22–26+6 weeks GA cared for in centers that are part of the German Neonatal Network.143 In this population, LISA was associated with reduced risks of all-cause death [OR: 0.74, 95% CI: 0.61–0.9.0; p=0.02], BPD (OR: 0.69, 95% CI: 0.62–0.78; p<0.001), and BPD or death (OR: 0.64, 95% CI: 0.57–0.72; p<0.001).143 Reduced risk for BPD and mortality remained significant in all GA groups even when adjusted for adverse perinatal characteristics.143 LISA has been readily adopted in Germany and other European countries as part of a bundled approach to transitional care that includes delayed cord clamping to support hemodynamic transition, minimal handling, and avoidance of procedural pain.144 Of note, Germany is one of the few developed countries where a decrease in the incidence of BPD has occurred despite increases in survival.19 The context in which LISA is used, together with the skill level of the clinician administering therapy are likely to have an important influence on outcome. Careful investment in training and guidelines for safe use of LISA are, however, necessary to avoid complications.145
DR Strategies: Use of O2 During DR Resuscitation
The amount of O2 required during DR resuscitation and during the first few hours of life has been linked to increased risk for BPD. To date, there remains little evidence linking specific regimens of O2 titration with reduced risk for BPD. Failure to achieve saturations >85% and/or heart rate >100 bpm by 5 min of life was linked with higher risk of both BPD and death in a recent multi-center RCT.146 Therefore, clinicians attending deliveries of extreme preterm infants should approach resuscitation with the goal of achieving cardio-pulmonary stability within this timeframe.
Early Course: First Week of Life – Early NRS Strategies to Reduce BPD
Retrospective study data suggest early attempts at extubation have potential to prevent BPD in the most immature infants.147,148 However, other reports indicate that the success of these attempts plays a pivotal role in determining outcome.149 Hence, efforts to maximize success of extubation are important; however, the most optimal NRS strategy for use in preterm infants at risk of BPD has not been conclusively identified. In “Bubble CPAP”, continuous distending pressure is generated by submersion of the expiratory limb of the circuit in varying levels of water.150 The variability in pressure caused by bubbling in the water-seal chamber has been linked to enhanced alveolar recruitment and surfactant production in animal studies.150 Results from pooled RCTs have not, however, shown any advantage of bubble CPAP above flow-driver delivered CPAP in terms of reducing pneumothorax or BPD.151 Nasal intermittent positive pressure ventilation (NIPPV) is a technique where an increased positive inspiratory pressure (PIP) is provided transiently, typically between 20 and 40 times a minute, over a set of positive end expiratory pressure (PEEP).116 These cycles are not synchronized with the infant’s spontaneous breaths but act to improve recruitment of small airspaces and enhance respiratory drive.116 Although meta-analysis data suggest that NIPPV may be superior to CPAP in terms of prevention of BPD and/or the composite outcome of BPD and mortality,51 this advantage may be due to the higher mean airway pressure (MAP) generated by intermittent rises in PIP rather than the support strategy itself. Increased use of NIPPV was not associated with increased survival without moderate-to-severe BPD in a recent multicenter cohort study evaluating the outcomes of 6735 VLBW infants who did not require intubation during their NICU stay.152 In a recent Cochrane review, early NIPPV (ie within 6 hr after birth) reduced the risk of respiratory failure and the need for intubation and IMV in very preterm infants (GA ≥28 weeks) with RDS, compared to CPAP. It was also noted that there was a slight decrease in the rate of BPD (RR 0.70, 95% CI 0.52–0.92).92 However, this may not apply to extremely preterm infants that are most at-risk of BPD.92 Hence, additional studies are required to evaluate the efficacy of NIPPV in preventing BPD in extreme preterm populations.
Nasal high-frequency ventilation (NHFV) employs rapid oscillatory changes in pressure around a set MAP to enhance recruitment of small airways in preterm infants.150 This technique, although less widely used than either NIPPV and nasal or NCPAP, has been shown to be a safe option in preterm infants. Results from a recent RCT comparing NHFV to CPAP and NIPPV as a post-extubation support modality, suggested that NHFV may be more effective in reducing the duration of IMV than either CPAP or NIPPV.153 Subgroup analysis of participants in the treatment group who either were born at <29 weeks GA and/or had evidence of a more severe degree of respiratory failure demonstrated significantly reduced incidence of moderate-severe BPD relative to infants in the CPAP group (range, −12% to −10%; NNT 8–9 infants).154 These results suggest that NHFV is safe and may have some advantages over more frequently used NRS modalities.
Neurally adjusted ventilatory assist (NAVA) utilizes electrical signals from the diaphragm detected by a sensor implanted in a specially designed feeding tube to adjust the intensity of inflations and synchronize these cycles to the patient’s own respiratory effort.150 This strategy can be applied to infants receiving IMV or NRS (non-invasive ventilation or NIV-NAVA). Small crossover trials have shown that NIV-NAVA is associated with reduced work of breathing and improved oxygenation at a lower delivered PIP when compared with other NRS strategies.155,156 Two small prospective RCTs associated NIV-NAVA with reduced rates of extubation failure when compared to CPAP157 and NIPPV;158 however, no long-term benefits in terms of a reduction in BPD have been noted.157 While NIV-NAVA has been adopted with apparent success in several centers,83,159 evidence from larger prospective trials is needed in order to demonstrate a clear advantage of NIV-NAVA over other NRS modalities.
Early Course: First Week of Life – Early Invasive Respiratory Support Modalities
Infants who require initiation of IMV during the first week of life represent a group at high risk for BPD.27,31 Delivery of excessive tidal volume is known to be highly injurious to the developing lung and opening of atelectatic areas also leads to a potent inflammatory response.160 Volume targeted conventional mechanical ventilator modalities aim to avoid both excessive airspace distension and collapse through automated adjustment of PIPs to achieve a set tidal volume. Interventions that lead to improvements in lung compliance such as surfactant administration can therefore lead to automated weaning of inspiratory pressure. Meta-analysis data indicate that volume targeted ventilation is associated with reduced risk for BPD when compared to pressure limited ventilation.93 Retrospective study data suggest that this advantage may also be applicable to infants born between 22 and 25 weeks GA, therefore a volume targeted strategy on a conventional ventilator is a reasonable first-line approach in this population.161 High-frequency oscillatory ventilation (HFOV) represents an alternative lung protective support strategy in preterm infants at risk for BPD. Movement of a piston is used to generate rapid low volume oscillations that are superimposed over a constant MAP at rates of 300–900 cycles per minute.162 A Cochrane review comparing HFOV to conventional IMV demonstrated an association between reduced risk of BPD and HFOV use (RR: 0.86, 95% CI: 0.78–0.96) but increased risk for pulmonary air leak (RR: 1.19, 95% CI: 1.05–1.34).94 BPD outcomes were noted to be highly inconsistent between studies even after subgroup analysis was performed to take into account the use of different lung recruitment strategies.94 As most of the trials compared HFOV to pressure limited conventional IMV, it is not clear if this strategy offers superior lung protection to volume targeted IMV strategies. A follow-up study of participants in the HIFI trial163 demonstrated an association between HFOV and improved small airway function at 11–14 years.164 No significant differences in lung function parameters were, however, noted when trial subjects were assessed at 18–21 years of age.165 Despite these caveats, primary use of HFOV has been adopted as a QI bundle intervention in several units that have successfully reduced their BPD rates.83,84 Earlier initiation of a higher frequency (15–20 Hz), tidal volume limited HFOV strategy as a rescue therapy for preterm infants failing conventional IMV was associated with increased survival free of grades 2/3 BPD (OR 2.93, 95% CI: 1.41–6.05) and reduced hospital admissions and respiratory treatments post discharge (aOR 2.33, 95% CI: 1.10–4.93).166 Further application of this HFOV-volume guarantee approach may be helpful in limiting early lung damage and preventing BPD; however, efficacy of this modality is yet to be evaluated in a prospective trial.
High-frequency jet ventilation (HFJV) has been frequently considered by several centers as an alternative lung protective strategy for use in high-risk preterm infants.159 PEEP delivered by a conventional ventilator is used to maintain lung recruitment, while high-velocity pulsations generated by a pinch valve are delivered via a second ETT attachment.162 These pulsations generate turbulence and encourage molecular diffusion to occur in small airspaces. Although there is no evidence from prospective RCTs that primary application of HFJV prevents BPD, centers that use this strategy have reported improved outcomes, particularly in the most immature infants 22–25 weeks GA.167,168
A proactive approach to weaning support should be strongly considered as continued IMV dependency on day of life (DOL) 7 has been consistently associated with increased risk for BPD.28,148,169 Use of NAVA has been associated with more rapid weaning of the ventilator when compared to the use of pressure limited strategies,170 suggesting this could be a helpful transitional strategy. Readiness for extubation should be considered carefully in the most immature infants, given the association with early failure and multiple adverse outcomes.149
Early Course: First Week of Life – Caffeine
Caffeine is a methylxanthine medication that has been used for over 5 decades with the purpose of preventing central apnea in preterm infants. Data from a multicenter RCT demonstrated an association between early caffeine use and reduced risk for BPD (OR: 0.63, 95% CI: 0.52–0.76; p< 0.001)100 with follow-up studies also demonstrating positive effects on neurodevelopmental outcome.102,171 The protective effects of caffeine appear to be most apparent in infants treated within the first 3 days of life.101,172–175 Early initiation of caffeine is, to date, the only BPD preventive strategy that has been associated with both improved respiratory and neurodevelopmental outcomes.171,176,177 Long-term benefits include increased survival without disability at 18–21 months of age171 and reduced incidence of developmental co-ordination disorder along with improved lung function at 11 years of age.177,178
Caffeine use has been associated with increased likelihood of successful extubation179 and reduced duration of IMV.101,173 These observations are likely due to enhancement of respiratory drive and diaphragmatic contractility.180 Early caffeine use is also associated with a reduction in medical management of the patent ductus arteriosus (PDA)100,172,173 as well as diuretic, anti-inflammatory,181,182 pro-angiogenic183,184 and antioxidant184–186 effects. Caffeine citrate is typically initiated with a loading dose of 20 mg/kg with maintenance therapy, then started 24 hr later at 5–10 mg/kg/day.187 For infants either weaning on IMV or receiving NRS, dose is usually titrated according to the frequency and severity of apneic events. Such a titration does not usually occur in the most immature, critically ill infants who require higher levels of IMV support. Several studies indicate that increased loading (>20 mg/kg) and/or maintenance doses (>5 mg/kg/day) may be more beneficial in preventing BPD.188 Some studies have reported increased rates of tachycardia with higher caffeine doses,189,190 with other side-effects including feeding intolerance, hyponatremia, hypertension and hyperglycemia.191 In some centers, a maintenance dose of 8–10 mg/kg/day is used with weekly weight adjustment of maintenance dose in the range of 8–20 mg/kg/day to prevent extubation failure.192 Further work evaluating the pharmacodynamics of caffeine along with outcomes in different populations is needed to optimize the preventive properties of this treatment.
Early Course: First Week of Life – Vitamin A
A RCT conducted in ELBW infants demonstrated an association between early vitamin A administration and reduced risk for the composite outcome of death prior to 36 weeks PMA and BPD (55% versus 62%, RR: 0.89, 95% CI: 0.80–0.990, NNT 14–15).105 Despite the significant effects noted by this trial, differences in the perceived benefits of vitamin A by clinicians have led to inconsistent uptake of this approach in clinical practice.193 Pain from repeated intramuscular injections together with the significant financial cost of vitamin A may also contribute to this finding. The failure of subsequent studies to demonstrate long-term pulmonary benefits from parenteral vitamin A supplementation,106 coupled with the observation that BPD rates remained stable during a national vitamin A shortage,194 adds weight to the argument that this is a less efficacious strategy to prevent BPD. Even a small reduction in the risk of BPD in a high-risk population may, however, offset a substantial part of the cost of this therapy. A more recent meta-analysis not only showed an association between vitamin A and reduced dependency on supplemental O2 at 36 weeks PMA (pooled risk ratio: 0.88, 95% CI: 0.77–0.99; 4 trials, 841 infants, moderate certainty of evidence) but also suggested that vitamin A use is linked to reduced length of hospital stay (mean difference, −49.9; 95% CI: −88.78 to −11.02; 1 trial, 20 infants, low certainty of evidence).195 This may be another area where this treatment may prove cost-effective. Although vitamin A should be strongly considered as part of a bundle for BPD prevention in ELBW infants, this strategy is unlikely to be pivotal in reducing adverse respiratory outcomes.
Early Course: First Week of Life – Low Dose Hydrocortisone
Hydrocortisone initiated at low doses to counteract relative adrenal insufficiency during the first week of life is associated with a reduction in the composite outcome of mortality and BPD (RR: 0.90, 95% CI 0.82–0.99).107 Low dose hydrocortisone is not, however, associated with a reduction in BPD (RR: 0.92, 95% CI: 0.81–1.06).107 A single patient meta-analysis indicated that female sex, ≥26 weeks GA and exposure to chorioamnionitis were factors linked to increased odds of BPD-free survival following receipt of hydrocortisone.196 Risks associated with early low-dose hydrocortisone therapy include gastrointestinal perforation, which is more likely with concomitant use of indomethacin and early onset sepsis, which was noted more frequently in infants <26 weeks GA.197 A cortisol nomogram developed using data compiled from a large RCT of early hydrocortisone therapy197 suggested that infants with high baseline cortisol levels exposed to hydrocortisone were at increased risk for spontaneous intestinal perforation and severe IVH compared to those with comparable results who received placebo.198 These findings suggest the presence of a “Goldilocks phenomenon” where either inadequate199 or excessive198 mineralocorticoid stimulation is associated with adverse effects. Until there is improved delineation of the patients most likely to benefit from treatment, early hydrocortisone therapy should be approached with caution.
Early Course: First Week of Life – Targeted Medical Management of the PDA
Although there is considerable evidence to suggest that prolonged patency of the ductus arteriosus contributes to adverse respiratory outcomes in preterm infants,200–203 recent RCTs and QI studies have not demonstrated that use of cyclo-oxygenase inhibitors to induce earlier closure of a hemodynamically significant PDA (hsPDA) reduces BPD.204–206 Failure of medications to reliably induce ductal closure in preterm infants <26 weeks GA, combined with significant rates of spontaneous closure and open-label treatment, pose limitations on the accuracy of information obtained from RCTs.204 Of note, data from a recent QI project that implemented a “Targeted Prophylactic Indomethacin” treatment approach for infants either born <25 weeks GA or ≥25 weeks GA and requiring IMV at 72 hr of life did show a significant decrease in the duration of ductal patency when compared with expectant management.204 Reduction in exposure to a PDA in this study was not, however, associated with decreased incidence of BPD.204 The impact of a hsPDA does, however, seem to be modulated by the duration of IMV.207 Infants exposed to IMV for <10 days were found to have a reduced risk for grade 2–3 BPD regardless of the presence of a hsPDA. Exposure to a hsPDA for >11 days was, however, associated with almost 2 times the rate of a Grade 2–3 BPD diagnosis in infants who required >10 days of IMV (PDA exposure >11 days 79% with Grade 2–3 BPD versus 40% with PDA exposure <11 days, p< 0.01).207 It is possible that longer exposure to IMV sensitizes the airspaces and pulmonary vasculature to the additional stress of a significant left-to-right shunt. Closure of a PDA should therefore be considered in patients likely to require IMV beyond the first week of life.
Results from centers who have adopted and developed techniques in early hemodynamic screening suggest that proactive identification and management of a PDA may benefit infants <24 weeks GA who have been underrepresented in RCTs.208 Ongoing efforts to further define PDA-related risk factors for BPD,209 to develop alternative therapies to induce ductal closure210 and to identify subgroups of infants who may benefit from treatment211 may, however, have a significant impact in improving respiratory morbidity.
Early Course: First Week of Life – Judicious Use of Antimicrobial Therapy
A recently published retrospective study that included 196 infants <28 weeks GA suggested that babies with airway cultures or PCR positive for Ureaplasma spp. on admission may have over 4 times the risk of developing BPD compared to those without a positive test.212 These findings are supported by data from previous clinical and pre-clinical studies that link Ureaplasma spp. infection to increased risk for BPD.213,214 RCTs evaluating the strategy of early eradication of Ureaplasma spp. with azithromycin have not, however, shown consistent benefit in reducing BPD.215–217 Although there is some evidence that treatment of infants who test positive for Ureaplasma spp. can reduce the risk of BPD,217 a prophylactic approach could lead to many more infants being exposed to a therapy that could be harmful. Disturbances in the airway microbiome at birth, with increased Gram-negative Gammaproteobacteria relative to Gram positive Lactobacilli has been associated with BPD.218 The combination of dysbiosis and hyperoxia has also been linked to more severe disturbances in lung architecture in animal models.219,220 Exposure to antibiotics both in the first week of life221,222 and throughout the NICU stay119 is associated with BPD, with evidence of a dose–response relationship.119 Significantly higher odds of moderate-severe BPD were noted in a cohort of infants <32 weeks GA without risk factors for infection who were exposed to antibiotics within the 24 hr of life (OR: 2.30, 95% CI: 1.21–4.38).108 These results suggest that movement towards a selective approach to early antibiotic treatment may be a more effective BPD preventive strategy. In centers where turnaround time for Ureaplasma spp. detection is less than 48 hr, prophylaxis with azithromycin may be reasonable. Treatment could also be considered if there is also high clinical and radiographic suspicion of active infection.223
Early Course: First Week of Life – Fluid Management and Nutritional Strategies
Subgroup analysis of the multicenter Preterm Erythropoietin Neuroprotection Trial (PENUT) revealed that higher peak fluid balance (calculated by percentage change in weight from BW) was associated with increased risk for IMV on DOL 14 and in the combined outcome for BPD and mortality.109 Every 10% increase in peak fluid balance was associated with 103% increased odds of receipt of IMV on DOL 14 (aOR: 2.03, 95% CI: 1.64–2.51).109 Current evidence suggests that keeping the baby “dry” (ie BW loss in the range of 6–15% and maintaining serum sodium levels at the lower end of normal) from DOL 1–10 might be helpful in the prevention of BPD.224 Additional information from prospective trials is required to identify optimal fluid management practices preventing BPD.
Suboptimal nutritional intake in the first 2 weeks of life has been linked to both short-110,225 and long-term112 pulmonary outcomes. Standardized feeding protocols that optimize components of parenteral nutrition whilst promoting timely advancements in enteral feeding have been shown to be effective in reducing time to full enteral feeds and improving growth parameters in preterm infants.226–228 Human milk, particularly colostrum, contains many immuno-modulatory protective components that act to promote a more favorable microbiome and help prevent BPD. Results from a prospective cohort study investigating the impact of own mother’s milk (OMM) on neonatal outcomes reported a 9.5% reduction in the odds of developing BPD for every 10% increase in receipt of OMM [aOR 0.905, 95% CI: 0.824–0.995].229 Systemic racism and socioeconomic hardship have been shown to have a negative impact on provision of OMM.230 It is imperative that these disparities are considered when planning initiatives to encourage provision of OMM to ensure equitable improvement in outcome.
Evolving Course: DOL 7 – 36 Weeks PMA
Evidence strongly points towards the perinatal period and first post-natal week as being an exquisitely sensitive time for determination of both short- and long-term pulmonary outcomes. Interventions adopted to reduce BPD beyond this time-period have typically focused on reducing duration of IMV as this represents a major risk factor for respiratory morbidity.29,231 Results of recent studies that have shown increasing trends in BPD rates, even with similar to reduced duration of IMV and increased usage of NRS,5,25 indicate that other factors, such as maintenance of an adequate growth trajectory and avoidance of atelectasis may also be important. The use of invasive ventilatory strategies that limit volutrauma while maintaining adequate functional residual capacity (FRC) such as volume targeted ventilation93 and HFV159,168 may be more helpful in avoiding respiratory morbidity infants who are not yet ready to tolerate extubation and/or are <23 weeks GA.232 Tolerating a mild degree of permissive hypercapnia (PaCO2 55–65 mmHg), particularly in infants maintained on NRS should be strongly considered, but not at the expense of maintaining adequate lung recruitment and growth.33
Selective treatment of preterm infants dependent on IMV after 7 days of life with low-dose dexamethasone has been shown to be effective in facilitating extubation.113 This specific benefit of steroid treatment may underly findings from the latest Cochrane review that suggest an association between dexamethasone treatment after 7 days of life and a reduction in O2 dependency at 36 weeks PMA [RR 0.76, 95% CI: 0.66–0.870].114 Unlike treatment during the first 7 days of life, exposure to dexamethasone after 7 days was not associated with increased risk of the combined outcome of cerebral palsy and mortality.114 This may reflect key differences in the characteristics of infants undergoing late treatment, as exposure to dexamethasone reduces the composite risk for death or neuro-disability in patients at high risk of developing BPD.115,233 Given concerns regarding adverse effects on neurodevelopmental outcomes noted with early use of dexamethasone, treatment is rarely given before 21 days of life and often postponed further. Of concern, increasing trends in the use of post-natal steroids were not associated with reduced incidence of BPD, indicating a need for further study and refinement of treatment strategies.23 Evidence from a retrospective analysis indicates that infants who received dexamethasone between 21 and 28 days of life were more likely to avoid a diagnosis of severe BPD than those treated after DOL 50.234 No significant differences in neurodevelopmental outcomes were noted between infants in the earlier and later treatment groups.234 Receipt of steroids >36 days of life was associated with approximately twice the risk for Grade 2 or 3 BPD when compared to exposure between 8 and 21 days of life [aOR: 2.0, 95% CI: 1.1–3.70] in a retrospective study conducted by centers enrolled in the Children’s Hospital Neonatal Consortium (CHNC).235 Although it seems surprising that almost half of the patients in this cohort received their first dose of systemic corticosteroids in the 8–21-day timeframe, a much higher proportion of infants in this group received hydrocortisone (77.9%) versus dexamethasone (22.1%) when compared with those that received later treatment.235 Clinicians may be more willing to use hydrocortisone before 21 days of life as unlike dexamethasone, early postnatal hydrocortisone use has not been linked to neurodevelopmental impairment.107,236,237 No evidence of adverse outcomes has been noted in the follow-up of participants in the SToP BPD Trial, who received later, higher doses of hydrocortisone;237 however, there is as yet no evidence from RCTs that treatment with hydrocortisone beyond the first week of life is helpful in reducing BPD even for higher risk populations.238–240 In summary, treatment with systemic steroids with the goal of reducing risk for BPD should be strongly considered in preterm infants who remain IMV dependent at or approaching 21 days of life.
Diuretic therapy is another strategy that is frequently used to improve lung compliance and reduce respiratory support requirements. In a large retrospective analysis, longer duration of furosemide exposure from DOL 7 to 36 weeks PMA was associated with a significantly lower risk of BPD, suggesting a potential benefit.241 Unfortunately, electrolyte losses caused by loop diuretics also increase the risk for metabolic bone disease, nephrocalcinosis and growth failure.242 In the absence of RCT data indicating a relationship between diuretic use and reduced risk for BPD, short-term use of these medications should be restricted to infants with clinical or radiographic evidence of pulmonary edema.33 Long-term use of diuretics should be restricted to patients who have demonstrated objective evidence of improvement. Assessment of pulmonary edema using lung US is a potentially useful strategy to guide selective medication administration and to determine the degree of response.243
Initiation of inhaled bronchodilators and inhaled steroids between 7 days to 36 weeks PMA varies widely between different neonatal units. To date, the use of these medications during this time-period has not been either associated with a reduction in BPD or an increase in any specific adverse outcome. For this reason, no firm recommendations on their use can be made.
Evolving Course: DOL 7 – 36 Weeks PMA – Maintain Growth Trajectory
Strategies that promote growth in preterm infants must involve measures to reduce unnecessary energy expenditure such as adherence to incubator weaning protocols and provision of adequate respiratory support. The practice of maintaining extremely preterm infants on NRS until they reach 32 weeks PMA has been included as a bundle intervention in quality improvement projects that successfully reduced BPD.83,85 This approach could be explained by data suggesting that weaning by transition to high flow nasal cannula (HFNC) is associated with increased duration of O2 exposure and increased length of stay.244–246 Another assumption behind the practice of routine prolonged CPAP administration is that this may help conserve energy and facilitate pulmonary growth and repair. This is supported by the results of a small RCT (n=44 analyzed) that linked extended CPAP with improvement in FRC measurements.117 There are, however, legitimate concerns regarding the impact of long-term continuous distending pressure on the developing lung.247 The utility of routine extension of CPAP until 32 weeks PMA or 1250g versus gradual weaning from CPAP to HFNC will be tested objectively in a clinical trial that is currently recruiting patients (NCT05557139). Information from this study may help further inform NRS practices used beyond 7 days of life.
Feeding protocols that standardize optimization of nutritional intake throughout the NICU stay, with continued daily tracking of caloric intake and weight gain, have not been directly associated with a reduction in BPD but are likely to be of benefit.248 Continued research to identify specific nutritional strategies to optimize growth is necessary to inform practice and mitigate long-term health outcomes.
Emerging Treatments
Increasing trends in the incidence of BPD reflect the need for novel therapies to protect the developing lung and promote growth and repair. Current treatments have relatively non-specific effects and often do not show the same degree of benefit in infants <25 weeks GA. Table 4 provides a summary of emerging therapies that are currently being evaluated in RCTs. Parallel research to identify important predictive markers of specific BPD phenotypes is also important for direct interventions towards patients who will demonstrate the most benefit.7
 |
Table 4 A Summary of Emerging Therapies for BPD Currently Being Evaluated in Clinical Trials |
Established BPD: > 36 Weeks PMA – Multidisciplinary Approach
Infants with established BPD, especially those with moderate-to-severe disease, benefit from a multidisciplinary approach. The core team should at a minimum include neonatologists experienced in the care of infants with BPD, a dedicated clinical pharmacist and dietician in addition to support from physical and occupational therapist. The expertise of other pediatric subspecialists is also required to optimize outcomes. Pediatric pulmonologists well-versed in the care of infants with established BPD can help determine the most appropriate long-term ventilatory strategy, evaluate for airway pathology with bronchoscopy and ease the transition from inpatient care to outpatient management. Infants with either concern for or established BPD-PH should be followed closely by a pediatric cardiologist to determine the frequency of surveillance and need for medications and/or cardiac catheterization. Pediatric otolaryngologists play an essential role in the evaluation and management of infants who have airway complications necessitating corrective surgery or a tracheostomy. Involvement of a pediatric general surgeon may also be necessary, since infants may develop oral aversion or have aspirations that require a gastrostomy tube.262 This interdisciplinary team-based approach that begins during the initial hospitalization and continues post-discharge has been shown to decrease the incidence of moderate-to-severe neurocognitive impairment and decrease the rates of readmission.263 A US institution that adopted a multidisciplinary approach to infants with BPD-PH was able to achieve resolution of PH in all their patients, with minimal vasodilator therapy (8%) and low mortality (5%).264 Various institutions have also found that a multidisciplinary approach to the care of infants with ventilator-dependent BPD results in improved survival until discharge.262,265 Development of a multi-disciplinary approach is therefore deemed to be essential in delivering optimal care to infants with BPD who require long-term IMV. Physical, Occupational and Speech therapy should all be instituted, while infants with established BPD are still in the hospital to provide early, age-appropriate interventions that optimize neurodevelopmental outcomes. Physical therapists can provide input regarding tolerance and engagement with age-appropriate activities that help determine the adequacy of respiratory support. Feeding difficulties are common in infants with established BPD and early referral to a speech therapy team should be considered. Some infants with established BPD may have recurrent aspiration further compromising pulmonary status. As aspiration can occur without clinical symptoms; therefore, a swallow study may be necessary to evaluate the safety of oral feedings and to prevent continuing lung injury. After discharge, infants require ongoing neurodevelopmental follow-up to promptly identify and address areas of concern.262
Established BPD: > 36 Weeks PMA – Respiratory Support
Optimization of respiratory support in the phase of established BPD requires a shift from a preventive-focused approach to a strategy that prioritizes somatic growth and developmental progress. Rapid weaning and avoidance of IMV are no longer the primary aims and may be counterproductive. The optimal approach to delivering respiratory support in this population has not been extensively studied, and therefore the reliance is on physiologically sound approaches informed by results from high-volume centers.262,266 Infants with a milder degree of respiratory compromise can be adequately supported with low-flow NC or NRS; however, infants with severe forms of BPD may require IMV for months to years.267 Weaning from their current level of support should only be initiated once comfortable breathing, growth and tolerance of developmental therapies have been demonstrated.262,266 Any reduction in settings should be performed in incremental steps, no more frequently than once or twice a week.262,266
Chronic exposure to supplemental oxygen and positive pressure during the evolving phase of the disease transforms the properties of the lungs of an infant with severe BPD to the extent that IMV strategies that were previously successful are unlikely to be effective.268 Although specific respiratory phenotypes vary between patients, common pathologic findings in infants with grade 3 BPD include parenchymal edema, inflammation of the small airways, and alveolar oversimplification.269,270 These factors combine to create the heterogeneous pattern of a multicompartment lung. The fast compartment of the lung consists of areas that have relatively normal alveolar dead space, airway resistance, and emptying times. In contrast, the slow compartment of the lung is characterized by larger alveolar dead space, increased airway resistance, and longer emptying times.271 In most infants with severe BPD, the slow compartment predominates; therefore, tailoring strategies to the properties of this part of the lung is ideal.266,268 Moreover, infants may suffer from additional comorbidities, such as airway disease or PH, that need to be addressed when selecting the appropriate IMV strategy.271
Most experts who treat a larger number of infants with grade 3 BPD agree that synchronized IMV (SIMV), whether volume or pressure controlled, is the preferred mode of delivery of IMV for a large part of this severe BPD population.266 HFOV or HFJV do not allow for sufficient expiration and ultimately can lead to more air trapping and impaired ventilation. Most often, infants with established BPD need large target volumes with long inspiratory times and a low rate. Larger targeted volumes (8–15 mL/kg)272 delivered with a long inspiratory time (0.5–1 sec)266 should help overcome the increased alveolar dead space and airway resistance in the slow compartment. Likewise, the low respiratory rate (12–20 bpm) allows for the increased emptying time present in these slow compartments; an I:E ratio of 1:5 is preferable.272 The amount of PEEP (7–12 cmH2O) required will also vary based on disease phenotype. If an infant appears to have a significant component of airway malacia, higher PEEPs are often necessary.266,268 This may seem counterintuitive, but the presence of hyperinflation may be evidence that the current level of PEEP is insufficient to keep the airway open during expiration, leading to increased air trapping.266
No large-scale or long-term RCTs have been aimed at determining the most effective NRS strategies for established BPD. A small study looking at 20 infants with evolving and established BPD found no difference in the work of breathing infants when comparing CPAP and HFNC;273 however, long-term use of these modalities was not assessed. Successful use of both invasive and NIV-NAVA, defined as achievement of respiratory stability at a lower level of respiratory support, has been reported in several centers caring for high volumes of infants with severe BPD.274 This approach has not, however, been evaluated in prospective clinical trials.
Established BPD: > 36 Weeks PMA – Approach to Tracheostomy
Severe BPD has been noted to be the most frequent indication for tracheostomy in infants, accounting for almost 50% of cases.267,275 There is no unifying guideline as to when and in whom tracheostomy is indicated. A retrospective cohort study from 16 centers of the NICHD NRN found that 83% of patients with tracheostomies experienced the composite outcome of death or neurodevelopmental impairment compared with 40% of patients without a tracheostomy [aOR 3.3, 95% CI: 2.4–4.6].276 In a retrospective study, a tracheostomy was performed on average on DOL 118 (95% CI: 107–128).277 Infants who had a tracheostomy before, rather than after, 120 days of life had a lower risk of death or neurodevelopmental impairment (aOR 0.5, 95% CI: 0.3–0.9), suggesting that timing has an important influence on outcome.276 A survey of providers in the CHNC revealed that the most common factors prompting consideration of tracheostomy were as follows: airway malacia (13%), need for higher PEEP (8%), endotracheal positive pressure (11%); PH (16%); multiple courses of systemic corticosteroids (11%); failure to thrive on non-invasive support (11%); and poor growth and feeding (10%).275 Some of the baseline clinical parameters that would prompt providers to begin considering tracheostomy in patients included PCO2 ≥76–85 mmHg, FiO2 ≥0.6, PEEP ≥9–11 cmH2O, respiratory rate ≥61–70 bpm, PMA ≥ 44 weeks, and weight <10th percentile at 44 weeks PMA.275 These findings demonstrate factors that are important to clinicians; however, the decision as to whether a tracheostomy is the best option requires consideration and input from various members of the team and the patient’s family. Factors that should be deliberated include the uncertain duration of the need for IMV, risks associated with surgery, the need to commit the infant to multiple serial procedures, together with the risk of accidental decannulation or obstruction at home.267,278
Established BPD: > 36 Weeks PMA – Long-Term O2 Therapy
Long-term home O2 therapy in infants with established BPD has not been sufficiently studied. Both the American Thoracic Society (ATS)279 and the European Respiratory Society (ERS)121 have recommended the use of home O2 in infants with BPD complicated by chronic hypoxemia. This guideline is based on very low-quality evidence from a few small studies that showed better growth, improved mean pulmonary artery pressures and improved sleep quality and duration for hypoxemic infants who receive supplemental O2. The use of low flow supplemental O2 also allows patients to be discharged home. The ERS suggests a target goal of 90% SpO2 or higher extrapolating from the BOOST II trial that showed an increased mortality in infants born <28 weeks who had a target of 85–89% SpO2.280 While the ATS suggests SpO2 goal of ≥92%, the lower threshold of 90% of the ERS limits unnecessarily prolonging hospitalization and the added associated risks.121
Established BPD: > 36 Weeks PMA – Use of Medications
The paucity of evidence regarding optimal pharmacological management of established BPD has led to marked variations in prescribing patterns between centers.281,282 Patients with different clinical phenotypes of BPD vary considerably in their response to specific therapies,57 therefore an individualized approach to care is strongly recommended.33 Infants with extensive small airway involvement may respond favorably to the anti-inflammatory effects of inhaled corticosteroids. However, two RCTs evaluating the effects of inhaled fluticasone failed to demonstrate objective evidence of long-term benefit.283 Although obstructive patterns of lung disease are common in patients with BPD,52 response to bronchodilator therapy is variable.284–286 Unlike in asthma, where reversible airway narrowing arises due to eosinophilic inflammation, the obstructive lung disease seen in BPD is more closely related to fixed structural abnormalities that limit small airway caliber.52 Trials of inhaled medications should therefore be considered in infants with established BPD; however, continuing therapy in the absence of a clinical response is not recommended.287
Use of both loop and thiazide diuretics has been shown to result in transient improvements in lung compliance; however, these changes have not been linked to weaning from respiratory support.118,288 Adverse reactions to chronic diuretic use include electrolyte imbalances, calciuria, nephrocalcinosis, ototoxicity, and delayed closure of PDA.283 As in the evolving phase of BPD, use of diuretics should be targeted at patients who demonstrate a response to therapy and weaned as tolerated following discharge.287
The use of systemic corticosteroids in the management of infants with established BPD has not been well studied. Short courses of prednisolone have been effectively used to wean off supplemental oxygen prior to discharge122 and short courses of corticosteroids can also be used during BPD exacerbations due to viral illnesses to decrease airway inflammation and reduce work of breathing.287 A pulse dose of methylprednisolone has also been shown to improve pulmonary severity score, in the 5 months following administration,289 with benefits most marked in infants with grades 2–3 BPD.289 Adverse side effects of chronic systemic corticosteroid use include significant hyperglycemia, hypertension, immune suppression, intestinal perforation, cardiomyopathy, growth retardation, and adverse neurodevelopmental outcome. There is also evidence from animal studies that alveolar development is impaired following chronic glucocorticoid exposure.290 For these reasons, prolonged use of systemic corticosteroids is not recommended.
Established BPD: > 36 Weeks PMA – Surveillance for BPD-PH
It is estimated that 25–37% of infants with BPD will develop BPD-PH, a diagnosis that is associated with increased risk for mortality291–293 and tracheostomy placement.58,294 A proactive approach to the detection of BPD-PH is therefore of critical importance. Different screening algorithms have been described.120,295 The European Pediatric Pulmonary Vascular Disease Network (EPPVDN) recommends screening all infants with established BPD with transthoracic echocardiography at 36 weeks PMA and before discharge.120 Screening should also be considered in any infant with chronic O2 dependency and in those who demonstrate suboptimal growth or lack of expected clinical improvement.120 Confirmation of BPD-PH should lead to consultation with cardiology and consideration for pulmonary vasodilator therapy with or without cardiac catheterization.120 Screening for PH should continue post-discharge. The Pediatric PH Network recommends evaluation with an echocardiogram every 4–6 months if the patient is requiring supplemental O2 or develops increasing respiratory support needs.296
Established BPD: > 36 Weeks PMA – Nutritional Strategies
A specific nutritional approach to an infant with established BPD should be informed by disease severity, respiratory support requirement, age and level of activity.124 Enteral feeding should be maintained as long as possible as this provides the most optimal source of energy, vitamins and minerals and contributes to maintenance of gut integrity. Continuing enteral feeds during acute deteriorations in status and in patients requiring sedation and paralysis has been recommended by experts who manage high volume of patients with severe BPD.124 This team also advocate for an approach that is sensitive to the various clinical phases of severe BPD that they describe as the acute, transitional, and pro-growth stages.124 Table 5 provides a brief description of each phase with specific expert recommendations.124
 |
Table 5 Summary of the Approach Used for Patients with Established BPD at Nationwide Children’s Hospital, Columbus, Ohio, USA |
Ongoing monitoring of anthropometric data should occur throughout both inpatient and outpatient phases of care along with screening for metabolic bone disease297 and anemia of prematurity. Electrolyte abnormalities are common in patients receiving diuretic therapy and should be identified and treated accordingly. Recommended goals for daily weight gain in preterm infants are 15–20 g/day with weekly linear growth of 0.9–1.1 cm per week and should be considered when tracking the progress of infants with established BPD. Maintenance of adequate growth should be a priority for infants with established BPD and multi-center trials on nutritional practices should be strongly considered.
Established BPD: > 36 Weeks PMA – Lung Transplantation
Between the years 2000–2020, a total of 37 patients with BPD as a diagnosis underwent a lung transplant.298 Overall, these patients had a younger median age at the time of transplant when compared to other lung transplant recipients, 22 years (interquartile range (IQR) 7–34 years) versus 58 years (IQR 48–64 years), respectively. When comparing the BPD lung transplant recipients born in the pre-surfactant era (SE) to the post-SE, the post-SE patients were significantly younger, 7.5 years (IQR 1–13.35 years) versus 34 years (IQR 29–37 years). Post-SE patients were also more likely to be on ventilator support 43.4% versus 0%.298 There were no statistically significant differences in survival post-lung transplant when comparing the BPD group to the group with other diagnoses nor was there a difference when comparing pre-SE and post-SE BPD patients.298 While an extreme option, lung transplant could be considered for patients with progressive, life-limiting lung disease secondary to BPD.
Conclusions
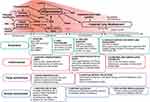
BPD is a complex multifactorial lung injury syndrome that has proven to be extremely challenging to diagnose, prevent and manage. Current strategies used for the prevention and management of BPD, summarized in Figure 1, have shown efficacy in preventing and mitigating the extent of lung injury. The impact of these treatments has not, however, been sufficient to keep pace with improvements in survival. Novel treatments are needed to address the needs of a growing population of peri-viable infants. In the meantime, QI frameworks need to be leveraged for optimal delivery of existing therapeutic strategies. Continued investigation, identification and action are required to urgently address racial and socio-economic disparities in respiratory outcomes. Finally, collaboration between basic scientists, clinical investigators, and clinicians in a range of subspecialties is necessary to close gaps in knowledge and optimize outcomes for patients at all stages of life.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Northway WH, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368. doi:10.1056/NEJM196702162760701
2. Allen J, Panitch H. Bronchopulmonary dysplasia-A historical perspective. Pediatr Pulmonol. 2021;56(11):3478–3489. doi:10.1002/ppul.25341
3. Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46(6):641–643. doi:10.1203/00006450-199912000-00007
4. Bell EF, Hintz SR, Hansen NI, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA. 2022;327(3):248–263. doi:10.1001/jama.2021.23580
5. Nakashima T, Inoue H, Sakemi Y, et al. Trends in bronchopulmonary dysplasia among extremely preterm infants in Japan, 2003–2016. J Pediatr. 2021;230:119–125.e7. doi:10.1016/j.jpeds.2020.11.041
6. Gilfillan M, Bhandari V. Moving bronchopulmonary dysplasia research from the bedside to the bench. Am J Physiol Lung Cell Mol Physiol. 2022;322(6):L804–L821. doi:10.1152/ajplung.00452.2021
7. Mandell EW, Kratimenos P, Abman SH, Steinhorn RH. Drugs for the prevention and treatment of bronchopulmonary dysplasia. Clin Perinatol. 2019;46(2):291–310. doi:10.1016/j.clp.2019.02.011
8. Lui K, Lee SK, Kusuda S, et al. Trends in outcomes for neonates born very preterm and very low birth weight in 11 high-income countries. J Pediatr. 2019;215:32–40.e14. doi:10.1016/j.jpeds.2019.08.020
9. van Katwyk S, Augustine S, Thebaud B, Thavorn K. Lifetime patient outcomes and healthcare utilization for Bronchopulmonary dysplasia (BPD) and extreme preterm infants: a microsimulation study. BMC Pediatr. 2020;20(1):136. doi:10.1186/s12887-020-02037-5
10. Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. 2018;42(7):478–484. doi:10.1053/j.semperi.2018.09.013
11. Shin JE, Jang H, Han JH, et al. Association between bronchopulmonary dysplasia and early respiratory morbidity in children with respiratory distress syndrome: a case-control study using nationwide data. Sci Rep. 2022;12(1):7578. doi:10.1038/s41598-022-11657-z
12. Humayun J, Lofqvist C, Ley D, Hellstrom A, Gyllensten H. Systematic review of the healthcare cost of bronchopulmonary dysplasia. BMJ Open. 2021;11(8):e045729. doi:10.1136/bmjopen-2020-045729
13. Thunqvist P, Tufvesson E, Bjermer L, et al. Lung function after extremely preterm birth-A population-based cohort study (EXPRESS). Pediatr Pulmonol. 2018;53(1):64–72. doi:10.1002/ppul.23919
14. Keller RL, Feng R, DeMauro SB, et al. Bronchopulmonary dysplasia and perinatal characteristics predict 1-year respiratory outcomes in newborns born at extremely low gestational age: a prospective cohort study. J Pediatr. 2017;187:89–97.e3. doi:10.1016/j.jpeds.2017.04.026
15. Mowitz ME, Mangili A, Han L, et al. Prevalence of chronic respiratory morbidity, length of stay, inpatient readmissions, and costs among extremely preterm infants with bronchopulmonary dysplasia. Expert Rev Pharmacoecon Outcomes Res. 2021;21(5):1117–1125. doi:10.1080/14737167.2021.1848554
16. Nobile S, Marchionni P, Gidiucci C, et al. Oxygen saturation/FIO2 ratio at 36 weeks’ PMA in 1005 preterm infants: effect of gestational age and early respiratory disease patterns. Pediatr Pulmonol. 2019;54(5):637–643. doi:10.1002/ppul.24265
17. Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135(4):607–616. doi:10.1542/peds.2014-3060
18. Morrow LA, Wagner BD, Ingram DA, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med. 2017;196(3):364–374. doi:10.1164/rccm.201612-2414OC
19. Bührer C, Heller G, Thome UH. Population-based outcome data of extremely preterm infants in Germany during 2010–2017. Neonatology. 2022;119(3):370–376. doi:10.1159/000524455
20. Lundgren P, Morsing E, Hård AL, et al. National cohort of infants born before 24 gestational weeks showed increased survival rates but no improvement in neonatal morbidity. Acta Paediatr. 2022;111(8):1515–1525. doi:10.1111/apa.16354
21. Shukla VV, Souder JP, Imbrock G, et al. Hospital and neurodevelopmental outcomes in nano-preterm infants receiving invasive vs noninvasive ventilation at birth. JAMA Netw Open. 2022;5(8):e2229105. doi:10.1001/jamanetworkopen.2022.29105
22. Chung J, Iyengar A, Santry L, Swanson E, Davis JM, Volpe MV. Changes in respiratory management and the impact on bronchopulmonary dysplasia. Pediatr Pulmonol. 2022;57(10):2327–2334. doi:10.1002/ppul.26035
23. Yao S, Uthaya S, Gale C, Modi N, Battersby C; (UKNC) UNC. Postnatal corticosteroid use for prevention or treatment of bronchopulmonary dysplasia in England and Wales 2012–2019: a retrospective population cohort study. BMJ Open. 2022;12(11):e063835. doi:10.1136/bmjopen-2022-063835
24. Lee SM, Sie L, Liu J, Profit J, Lee HC. Evaluation of trends in bronchopulmonary dysplasia and respiratory support practice for very low birth weight infants: a population-based cohort study. J Pediatr. 2022;243:47–52.e2. doi:10.1016/j.jpeds.2021.11.049
25. Regin Y, Gie A, Eerdekens A, Toelen J, Debeer A. Ventilation and respiratory outcome in extremely preterm infants: trends in the new millennium. Eur J Pediatr. 2022;181(5):1899–1907. doi:10.1007/s00431-022-04378-y
26. Jensen EA, Edwards EM, Greenberg LT, Soll RF, Ehret DEY, Horbar JD. Severity of bronchopulmonary dysplasia among very preterm infants in the United States. Pediatrics. 2021;148(1). doi:10.1542/peds.2020-030007
27. Sucasas Alonso A, Pértega Diaz S, Sáez Soto R, Avila-Alvarez A. Epidemiology and risk factors for bronchopulmonary dysplasia in preterm infants born at or less than 32 weeks of gestation. An Pediatr. 2022;96(3):242–251. doi:10.1016/j.anpede.2021.03.006
28. Geetha O, Rajadurai VS, Anand AJ, et al. New BPD-prevalence and risk factors for bronchopulmonary dysplasia/mortality in extremely low gestational age infants ≤28 weeks. J Perinatol. 2021;41(8):1943–1950. doi:10.1038/s41372-021-01095-6
29. Ramos-Navarro C, Maderuelo-Rodríguez E, Concheiro-Guisán A, et al. Risk factors and bronchopulmonary dysplasia severity: data from the Spanish Bronchopulmonary Dysplasia Research Network. Eur J Pediatr. 2022;181(2):789–799. doi:10.1007/s00431-021-04248-z
30. He W, Zhang L, Feng R, et al. Risk factors and machine learning prediction models for bronchopulmonary dysplasia severity in the Chinese population. World J Pediatr. 2023;19(6):568–576. doi:10.1007/s12519-022-00635-0
31. Dou C, Yu Y-H, Zhuo Q-C, et al. Longer duration of initial invasive mechanical ventilation is still a crucial risk factor for moderate-to-severe bronchopulmonary dysplasia in very preterm infants: a multicentrer prospective study. World J Pediatr. 2023;19(6):577–585. doi:10.1007/s12519-022-00671-w
32. Greenberg RG, McDonald SA, Laughon MM, et al. Online clinical tool to estimate risk of bronchopulmonary dysplasia in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed. 2022;107(6):638–643. doi:10.1136/archdischild-2021-323573
33. Gilfillan M, Bhandari A, Bhandari V. Diagnosis and management of bronchopulmonary dysplasia. BMJ. 2021;375:n1974. doi:10.1136/bmj.n1974
34. Gobec K, Mukenauer R, Keše D, Erčulj V, Grosek Š, Perme T. Association between colonization of the respiratory tract with Ureaplasma species and bronchopulmonary dysplasia in newborns with extremely low gestational age: a retrospective study. Croat Med J. 2023;64(2):75–83. doi:10.3325/cmj.2023.64.75
35. Pierro M, Villamor-Martinez E, van Westering-Kroon E, Alvarez-Fuente M, Abman SH, Villamor E. Association of the dysfunctional placentation endotype of prematurity with bronchopulmonary dysplasia: a systematic review, meta-analysis and meta-regression. Thorax. 2022;77(3):268–275. doi:10.1136/thoraxjnl-2020-216485
36. Sheth S, Goto L, Bhandari V, Abraham B, Mowes A. Factors associated with development of early and late pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. J Perinatol. 2020;40(1):138–148. doi:10.1038/s41372-019-0549-9
37. Jensen EA, Dysart K, Gantz MG, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. 2019;200(6):751–759. doi:10.1164/rccm.201812-2348OC
38. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi:10.1164/ajrccm.163.7.2011060
39. Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82(4):527–532. doi:10.1542/peds.82.4.527
40. Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114(5):1305–1311. doi:10.1542/peds.2004-0204
41. Isayama T, Lee SK, Yang J, et al. Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 2017;171(3):271–279. doi:10.1001/jamapediatrics.2016.4141
42. Adler-Haltovsky T, Gileles-Hillel A, Erlichman I, Eventov-Friedman S. Changes in ventilation modes in the last decade and their impact on the prevalence of bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol. 2023;58(7):1959–1966. doi:10.1002/ppul.26418
43. Higgins RD, Jobe AH, Koso-Thomas M, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300–308. doi:10.1016/j.jpeds.2018.01.043
44. Kim F, Bateman DA, Goldshtrom N, Sahni R, Wung JT, Wallman-Stokes A. Revisiting the definition of bronchopulmonary dysplasia in premature infants at a single center quaternary neonatal intensive care unit. J Perinatol. 2021;41(4):756–763. doi:10.1038/s41372-021-00980-4
45. Kurihara C, Zhang L, Mikhael M. Newer bronchopulmonary dysplasia definitions and prediction of health economics impacts in very preterm infants. Pediatr Pulmonol. 2021;56(2):409–417. doi:10.1002/ppul.25172
46. Guaman MC, Pishevar N, Abman SH, et al. Invasive mechanical ventilation at 36 weeks post-menstrual age, adverse outcomes with a comparison of recent definitions of bronchopulmonary dysplasia. J Perinatol. 2021;41(8):1936–1942. doi:10.1038/s41372-021-01102-w
47. Oluwole I, Tan JBC, DeSouza S, et al. The association between bronchopulmonary dysplasia grade and risks of adverse neurodevelopmental outcomes among preterm infants born at less than 30 weeks of gestation. J Matern Fetal Neonatal Med. 2023;36(1):2167074. doi:10.1080/14767058.2023.2167074
48. Jeon GW, Oh M, Chang YS. Definitions of bronchopulmonary dysplasia and long-term outcomes of extremely preterm infants in Korean Neonatal Network. Sci Rep. 2021;11(1):24349. doi:10.1038/s41598-021-03644-7
49. Jeon GW, Oh M, Lee J, Jun YH, Chang YS. Comparison of definitions of bronchopulmonary dysplasia to reflect the long-term outcomes of extremely preterm infants. Sci Rep. 2022;12(1):18095. doi:10.1038/s41598-022-22920-8
50. Katz TA, van Kaam AH, Schuit E, et al. Comparison of new bronchopulmonary dysplasia definitions on long-term outcomes in preterm infants. J Pediatr. 2022. doi:10.1016/j.jpeds.2022.09.022
51. Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA. 2016;316(6):611–624. doi:10.1001/jama.2016.10708
52. Collaco JM, McGrath-Morrow SA. Bronchopulmonary dysplasia as a determinant of respiratory outcomes in adult life. Pediatr Pulmonol. 2021;56(11):3464–3471. doi:10.1002/ppul.25301
53. Collaco JM, Tracy MC, Sheils CA, et al. Insurance coverage and respiratory morbidities in bronchopulmonary dysplasia. Pediatr Pulmonol. 2022;57(7):1735–1743. doi:10.1002/ppul.25933
54. Banwell E, Collaco JM, Oates GR, et al. Area deprivation and respiratory morbidities in children with bronchopulmonary dysplasia. Pediatr Pulmonol. 2022;57(9):2053–2059. doi:10.1002/ppul.25969
55. Deschamps J, Boucekine M, Fayol L, et al. Neighborhood disadvantage and early respiratory outcomes in very preterm infants with bronchopulmonary dysplasia. J Pediatr. 2021;237:177–182.e1. doi:10.1016/j.jpeds.2021.06.061
56. Smith MA, Steurer MA, Mahendra M, Zinter MS, Keller RL. Sociodemographic factors associated with tracheostomy and mortality in bronchopulmonary dysplasia. Pediatr Pulmonol. 2023;58(4):1237–1246. doi:10.1002/ppul.26328
57. Pierro M, Van Mechelen K, van Westering-Kroon E, Villamor-Martínez E, Villamor E. Endotypes of prematurity and phenotypes of bronchopulmonary dysplasia: toward personalized neonatology. J Pers Med. 2022;12(5):687. doi:10.3390/jpm12050687
58. Wu KY, Jensen EA, White AM, et al. Characterization of disease phenotype in very preterm infants with severe bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2020;201(11):1398–1406. doi:10.1164/rccm.201907-1342OC
59. Hysinger EB, Friedman NL, Padula MA, et al. Tracheobronchomalacia is associated with increased morbidity in bronchopulmonary dysplasia. Ann Am Thorac Soc. 2017;14(9):1428–1435. doi:10.1513/AnnalsATS.201702-178OC
60. Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann Am Thorac Soc. 2018;15(5):530–538. doi:10.1513/AnnalsATS.201709-756FR
61. Gilfillan M, Bhandari V. Pulmonary phenotypes of bronchopulmonary dysplasia in the preterm infant. Semin Perinatol. 2023;47(6):151810. doi:10.1016/j.semperi.2023.151810
62. Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129(3):e682–9. doi:10.1542/peds.2011-1827
63. Hysinger EB. Central airway issues in bronchopulmonary dysplasia. Pediatr Pulmonol. 2021;56(11):3518–3526. doi:10.1002/ppul.25417
64. Praprotnik M, Stucin Gantar I, Krivec U, Lucovnik M, Rodman Berlot J, Starc G. Physical fitness trajectories from childhood to adolescence in extremely preterm children: a longitudinal cohort study. Pediatr Pulmonol. 2023;58(7):1904–1911. doi:10.1002/ppul.26410
65. Peralta GP, Piatti R, Haile SR, et al. Respiratory morbidity in preschool and school-age children born very preterm and its association with parents’ health-related quality of life and family functioning. Eur J Pediatr. 2023;182(3):1201–1210. doi:10.1007/s00431-022-04783-3
66. Sriram S, Schreiber MD, Msall ME, et al. Cognitive development and quality of life associated with BPD in 10-year-olds born preterm. Pediatrics. 2018;141(6). doi:10.1542/peds.2017-2719
67. Doyle LW, Irving L, Haikerwal A, Lee K, Ranganathan S, Cheong J. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax. 2019;74(12):1147–1153. doi:10.1136/thoraxjnl-2019-213757
68. Doyle LW, Ranganathan S, Cheong J; Group VICS. Bronchopulmonary dysplasia and expiratory airflow at 8 years in children born extremely preterm in the post-surfactant era. Thorax. 2023;78(5):484–488. doi:10.1136/thoraxjnl-2022-218792
69. Harris C, Morris S, Lunt A, Peacock J, Greenough A. Influence of bronchopulmonary dysplasia on lung function in adolescents who were born extremely prematurely. Pediatr Pulmonol. 2022;57(12):3151–3157. doi:10.1002/ppul.26151
70. Tan S, Szatkowski L, Moreton W, et al. Early childhood respiratory morbidity and antibiotic use in ex-preterm infants: a primary care population-based cohort study. Eur Respir J. 2020;56(1):2000202. doi:10.1183/13993003.00202-2020
71. Kim K, Lee JY, Kim YM, et al. Prevalence of asthma in preterm and associated risk factors based on prescription data from the Korean National Health Insurance database. Sci Rep. 2023;13(1):4484. doi:10.1038/s41598-023-31558-z
72. Siffel C, Hirst AK, Sarda SP, et al. The clinical burden of extremely preterm birth in a large medical records database in the United States: complications, medication use, and healthcare resource utilization. J Matern Fetal Neonatal Med. 2022;35(26):10271–10278. doi:10.1080/14767058.2022.2122035
73. Doyle LW, Andersson S, Bush A, et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: a meta-analysis of individual participant data. Lancet Respir Med. 2019;7(8):677–686. doi:10.1016/S2213-2600(18)30530-7
74. Bårdsen T, Røksund OD, Benestad MR, et al. Tracking of lung function from 10 to 35 years after being born extremely preterm or with extremely low birth weight. Thorax. 2022;77(8):790–798. doi:10.1136/thoraxjnl-2021-218400
75. Prenzel F, Vogel M, Siekmeyer W, Körner A, Kiess W, Vom Hove M. Exercise capacity in children with bronchopulmonary dysplasia at school age. Respir Med. 2020;171:106102. doi:10.1016/j.rmed.2020.106102
76. McGrath-Morrow SA, Collaco JM. Bronchopulmonary dysplasia: what are its links to COPD? Ther Adv Respir Dis. 2019;13:1753466619892492. doi:10.1177/1753466619892492
77. DeMauro SB. Neurodevelopmental outcomes of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2021;56(11):3509–3517. doi:10.1002/ppul.25381
78. Martin M, Smith L, Hofheimer JA, et al. Bronchopulmonary dysplasia and neurobehavioural outcomes at birth and 2 years in infants born before 30 weeks. Arch Dis Child Fetal Neonatal Ed. 2023;108(2):142–148. doi:10.1136/archdischild-2021-323405
79. Tréluyer L, Nuytten A, Guellec I, et al. Neurodevelopment and healthcare utilisation at age 5–6 years in bronchopulmonary dysplasia: an EPIPAGE-2 cohort study. Arch Dis Child Fetal Neonatal Ed. 2023:325376. doi:10.1136/archdischild-2023-325376
80. Carregã M, Sousa P, Rocha G, Ferreira-Magalhães M, Azevedo I. Respiratory and non-respiratory outcomes of bronchopulmonary dysplasia in adolescents: a systematic review. Early Hum Dev. 2023;180:105756. doi:10.1016/j.earlhumdev.2023.105756
81. Lapcharoensap W, Bennett MV, Xu X, Lee HC, Dukhovny D. Hospitalization costs associated with bronchopulmonary dysplasia in the first year of life. J Perinatol. 2020;40(1):130–137. doi:10.1038/s41372-019-0548-x
82. Lai KC, Lorch SA. Healthcare costs of major morbidities associated with prematurity in US children’s hospitals. J Pediatr. 2023;256:53–62.e4. doi:10.1016/j.jpeds.2022.11.038
83. Villosis MFB, Barseghyan K, Ambat MT, Rezaie KK, Braun D. Rates of bronchopulmonary dysplasia following implementation of a novel prevention bundle. JAMA Netw Open. 2021;4(6):e2114140. doi:10.1001/jamanetworkopen.2021.14140
84. Ratliff-Crain D, Wallingford B, Jorgenson L. Using a bundle approach to prevent bronchopulmonary dysplasia in very premature infants. Adv Neonatal Care. 2022;22(4):300–308. doi:10.1097/ANC.0000000000000920
85. White H, Merritt K, Martin K, Lauer E, Rhein L. Respiratory support strategies in the prevention of bronchopulmonary dysplasia: a single center quality improvement initiative. Front Pediatr. 2022;10:1012655. doi:10.3389/fped.2022.1012655
86. Oei JL, Vento M, Rabi Y, et al. Higher or lower oxygen for delivery room resuscitation of preterm infants below 28 completed weeks gestation: a meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2017;102(1):F24–F30. doi:10.1136/archdischild-2016-310435
87. Askie LM, Darlow BA, Davis PG, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev. 2017;4:CD011190. doi:10.1002/14651858.CD011190.pub2
88. Bahadue FL, Soll R. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2012;11(11):CD001456. doi:10.1002/14651858.CD001456.pub2
89. Bhandari V, Black R, Gandhi B, et al. RDS-NExT workshop: consensus statements for the use of surfactant in preterm neonates with RDS. J Perinatol. 2023;43(8):982–990. doi:10.1038/s41372-023-01690-9
90. Kakkilaya V, Wagner S, Mangona KLM, et al. Early predictors of continuous positive airway pressure failure in preterm neonates. J Perinatol. 2019;39(8):1081–1088. doi:10.1038/s41372-019-0392-z
91. Gulczyńska E, Szczapa T, Hożejowski R, Borszewska-Kornacka MK, Rutkowska M. Fraction of inspired oxygen as a predictor of CPAP failure in preterm infants with respiratory distress syndrome: a prospective multicenter study. Neonatology. 2019;116(2):171–178. doi:10.1159/000499674
92. Lemyre B, Deguise MO, Benson P, Kirpalani H, Ekhaguere OA, Davis PG. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database Syst Rev. 2023;7(7):CD005384. doi:10.1002/14651858.CD005384.pub3
93. Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;10(10):CD003666. doi:10.1002/14651858.CD003666.pub4
94. Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015;3:CD000104. doi:10.1002/14651858.CD000104.pub4
95. Kamlin C, Davis PG. Long versus short inspiratory times in neonates receiving mechanical ventilation. Cochrane Database Syst Rev. 2004;4:CD004503. doi:10.1002/14651858.CD004503.pub2
96. Ambalavanan N, Carlo WA. Ventilatory strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):192–199. doi:10.1053/j.semperi.2006.05.006
97. Thome UH, Ambalavanan N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin Fetal Neonatal Med. 2009;14(1):21–27. doi:10.1016/j.siny.2008.08.005
98. Thome UH, Genzel-Boroviczeny O, Bohnhorst B, et al. Permissive hypercapnia in extremely low birthweight infants (PHELBI): a randomised controlled multicentre trial. Lancet Respir Med. 2015;3(7):534–543. doi:10.1016/S2213-2600(15)00204-0
99. Bhandari V. Nasal intermittent positive pressure ventilation in the newborn: review of literature and evidence-based guidelines. J Perinatol. 2010;30(8):505–512. doi:10.1038/jp.2009.165
100. Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006;354(20):2112–2121. doi:10.1056/NEJMoa054065
101. Davis PG, Schmidt B, Roberts RS, et al. Caffeine for Apnea of Prematurity trial: benefits may vary in subgroups. J Pediatr. 2010;156(3):382–387. doi:10.1016/j.jpeds.2009.09.069
102. Doyle LW, Schmidt B, Anderson PJ, et al. Reduction in developmental coordination disorder with neonatal caffeine therapy. J Pediatr. 2014;165(2):356–359.e2. doi:10.1016/j.jpeds.2014.04.016
103. Henderson-Smart DJ, Davis PG. Prophylactic methylxanthines for endotracheal extubation in preterm infants. Cochrane Database Syst Rev. 2010;12:CD000139. doi:10.1002/14651858.CD000139.pub2
104. Yeh TF, Chen CM, Wu SY, et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193(1):86–95. doi:10.1164/rccm.201505-0861OC
105. Tyson JE, Wright LL, Oh W, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National institute of child health and human development neonatal research network. N Engl J Med. 1999;340(25):1962–1968. doi:10.1056/NEJM199906243402505
106. Ambalavanan N, Tyson JE, Kennedy KA, et al. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics. 2005;115(3):e249–54. doi:10.1542/peds.2004-1812
107. Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Early (< 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;10(10):CD001146. doi:10.1002/14651858.CD001146.pub6
108. Letouzey M, Lorthe E, Marchand-Martin L, et al. Early antibiotic exposure and adverse outcomes in very preterm infants at low risk of early-onset sepsis: the EPIPAGE-2 cohort study. J Pediatr. 2022;243:91–98.e4. doi:10.1016/j.jpeds.2021.11.075
109. Starr MC, Griffin R, Gist KM, et al. Association of fluid balance with short- and long-term respiratory outcomes in extremely premature neonates: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2022;5(12):e2248826. doi:10.1001/jamanetworkopen.2022.48826
110. Uberos J, Jimenez-Montilla S, Molina-Oya M, García-Serrano JL. Early energy restriction in premature infants and bronchopulmonary dysplasia: a cohort study. Br J Nutr. 2020;123(9):1024–1031. doi:10.1017/S0007114520000240
111. Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi:10.1542/peds.2004-1974
112. Uberos-Fernández J, Ruiz-López A, Carrasco-Solis M, Fernandez-Marín E, Garcia-Cuesta A, Campos-Martínez A. Extrauterine growth restriction and low energy intake during the early neonatal period of very low birth weight infants are associated with decreased lung function in childhood. Br J Nutr. 2023;1–9. doi:10.1017/S0007114523001332
113. Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, Investigators DS. Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics. 2006;117(1):75–83. doi:10.1542/peds.2004-2843
114. Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2021;11(11):CD001145. doi:10.1002/14651858.CD001145.pub5
115. Jensen EA, Wiener LE, Rysavy MA, et al. Assessment of corticosteroid therapy and death or disability according to pretreatment risk of death or bronchopulmonary dysplasia in extremely preterm infants. JAMA Netw Open. 2023;6(5):e2312277. doi:10.1001/jamanetworkopen.2023.12277
116. Dumpa V, Avulakunta I, Bhandari V. Respiratory management in the premature neonate. Expert Rev Respir Med. 2023;17(2):155–170. doi:10.1080/17476348.2023.2183843
117. Lam R, Schilling D, Scottoline B, et al. The effect of extended continuous positive airway pressure on changes in lung volumes in stable premature infants: a randomized controlled trial. J Pediatr. 2020;217:66–72.e1. doi:10.1016/j.jpeds.2019.07.074
118. Stewart A, Brion LP, Ambrosio-Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2011;9:CD001817. doi:10.1002/14651858.CD001817.pub2
119. Hou S, Yu Y, Wu Y, et al. Association between antibiotic overexposure and adverse outcomes in very-low-birth-weight infants without culture-proven sepsis or necrotizing enterocolitis: a multicenter prospective study. Indian J Pediatr. 2022;89(8):785–792. doi:10.1007/s12098-021-04023-w
120. Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. 2019;38(9):879–901. doi:10.1016/j.healun.2019.06.022
121. Duijts L, van Meel ER, Moschino L, et al. European respiratory society guideline on long-term management of children with bronchopulmonary dysplasia. Eur Respir J. 2020;55(1):1900788. doi:10.1183/13993003.00788-2019
122. Bhandari A, Schramm CM, Kimble C, Pappagallo M, Hussain N. Effect of a short course of prednisolone in infants with oxygen-dependent bronchopulmonary dysplasia. Pediatrics. 2008;121(2):e344–9. doi:10.1542/peds.2006-3668
123. Miller AN, Moise AA, Cottrell L, Loomis K, Polak M, Gest A. Linear growth is associated with successful respiratory support weaning in infants with bronchopulmonary dysplasia. J Perinatol. 2022;42(4):544–545. doi:10.1038/s41372-022-01322-8
124. Miller AN, Curtiss J, Taylor SN, Backes CH, Kielt MJ. A review and guide to nutritional care of the infant with established bronchopulmonary dysplasia. J Perinatol. 2023;43(3):402–410. doi:10.1038/s41372-022-01578-0
125. Narayan O, Bentley A, Mowbray K, et al. Updated cost-effectiveness analysis of palivizumab (Synagis) for the prophylaxis of respiratory syncytial virus in infant populations in the UK. J Med Econ. 2020:1–13. doi:10.1080/13696998.2020.1836923
126. Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3(3):CD004454. doi:10.1002/14651858.CD004454.pub3
127. Chawla S, Wyckoff MH, Rysavy MA, et al. Association of antenatal steroid exposure at 21 to 22 weeks of gestation with neonatal survival and survival without morbidities. JAMA Netw Open. 2022;5(9):e2233331. doi:10.1001/jamanetworkopen.2022.33331
128. Parikh S, Reichman B, Kusuda S, et al. Trends, characteristic, and outcomes of preterm infants who received postnatal corticosteroid: a cohort study from 7 high-income countries. Neonatology. 2023:1–10. doi:10.1159/000530128
129. Kemp MW, Jobe AH, Usuda H, et al. Efficacy and safety of antenatal steroids. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):R825–R839. doi:10.1152/ajpregu.00193.2017
130. Daskalakis G, Pergialiotis V, Domellöf M, et al. European guidelines on perinatal care: corticosteroids for women at risk of preterm birth. J Matern Fetal Neonatal Med. 2023;36(1):2160628. doi:10.1080/14767058.2022.2160628
131. Dunn MS, Kaempf J, de Klerk A, et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069–76. doi:10.1542/peds.2010-3848
132. Morley CJ, Davis PG, Doyle LW, et al. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700–708. doi:10.1056/NEJMoa072788
133. Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–1979. doi:10.1056/NEJMoa0911783
134. Sandri F, Plavka R, Ancora G, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125(6):e1402–9. doi:10.1542/peds.2009-2131
135. Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347(oct17 3):f5980. doi:10.1136/bmj.f5980
136. Wright CJ, Glaser K, Speer CP, Härtel C, Roehr CC. Noninvasive ventilation and exogenous surfactant in times of ever decreasing gestational age: how do we make the most of these tools? J Pediatr. 2022;247:138–146. doi:10.1016/j.jpeds.2022.04.011
137. Dargaville PA, Gerber A, Johansson S, et al. Incidence and outcome of CPAP failure in preterm infants. Pediatrics. 2016;138(1). doi:10.1542/peds.2015-3985
138. Sweet DG, Carnielli VP, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. 2023;120(1):3–23. doi:10.1159/000528914
139. Capasso L, Pacella D, Migliaro F, et al. Can lung ultrasound score accurately predict surfactant replacement? A systematic review and meta-analysis of diagnostic test studies. Pediatr Pulmonol. 2023;58(5):1427–1437. doi:10.1002/ppul.26337
140. Abdel-Latif ME, Davis PG, Wheeler KI, De Paoli AG, Dargaville PA. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2021;5(5):CD011672. doi:10.1002/14651858.CD011672.pub2
141. Kesler H, Lohmeier K, Hoehn T, Kribs A, Peinemann F. Thin-catheter surfactant application for respiratory distress syndrome in spontaneously breathing preterm infants: a meta-analysis of randomized clinical trials. Curr Pediatr Rev. 2022;18(4):286–300. doi:10.2174/1573396318666220404194857
142. Dargaville PA, Kamlin COF, Orsini F, et al. Effect of minimally invasive surfactant therapy vs sham treatment on death or bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome: the OPTIMIST-A randomized clinical trial. JAMA. 2021;326(24):2478–2487. doi:10.1001/jama.2021.21892
143. Härtel C, Herting E, Humberg A, et al. Association of administration of surfactant using less invasive methods with outcomes in extremely preterm infants less than 27 weeks of gestation. JAMA Netw Open. 2022;5(8):e2225810. doi:10.1001/jamanetworkopen.2022.25810
144. Herting E, Härtel C, Göpel W. Less invasive surfactant administration: best practices and unanswered questions. Curr Opin Pediatr. 2020;32(2):228–234. doi:10.1097/MOP.0000000000000878
145. Kakkilaya V, Gautham KS. Should less invasive surfactant administration (LISA) become routine practice in US neonatal units? Pediatr Res. 2023;93(5):1188–1198. doi:10.1038/s41390-022-02265-8
146. Oei JL, Finer NN, Saugstad OD, et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch Dis Child Fetal Neonatal Ed. 2018;103(5):F446–F454. doi:10.1136/archdischild-2016-312366
147. Söderström F, Ågren J, Sindelar R. Early extubation is associated with shorter duration of mechanical ventilation and lower incidence of bronchopulmonary dysplasia. Early Hum Dev. 2021;163:105467. doi:10.1016/j.earlhumdev.2021.105467
148. Berger J, Mehta P, Bucholz E, Dziura J, Bhandari V. Impact of early extubation and reintubation on the incidence of bronchopulmonary dysplasia in neonates. Am J Perinatol. 2014;31(12):1063–1072. doi:10.1055/s-0034-1371702
149. Shalish W, Keszler M, Kovacs L, et al. Age at first extubation attempt and death or respiratory morbidities in extremely preterm infants. J Pediatr. 2023;252:124–130.e3. doi:10.1016/j.jpeds.2022.08.025
150. Sammour I, Karnati S. Non-invasive respiratory support of the premature neonate: from physics to bench to practice. Front Pediatr. 2020;8:214. doi:10.3389/fped.2020.00214
151. Prakash R, De Paoli AG, Davis PG, Oddie SJ, McGuire W. Bubble devices versus other pressure sources for nasal continuous positive airway pressure in preterm infants. Cochrane Database Syst Rev. 2023;3(3):CD015130. doi:10.1002/14651858.CD015130
152. Avila-Alvarez A, García-Muñoz Rodrigo F, Solís-García G, et al. Nasal intermittent positive pressure ventilation and bronchopulmonary dysplasia among very preterm infants never intubated during the first neonatal admission: a multicenter cohort study. Front Pediatr. 2022;10:896331. doi:10.3389/fped.2022.896331
153. Zhu X, Qi H, Feng Z, Shi Y, De Luca D, NOP-ENS G. Noninvasive high-frequency oscillatory ventilation vs nasal continuous positive airway pressure vs nasal intermittent positive pressure ventilation as postextubation support for preterm neonates in china: a randomized clinical trial. JAMA Pediatr. 2022;176(6):551–559. doi:10.1001/jamapediatrics.2022.0710
154. Zhu X, Li F, Shi Y, Feng Z, De Luca D; Group NOP-ENS. Effectiveness of nasal continuous positive airway pressure vs nasal intermittent positive pressure ventilation vs noninvasive high-frequency oscillatory ventilation as support after extubation of neonates born extremely preterm or with more severe respiratory failure: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(7):e2321644. doi:10.1001/jamanetworkopen.2023.21644
155. Latremouille S, Bhuller M, Shalish W, Sant’Anna G. Cardiorespiratory effects of NIV-NAVA, NIPPV, and NCPAP shortly after extubation in extremely preterm infants: a randomized crossover trial. Pediatr Pulmonol. 2021;56(10):3273–3282. doi:10.1002/ppul.25607
156. Goel D, Oei JL, Smyth J, Schindler T. Diaphragm-triggered non-invasive respiratory support in preterm infants. Cochrane Database Syst Rev. 2020;3:CD012935. doi:10.1002/14651858.CD012935.pub2
157. Shin SH, Kim SH, Song IG, Jung YH, Kim EK, Kim HS. Noninvasive neurally adjusted ventilation in postextubation stabilization of preterm infants: a randomized controlled study. J Pediatr. 2022;247:53–59.e1. doi:10.1016/j.jpeds.2022.04.025
158. Makker K, Cortez J, Jha K, et al. Comparison of extubation success using noninvasive positive pressure ventilation (NIPPV) versus noninvasive neurally adjusted ventilatory assist (NI-NAVA). J Perinatol. 2020;40(8):1202–1210. doi:10.1038/s41372-019-0578-4
159. Dagle JM, Rysavy MA, Hunter SK, et al. Cardiorespiratory management of infants born at 22 weeks’ gestation: the Iowa approach. Semin Perinatol. 2022;46(1):151545. doi:10.1016/j.semperi.2021.151545
160. Keszler M, Sant’Anna G. Mechanical Ventilation and Bronchopulmonary Dysplasia. Clin Perinatol. 2015;42(4):781–796. doi:10.1016/j.clp.2015.08.006
161. Wallström L, Sjöberg A, Sindelar R. Early volume targeted ventilation in preterm infants born at 22–25 weeks of gestational age. Pediatr Pulmonol. 2021;56(5):1000–1007. doi:10.1002/ppul.25271
162. Ackermann BW, Klotz D, Hentschel R, Thome UH, van Kaam AH. High-frequency ventilation in preterm infants and neonates. Pediatr Res. 2023;93(7):1810–1818. doi:10.1038/s41390-021-01639-8
163. The HIFI Study Group. High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants. N Engl J Med. 1989;320(2):88–93. doi:10.1056/NEJM198901123200204
164. Zivanovic S, Peacock J, Alcazar-Paris M, et al. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med. 2014;370(12):1121–1130. doi:10.1056/NEJMoa1309220
165. Harris C, Bisquera A, Lunt A, Peacock JL, Greenough A. Outcomes of the neonatal trial of high-frequency oscillation at 16 to 19 years. N Engl J Med. 2020;383(7):689–691. doi:10.1056/NEJMc2008677
166. Solís-García G, Ramos-Navarro C, González-Pacheco N, Sánchez-Luna M. Lung protection strategy with high-frequency oscillatory ventilation improves respiratory outcomes at two years in preterm respiratory distress syndrome: a before and after, quality improvement study. J Matern Fetal Neonatal Med. 2022;35(26):10698–10705. doi:10.1080/14767058.2022.2155040
167. Sindelar R, Nakanishi H, Stanford AH, Colaizy TT, Klein JM. Respiratory management for extremely premature infants born at 22 to 23 weeks of gestation in proactive centers in Sweden, Japan, and USA. Semin Perinatol. 2022;46(1):151540. doi:10.1016/j.semperi.2021.151540
168. Watkins PL, Dagle JM, Bell EF, Colaizy TT. Outcomes at 18 to 22 months of corrected age for infants born at 22 to 25 weeks of gestation in a center practicing active management. J Pediatr. 2020;217:52–58.e1. doi:10.1016/j.jpeds.2019.08.028
169. Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715–1722. doi:10.1164/rccm.201101-0055OC
170. Fang SJ, Su CH, Liao DL, et al. Neurally adjusted ventilatory assist for rapid weaning in preterm infants. Pediatr Int. 2023;65(1):e15360. doi:10.1111/ped.15360
171. Schmidt B, Roberts RS, Davis P, et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med. 2007;357(19):1893–1902. doi:10.1056/NEJMoa073679
172. Patel RM, Leong T, Carlton DP, Vyas-Read S. Early caffeine therapy and clinical outcomes in extremely preterm infants. J Perinatol. 2013;33(2):134–140. doi:10.1038/jp.2012.52
173. Dobson NR, Patel RM, Smith PB, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014;164(5):992–998.e3. doi:10.1016/j.jpeds.2013.12.025
174. Szatkowski L, Fateh S, Abramson J, et al. Observational cohort study of use of caffeine in preterm infants and association between early caffeine use and neonatal outcomes. Arch Dis Child Fetal Neonatal Ed. 2023;108(5):505–510. doi:10.1136/archdischild-2022-324919
175. Lodha A, Seshia M, McMillan DD, et al. Association of early caffeine administration and neonatal outcomes in very preterm neonates. JAMA Pediatr. 2015;169(1):33–38. doi:10.1001/jamapediatrics.2014.2223
176. Lodha A, Entz R, Synnes A, et al. Early Caffeine Administration and Neurodevelopmental Outcomes in Preterm Infants. Pediatrics. 2019;143(1). doi:10.1542/peds.2018-1348
177. Schmidt B, Roberts RS, Anderson PJ, et al. Academic performance, motor function, and behavior 11 years after neonatal caffeine citrate therapy for apnea of prematurity: an 11-year follow-up of the CAP randomized clinical trial. JAMA Pediatr. 2017;171(6):564–572. doi:10.1001/jamapediatrics.2017.0238
178. Doyle LW, Ranganathan S, Cheong JLY. Neonatal caffeine treatment and respiratory function at 11 years in children under 1251 g at birth. Am J Respir Crit Care Med. 2017;196(10):1318–1324. doi:10.1164/rccm.201704-0767OC
179. Ferguson KN, Roberts CT, Manley BJ, Davis PG. Interventions to Improve Rates of Successful Extubation in Preterm Infants: a Systematic Review and Meta-analysis. JAMA Pediatr. 2017;171(2):165–174. doi:10.1001/jamapediatrics.2016.3015
180. Kraaijenga JV, Hutten GJ, de Jongh FH, van Kaam AH. The effect of caffeine on diaphragmatic activity and tidal volume in preterm infants. J Pediatr. 2015;167(1):70–75. doi:10.1016/j.jpeds.2015.04.040
181. Chen S, Wu Q, Zhong D, Li C, Du L. Caffeine prevents hyperoxia-induced lung injury in neonatal mice through NLRP3 inflammasome and NF-κB pathway. Respir Res. 2020;21(1):140. doi:10.1186/s12931-020-01403-2
182. Zhao W, Ma L, Cai C, Gong X. Caffeine Inhibits NLRP3 inflammasome activation by suppressing MAPK/NF-κB and A2aR signaling in LPS-Induced THP-1 macrophages. Int J Biol Sci. 2019;15(8):1571–1581. doi:10.7150/ijbs.34211
183. Dumpa V, Nielsen L, Wang H, Kumar VHS. Caffeine is associated with improved alveolarization and angiogenesis in male mice following hyperoxia induced lung injury. BMC Pulm Med. 2019;19(1):138. doi:10.1186/s12890-019-0903-x
184. Tian C, Li D, Fu J. Molecular mechanism of caffeine in preventing bronchopulmonary dysplasia in premature infants. Front Pediatr. 2022;10:902437. doi:10.3389/fped.2022.902437
185. Endesfelder S, Strauß E, Scheuer T, Schmitz T, Bührer C. Antioxidative effects of caffeine in a hyperoxia-based rat model of bronchopulmonary dysplasia. Respir Res. 2019;20(1):88. doi:10.1186/s12931-019-1063-5
186. Yuan Y, Yang Y, Lei X, Dong W. Caffeine and bronchopulmonary dysplasia: clinical benefits and the mechanisms involved. Pediatr Pulmonol. 2022;57(6):1392–1400. doi:10.1002/ppul.25898
187. Moschino L, Zivanovic S, Hartley C, Trevisanuto D, Baraldi E, Roehr CC. Caffeine in preterm infants: where are we in 2020? ERJ Open Res. 2020;6(1):00330–2019. doi:10.1183/23120541.00330-2019
188. Brattström P, Russo C, Ley D, Bruschettini M. High-versus low-dose caffeine in preterm infants: a systematic review and meta-analysis. Acta Paediatr. 2019;108(3):401–410. doi:10.1111/apa.14586
189. Chen J, Jin L, Chen X. Efficacy and safety of different maintenance doses of caffeine citrate for treatment of apnea in premature infants: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:9061234. doi:10.1155/2018/9061234
190. Mohammed S, Nour I, Shabaan AE, Shouman B, Abdel-Hady H, Nasef N. High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur J Pediatr. 2015;174(7):949–956. doi:10.1007/s00431-015-2494-8
191. Puia-Dumitrescu M, Smith PB, Zhao J, et al. Dosing and safety of off-label use of caffeine citrate in premature infants. J Pediatr. 2019;211:27–32.e1. doi:10.1016/j.jpeds.2019.04.028
192. Long JY, Guo HL, He X, et al. Caffeine for the pharmacological treatment of apnea of prematurity in the NICU: dose selection conundrum, therapeutic drug monitoring and genetic factors. Front Pharmacol. 2021;12:681842. doi:10.3389/fphar.2021.681842
193. Ambalavanan N, Kennedy K, Tyson J, Carlo WA. Survey of vitamin A supplementation for extremely-low-birth-weight infants: is clinical practice consistent with the evidence? J Pediatr. 2004;145(3):304–307. doi:10.1016/j.jpeds.2004.04.046
194. Tolia VN, Murthy K, McKinley PS, Bennett MM, Clark RH. The effect of the national shortage of vitamin A on death or chronic lung disease in extremely low-birth-weight infants. JAMA Pediatr. 2014;168(11):1039–1044. doi:10.1001/jamapediatrics.2014.1353
195. Araki S, Kato S, Namba F, Ota E, Ehrhardt H. Vitamin A to prevent bronchopulmonary dysplasia in extremely low birth weight infants: a systematic review and meta-analysis. PLoS One. 2018;13(11):e0207730. doi:10.1371/journal.pone.0207730
196. Shaffer ML, Baud O, Lacaze-Masmonteil T, Peltoniemi OM, Bonsante F, Watterberg KL. Effect of prophylaxis for early adrenal insufficiency using low-dose hydrocortisone in very preterm infants: an individual patient data meta-analysis. J Pediatr. 2019;207:136–142.e5. doi:10.1016/j.jpeds.2018.10.004
197. Baud O, Maury L, Lebail F, et al. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet. 2016;387(10030):1827–1836. doi:10.1016/S0140-6736(16)00202-6
198. Renolleau C, Toumazi A, Bourmaud A, et al. Association between Baseline cortisol serum concentrations and the effect of prophylactic hydrocortisone in extremely preterm infants. J Pediatr. 2021;234:65–70.e3. doi:10.1016/j.jpeds.2020.12.057
199. Watterberg KL, Scott SM. Evidence of early adrenal insufficiency in babies who develop bronchopulmonary dysplasia. Pediatrics. 1995;95(1):120–125. doi:10.1542/peds.95.1.120
200. El-Khuffash A, James AT, Corcoran JD, et al. A patent ductus arteriosus severity score predicts chronic lung disease or death before discharge. J Pediatr. 2015;167(6):1354–1361.e2. doi:10.1016/j.jpeds.2015.09.028
201. Clyman RI, Hills NK, Liebowitz M, Johng S. Relationship between duration of infant exposure to a moderate-to-large patent ductus arteriosus shunt and the risk of developing bronchopulmonary dysplasia or death before 36 weeks. Am J Perinatol. 2020;37(2):216–223. doi:10.1055/s-0039-1697672
202. Gentle SJ, Travers CP, Clark M, Carlo WA, Ambalavanan N. Patent ductus arteriosus and development of bronchopulmonary dysplasia with pulmonary hypertension. Am J Respir Crit Care Med. 2022. doi:10.1164/rccm.202203-0570OC
203. Relangi D, Somashekar S, Jain D, et al. Changes in patent ductus arteriosus treatment strategy and respiratory outcomes in premature infants. J Pediatr. 2021;235:58–62. doi:10.1016/j.jpeds.2021.04.030
204. Clyman RI, Hills NK. Patent ductus arteriosus (PDA) and pulmonary morbidity: can early targeted pharmacologic PDA treatment decrease the risk of bronchopulmonary dysplasia? Semin Perinatol. 2023;47(2):151718. doi:10.1016/j.semperi.2023.151718
205. El-Khuffash A, Bussmann N, Breatnach CR, et al. A pilot randomized controlled trial of early targeted patent ductus arteriosus treatment using a risk based severity score (The PDA RCT). J Pediatr. 2021;229:127–133. doi:10.1016/j.jpeds.2020.10.024
206. Hundscheid T, Onland W, Kooi EMW, et al. Expectant management or early ibuprofen for patent ductus arteriosus. N Engl J Med. 2023;388(11):980–990. doi:10.1056/NEJMoa2207418
207. Clyman RI, Kaempf J, Liebowitz M, et al. Prolonged tracheal intubation and the association between patent ductus arteriosus and bronchopulmonary dysplasia: a secondary analysis of the PDA-TOLERATE trial. J Pediatr. 2021;229:283–288.e2. doi:10.1016/j.jpeds.2020.09.047
208. Giesinger RE, Rios DR, Chatmethakul T, et al. Impact of early hemodynamic screening on extremely preterm outcomes in a high-performance center. Am J Respir Crit Care Med. 2023;208(3):290–300. doi:10.1164/rccm.202212-2291OC
209. El-Khuffash A, Rios DR, McNamara PJ. Toward a rational approach to patent ductus arteriosus trials: selecting the population of interest. J Pediatr. 2021;233:11–13. doi:10.1016/j.jpeds.2021.01.012
210. Shelton EL, Singh GK, Nichols CG. Novel drug targets for ductus arteriosus manipulation: looking beyond prostaglandins. Semin Perinatol. 2018;42(4):221–227. doi:10.1053/j.semperi.2018.05.004
211. Makoni M, Chatmethakul T, Giesinger R, McNamara PJ. Hemodynamic precision in the neonatal intensive care unit using targeted neonatal echocardiography. J Vis Exp. 2023;191. doi:10.3791/64257
212. Backes CH, Hill KD, Shelton EL, et al. Patent ductus arteriosus: a contemporary perspective for the pediatric and adult cardiac care provider. J Am Heart Assoc. 2022;11(17):e025784. doi:10.1161/JAHA.122.025784
213. Chu A, de St Maurice A, Sim MS, Kallapur SG. Neonatal mycoplasma and ureaplasma infections. Pediatr Ann. 2020;49(7):e305–e312. doi:10.3928/19382359-20200625-01
214. Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54(6):797–807. doi:10.1203/01.PDR.0000091284.84322.16
215. Nunes CR, Procianoy RS, Corso AL, Silveira RC. Use of azithromycin for the prevention of lung injury in mechanically ventilated preterm neonates: a randomized controlled trial. Neonatology. 2020;117(4):522–528. doi:10.1159/000509462
216. Viscardi RM, Terrin ML, Magder LS, et al. Randomised trial of azithromycin to eradicate. Arch Dis Child Fetal Neonatal Ed. 2020;105(6):615–622. doi:10.1136/archdischild-2019-318122
217. Ballard HO, Shook LA, Bernard P, et al. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr Pulmonol. 2011;46(2):111–118. doi:10.1002/ppul.21352
218. Lal CV, Travers C, Aghai ZH, et al. The airway microbiome at birth. Sci Rep. 2016;6(1):31023. doi:10.1038/srep31023
219. Freeman AE, Willis KA, Qiao L, et al. Microbial-induced redox imbalance in the neonatal lung is ameliorated by live biotherapeutics. Am J Respir Cell Mol Biol. 2023;68(3):267–278. doi:10.1165/rcmb.2021-0508OC
220. Willis KA, Siefker DT, Aziz MM, et al. Perinatal maternal antibiotic exposure augments lung injury in offspring in experimental bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2020;318(2):L407–L418. doi:10.1152/ajplung.00561.2018
221. Chen WY, Lo YC, Huang PH, et al. Increased antibiotic exposure in early life is associated with adverse outcomes in very low birth weight infants. J Chin Med Assoc. 2022;85(9):939–943. doi:10.1097/JCMA.0000000000000749
222. Yu W, Zhang L, Li S, et al. Early antibiotic use and neonatal outcomes among preterm infants without infections. Pediatrics. 2023;151(5). doi:10.1542/peds.2022-059427
223. Chen X, Huang X, Lin Y, Lin B, Yang C, Huang Z. Association of Ureaplasma infection pattern and azithromycin treatment effect with bronchopulmonary dysplasia in Ureaplasma positive infants: a cohort study. BMC Pulm Med. 2023;23(1):229. doi:10.1186/s12890-023-02522-4
224. Sharma R, Bhandari V. Fluid balance in early postnatal life: should we keep the babies dry to prevent bronchopulmonary dysplasia? Pediatr Res. 2021;90(2):240–241. doi:10.1038/s41390-021-01589-1
225. Thiess T, Lauer T, Woesler A, et al. Correlation of early nutritional supply and development of bronchopulmonary dysplasia in preterm infants <1000 g. Front Pediatr. 2021;9:741365. doi:10.3389/fped.2021.741365
226. Stefanescu BM, Gillam-Krakauer M, Stefanescu AR, Markham M, Kosinski JL. Very low birth weight infant care: adherence to a new nutrition protocol improves growth outcomes and reduces infectious risk. Early Hum Dev. 2016;94:25–30. doi:10.1016/j.earlhumdev.2016.01.011
227. Miller M, Donda K, Bhutada A, Rastogi D, Rastogi S. Transitioning preterm infants from parenteral nutrition: a comparison of 2 protocols. JPEN J Parenter Enteral Nutr. 2017;41(8):1371–1379. doi:10.1177/0148607116664560
228. Behnke J, Estreich V, Oehmke F, Zimmer KP, Windhorst A, Ehrhardt H. Compatibility of rapid enteral feeding advances and noninvasive ventilation in preterm infants-An observational study. Pediatr Pulmonol. 2022;57(5):1117–1126. doi:10.1002/ppul.25868
229. Patel AL, Johnson TJ, Robin B, et al. Influence of own mother’s milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):F256–F261. doi:10.1136/archdischild-2016-310898
230. Patel AL, Johnson TJ, Meier PP. Racial and socioeconomic disparities in breast milk feedings in US neonatal intensive care units. Pediatr Res. 2021;89(2):344–352. doi:10.1038/s41390-020-01263-y
231. Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015;169(11):1011–1017. doi:10.1001/jamapediatrics.2015.2401
232. Rysavy MA, Mehler K, Oberthür A, et al. An immature science: intensive care for infants born at ≤23 weeks of gestation. J Pediatr. 2021;233(233):16–25.e1. doi:10.1016/j.jpeds.2021.03.006
233. Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115(3):655–661. doi:10.1542/peds.2004-1238
234. Harmon HM, Jensen EA, Tan S, et al. Timing of postnatal steroids for bronchopulmonary dysplasia: association with pulmonary and neurodevelopmental outcomes. J Perinatol. 2020;40(4):616–627. doi:10.1038/s41372-020-0594-4
235. Cuna A, Lagatta JM, Savani RC, et al. Association of time of first corticosteroid treatment with bronchopulmonary dysplasia in preterm infants. Pediatr Pulmonol. 2021;56(10):3283–3292. doi:10.1002/ppul.25610
236. Baud O, Trousson C, Biran V, et al. Association between early low-dose hydrocortisone therapy in extremely preterm neonates and neurodevelopmental outcomes at 2 years of age. JAMA. 2017;317(13):1329–1337. doi:10.1001/jama.2017.2692
237. Halbmeijer NM, Onland W, Cools F, et al. Effect of systemic hydrocortisone in ventilated preterm infants on parent-reported behavioural outcomes at 2 years’ corrected age: follow-up of a randomised clinical trial. Arch Dis Child Fetal Neonatal Ed. 2023;108(4):373–379. doi:10.1136/archdischild-2022-324179
238. Onland W, Cools F, Kroon A, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321(4):354–363. doi:10.1001/jama.2018.21443
239. Watterberg KL, Walsh MC, Li L, et al. Hydrocortisone to Improve Survival without Bronchopulmonary Dysplasia. N Engl J Med. 2022;386(12):1121–1131. doi:10.1056/NEJMoa2114897
240. Gentle SJ, Rysavy MA, Li L, et al. Heterogeneity of treatment effects of hydrocortisone by risk of bronchopulmonary dysplasia or death among extremely preterm infants in the national institute of child health and human development neonatal research network trial: a secondary analysis of a randomized clinical trial. JAMA Netw Open. 2023;6(5):e2315315. doi:10.1001/jamanetworkopen.2023.15315
241. Greenberg RG, Gayam S, Savage D, et al. Furosemide exposure and prevention of bronchopulmonary dysplasia in premature infants. J Pediatr. 2019;208:134–140.e2. doi:10.1016/j.jpeds.2018.11.043
242. Segar JL. Rethinking furosemide use for infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 2020;55(5):1100–1103. doi:10.1002/ppul.24722
243. Thakur A, Fursule A. Lung ultrasound in neonates - An underused tool. J Med Imaging Radiat Oncol. 2022. doi:10.1111/1754-9485.13485
244. Abdel-Hady H, Shouman B, Aly H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: a randomized controlled trial. Early Hum Dev. 2011;87(3):205–208. doi:10.1016/j.earlhumdev.2010.12.010
245. Taha DK, Kornhauser M, Greenspan JS, Dysart KC, Aghai ZH. High flow nasal cannula use is associated with increased morbidity and length of hospitalization in extremely low birth weight infants. J Pediatr. 2016;173:50–55.e1. doi:10.1016/j.jpeds.2016.02.051
246. van Delft B, Van Ginderdeuren F, Lefevere J, van Delft C, Cools F. Weaning strategies for the withdrawal of non-invasive respiratory support applying continuous positive airway pressure in preterm infants: a systematic review and meta-analysis. BMJ Paediatr Open. 2020;4(1):e000858. doi:10.1136/bmjpo-2020-000858
247. Zhang EY, Bartman CM, Prakash YS, Pabelick CM, Vogel ER. Oxygen and mechanical stretch in the developing lung: risk factors for neonatal and pediatric lung disease. Front Med. 2023;10:1214108. doi:10.3389/fmed.2023.1214108
248. Kish MZ. Improving preterm infant outcomes: implementing an evidence-based oral feeding advancement protocol in the neonatal intensive care unit. Adv Neonatal Care. 2014;14(5):346–353. doi:10.1097/ANC.0000000000000099
249. McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074–2082. doi:10.1001/jama.2014.5217
250. McEvoy CT, Shorey-Kendrick LE, Milner K, et al. Vitamin C to pregnant smokers persistently improves infant airway function to 12 Months of age: a randomised trial. Eur Respir J. 2020;56(6):1902208. doi:10.1183/13993003.02208-2019
251. Shorey-Kendrick LE, McEvoy CT, O’Sullivan SM, et al. Impact of vitamin C supplementation on placental DNA methylation changes related to maternal smoking: association with gene expression and respiratory outcomes. Clin Epigenetics. 2021;13(1):177. doi:10.1186/s13148-021-01161-y
252. Buhimschi CS, Bahtiyar MO, Zhao G, et al. Antenatal N-acetylcysteine to improve outcomes of premature infants with intra-amniotic infection and inflammation (triple I): randomized clinical trial. Pediatr Res. 2021;89(1): 175–184. doi:10.1038/s41390-020-01106-w
253. McEvoy CT, Ballard PL, Ward RM, et al. Dose-escalation trial of budesonide in surfactant for prevention of bronchopulmonary dysplasia in extremely low gestational age high-risk newborns (SASSIE). Pediatr Res. 2020;88(4):629–636. doi:10.1038/s41390-020-0792-y
254. Hillman NH, Kemp MW, Fee E, et al. Budesonide with surfactant decreases systemic responses in mechanically ventilated preterm lambs exposed to fetal intra-amniotic lipopolysaccharide. Pediatr Res. 2021;90(2):328–334. doi:10.1038/s41390-020-01267-8
255. Hillman NH, Kothe TB, Schmidt AF, et al. Surfactant plus budesonide decreases lung and systemic responses to injurious ventilation in preterm sheep. Am J Physiol Lung Cell Mol Physiol. 2020;318(1):L41–L48. doi:10.1152/ajplung.00203.2019
256. Kothe TB, Royse E, Kemp MW, et al. Effects of budesonide and surfactant in preterm fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2018;315(2):L193–L201. doi:10.1152/ajplung.00528.2017
257. Ley D, Hallberg B, Hansen-Pupp I, et al. rhIGF-1/rhIGFBP-3 in preterm infants: a phase 2 randomized controlled trial. J Pediatr. 2019;206:56–65.e8. doi:10.1016/j.jpeds.2018.10.033
258. Seedorf G, Kim C, Wallace B, et al. rhIGF-1/BP3 preserves lung growth and prevents pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2020;201(9):1120–1134. doi:10.1164/rccm.201910-1975OC
259. Ahn SY, Chang YS, Lee MH, et al. Stem cells for bronchopulmonary dysplasia in preterm infants: a randomized controlled phase II trial. Stem Cells Transl Med. 2021;10(8):1129–1137. doi:10.1002/sctm.20-0330
260. Omar SA, Abdul-Hafez A, Ibrahim S, et al. Stem-cell therapy for bronchopulmonary dysplasia (BPD) in newborns. Cells. 2022;11(8):1275. doi:10.3390/cells11081275
261. Wu S, Benny M, Duara J, et al. Extracellular vesicles: pathogenic messengers and potential therapy for neonatal lung diseases. Front Pediatr. 2023;11:1205882. doi:10.3389/fped.2023.1205882
262. Abman SH, Collaco JM, Shepherd EG, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr. 2017;181:12–28.e1. doi:10.1016/j.jpeds.2016.10.082
263. Shepherd EG, Knupp AM, Welty SE, Susey KM, Gardner WP, Gest AL. An interdisciplinary bronchopulmonary dysplasia program is associated with improved neurodevelopmental outcomes and fewer rehospitalizations. J Perinatol. 2012;32(1):33–38. doi:10.1038/jp.2011.45
264. Yung D, Jackson EO, Blumenfeld A, et al. A multidisciplinary approach to severe bronchopulmonary dysplasia is associated with resolution of pulmonary hypertension. Front Pediatr. 2023;11:1077422. doi:10.3389/fped.2023.1077422
265. Hansen TP, Noel-MacDonnell J, Kuckelman S, Norberg M, Truog W, Manimtim W. A multidisciplinary chronic lung disease team in a neonatal intensive care unit is associated with increased survival to discharge of infants with tracheostomy. J Perinatol. 2021;41(8):1963–1971. doi:10.1038/s41372-021-00974-2
266. Gibbs K, Jensen EA, Alexiou S, Munson D, Zhang H. Ventilation strategies in severe bronchopulmonary dysplasia. Neoreviews. 2020;21(4):e226–e237. doi:10.1542/neo.21-4-e226
267. Akangire G, Manimtim W. Tracheostomy in infants with severe bronchopulmonary dysplasia: a review. Front Pediatr. 2022;10:1066367. doi:10.3389/fped.2022.1066367
268. Sindelar R, Shepherd EG, Ågren J, et al. Established severe BPD: is there a way out? Change of ventilatory paradigms. Pediatr Res. 2021;90(6):1139–1146. doi:10.1038/s41390-021-01558-8
269. Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29(7):710–717. doi:10.1016/S0046-8177(98)90280-5
270. Coalson JJ. Pathology of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):179–184. doi:10.1053/j.semperi.2006.05.004
271. Özkan H, Duman N, Tüzün F. Pathophysiologically based ventilatory management of severe bronchopulmonary dysplasia. Turk Arch Pediatr. 2022;57(4):385–390. doi:10.5152/TurkArchPediatr.2022.22112
272. Hysinger EB, Ahlfeld SK. Respiratory support strategies in the prevention and treatment of bronchopulmonary dysplasia. Front Pediatr. 2023;11:1087857. doi:10.3389/fped.2023.1087857
273. Shetty S, Hickey A, Rafferty GF, Peacock JL, Greenough A. Work of breathing during CPAP and heated humidified high-flow nasal cannula. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F404–7. doi:10.1136/archdischild-2015-309310
274. McKinney RL, Keszler M, Truog WE, et al. Multicenter experience with neurally adjusted ventilatory assist in infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2021;38(S 01):e162–e166. doi:10.1055/s-0040-1708559
275. Yallapragada S, Savani RC, Mūnoz-Blanco S, et al. Qualitative indications for tracheostomy and chronic mechanical ventilation in patients with severe bronchopulmonary dysplasia. J Perinatol. 2021;41(11):2651–2657. doi:10.1038/s41372-021-01165-9
276. DeMauro SB, D’Agostino JA, Bann C, et al. Developmental outcomes of very preterm infants with tracheostomies. J Pediatr. 2014;164(6):1303–10.e2. doi:10.1016/j.jpeds.2013.12.014
277. Levit OL, Shabanova V, Bazzy-Asaad A, Bizzarro MJ, Bhandari V. Risk factors for tracheostomy requirement in extremely low birth weight infants. J Matern Fetal Neonatal Med. 2018;31(4):447–452. doi:10.1080/14767058.2017.1287895
278. DeMauro SB, Wei JL, Lin RJ. Perspectives on neonatal and infant tracheostomy. Semin Fetal Neonatal Med. 2016;21(4):285–291. doi:10.1016/j.siny.2016.03.006
279. Hayes D, Wilson KC, Krivchenia K, et al. Home oxygen therapy for children. An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2019;199(3):e5–e23. doi:10.1164/rccm.201812-2276ST
280. Stenson BJ, Tarnow-Mordi WO, Darlow BA, et al. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368(22):2094–2104. doi:10.1056/NEJMoa1302298
281. Slaughter JL, Stenger MR, Reagan PB. Variation in the use of diuretic therapy for infants with bronchopulmonary dysplasia. Pediatrics. 2013;131(4):716–723. doi:10.1542/peds.2012-1835
282. Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR, Choonara I. Utilization of inhaled corticosteroids for infants with bronchopulmonary dysplasia. PLoS One. 2014;9(9):e106838. doi:10.1371/journal.pone.0106838
283. Sakaria RP, Dhanireddy R. Pharmacotherapy in Bronchopulmonary Dysplasia: what Is the Evidence? Front Pediatr. 2022;10:820259. doi:10.3389/fped.2022.820259
284. Nelin LD, Kielt MJ, Jebbia M, Jadcherla S, Shepherd EG. Bronchodilator responsiveness and dysanapsis in bronchopulmonary dysplasia. ERJ Open Res. 2022;8(3):00682–2021. doi:10.1183/23120541.00682-2021
285. Cousins M, Hart K, Williams EM, Kotecha S. Impaired exercise outcomes with significant bronchodilator responsiveness in children with prematurity-associated obstructive lung disease. Pediatr Pulmonol. 2022;57(9):2161–2171. doi:10.1002/ppul.26019
286. Baraldi E, Bonetto G, Zacchello F, Filippone M. Low exhaled nitric oxide in school-age children with bronchopulmonary dysplasia and airflow limitation. Am J Respir Crit Care Med. 2005;171(1):68–72. doi:10.1164/rccm.200403-298OC
287. Bhandari A, Panitch H. An update on the post-NICU discharge management of bronchopulmonary dysplasia. Semin Perinatol. 2018;42(7):471–477. doi:10.1053/j.semperi.2018.09.011
288. Rastogi A, Luayon M, Ajayi OA, Pildes RS. Nebulized furosemide in infants with bronchopulmonary dysplasia. J Pediatr. 1994;125(6 Pt 1):976–979. doi:10.1016/s0022-3476(05)82018-9
289. Billion E, Hadchouel A, Garcelon N, Delacourt C, Drummond D. Intravenous pulses of methylprednisolone for infants with severe bronchopulmonary dysplasia and respiratory support after 3 months of age. Pediatr Pulmonol. 2021;56(1):74–82. doi:10.1002/ppul.25109
290. Sahebjami H, Domino M. Effects of postnatal dexamethasone treatment on development of alveoli in adult rats. Exp Lung Res. 1989;15(6):961–973. doi:10.3109/01902148909069638
291. Hwang JK, Shin SH, Kim EK, Kim SH, Kim HS. Association of newer definitions of bronchopulmonary dysplasia with pulmonary hypertension and long-term outcomes. Front Pediatr. 2023;11:1108925. doi:10.3389/fped.2023.1108925
292. Branescu I, Shetty S, Richards J, Vladareanu S, Kulkarni A. Pulmonary hypertension in preterm infants with moderate-to-severe bronchopulmonary dysplasia (BPD). Acta Paediatr. 2023;112(9):1877–1883. doi:10.1111/apa.16863
293. Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics. 2007;120(6):1260–1269. doi:10.1542/peds.2007-0971
294. Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37(2):124–131. doi:10.1053/j.semperi.2013.01.009
295. Abman SH, Hansmann G, Archer SL, et al. Pediatric pulmonary hypertension: guidelines from the American heart association and American thoracic society. Circulation. 2015;132(21):2037–2099. doi:10.1161/CIR.0000000000000329
296. Chan S, Brugha R, Quyam S, Moledina S. Diagnosis and management of pulmonary hypertension in infants with bronchopulmonary dysplasia: a guide for paediatric respiratory specialists. Breathe. 2022;18(4):220209. doi:10.1183/20734735.0209-2022
297. Kehinde F, Marinescu A, Turchi R. Catch it before it breaks!: managing metabolic bone disease of prematurity. Curr Opin Pediatr. 2021;33(6):676–683. doi:10.1097/MOP.0000000000001060
298. Dani A, Hayes D, Guzman-Gomez A, et al. Lung transplantation for bronchopulmonary dysplasia. Chest. 2023;163(5):1166–1175. doi:10.1016/j.chest.2022.12.032
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.