Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Primary Sebaceous Carcinoma of the Eyebrow: A Case Report
Received 15 March 2023
Accepted for publication 10 June 2023
Published 29 June 2023 Volume 2023:16 Pages 1715—1720
DOI https://doi.org/10.2147/CCID.S412663
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Jiangping Ye,1 Qiaoyun Li2
1Department of Ophthalmology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Pathology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
Correspondence: Jiangping Ye, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, 261 Huansha Road, Hangzhou, People’s Republic of China, Email [email protected]
Abstract: Sebaceous carcinoma (SC) is an uncommon but aggressive malignancy and has a specific anatomic preference for the ocular region, especially the eyelids. However, periocular SC originated from the eyebrow is rare, which may cause poorer outcomes due to a greater likelihood of orbital invasion and excessive tumor volume. In the present case, we exhibited a 68-year-old male presenting with a large solid mass in his right eyebrow region developing in ten months. Based on the patient’s history, clinical conditions, orbital computed tomography (CT) and magnetic resonance imaging (MRI) scan results, a malignant tumor was suspected preliminarily. Excisional biopsy was performed, and the histopathologic examination and immunohistochemistry (IHC) staining of the tumor revealed SC. The patient declined the enlarged surgery recommended next and ended up with death caused by the distant metastasis of SC. The case highlighted the fact that despite its rarity, SC should be considered as a differential diagnosis of tumors located in the eyebrow region and histopathologic evaluation must be performed to reach a definite diagnosis. Ophthalmologists are supposed to have a comprehensive understanding of the clinicopathological characteristics of this disease and help patients accept the appropriate treatments promptly via properly and adequate communication if necessary.
Keywords: sebaceous carcinoma, eyebrow, diagnosis, prognosis, case report, SC
Introduction
Sebaceous carcinoma (SC) is an uncommon but aggressive malignancy that develops from cells comprising sebaceous glands.1–3 The clinical manifestations of eyelid SC are non-specific with the lesions having wide morphologic diversity and involving different periorbital parts, and can lead to an initial misdiagnosis in up to 50% of patients.4 Besides, the overall incidence of SC is increasing significantly in recent years.5 Therefore, ophthalmologists are urged to improve their knowledge and awareness of SC to increase the rate of early diagnosis and reduce misdiagnosis.
SC typically arises from meibomian glands of the eyelids, with the upper eyelid predominant due to the higher distribution of glands.1–3 Zeis glands of the eyelid (about 10%) and the lacrimal caruncle (<10%) are also periocular sites of SC.1,2 However, SC primarily originated from the eyebrow is rare, and to our knowledge there is little mention in the ophthalmic literature about SC of the eyebrow.1,2 In a retrospective study carried by Beijing Tongren Hospital in China, only one case was found arising from the eyebrow region among 354 eyelid SC patients.6 Herein, we reported a rare case of SC occurred in the eyebrow region which caused a fatal prognosis.
Case Report
A 68-year-old male was referred to our ophthalmology department for a large solid mass in his right eyebrow region. The mass developed in the past 10 months, causing compression to the eyeball as well as adjacent tissues gradually. The patient reported symptoms including restricted ocular motility and occasional stinging eyes. He had a 10-year history of hypertension and suffered a stroke 2 years ago which leaving with the sequelae of slurred speech. There was a mentionable history of orbital trauma that he hurt his right eyebrow at 10-years-old. He was previously diagnosed with a bilateral cataract and bilateral pterygium and denied any history of ocular surgery.
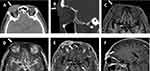
On ocular examination, an irregular and solid mass lesion measuring 40×20×15mm visually was observed spanning across the right eyebrow region with the main body located on the nasal side, causing ptosis of the upper eyelid and limitation of eye movements. The lesion had poor mobility and a lobulated appearance with a small depression and hyperpigmented area in the surface (Figure 1). Visual acuity of the patient was 20/100 in the right eye and 20/125 in the left eye, and both intraocular pressure and exophthalmos measurement were normal. Slit-lamp biomicroscopic examination revealed bilateral lens opacities (nuclear 3+/4+) and abnormal fibrovascular tissue of the conjunctiva encroaching onto the cornea. The remaining ophthalmic examination was unremarkable. Initial imaging of the orbit (computed tomography [CT] followed by magnetic resonance imaging [MRI]) revealed an irregular space-occupying lesion with a rich blood supply growing within soft tissue above the right orbit to the forehead which was considered to be a neoplastic lesion (Figure 2A-F). Based on the patient’s history, clinical conditions, CT and MRI scan results, a malignant tumor was suspected preliminarily.
 |
Figure 1 Clinical photo showed an irregular and lobulated mass lesion spanning across the right eyebrow region with a small depression and hyperpigmented area in the surface (white arrow). |
The patient underwent an excisional biopsy required for definite diagnosis in our hospital and resection was performed with a 4-mm surgical margin. The excised lesion consisted of a main part of 28×18×7mm in size and the other part of 10×8×8mm, and was suspicious for malignant cutaneous neoplasm originated from adnexal structures of the skin. The lesion was tumor-positive at partial resection margins assessed with intraoperative frozen pathological examination. The postoperative pathological report indicated the diagnosis of poorly-differentiated SC and demonstrated the neoplasm partially sarcomatoid differentiation and with focal basal cell carcinoma (BCC) component (Figure 3A–C). Immunohistochemistry (IHC) staining (EnVision method) and special staining of Alcian blue/Periodic acid-Schiff (AB-PAS) were performed subsequently to ensure the diagnosis. IHC stains were positive for cytokeratin (CK), P63, CK5/6, androgen receptor (AR), with a high Ki-67 expression (35%–40%); and negative for CK7, CK20, S-100, HMB45, MelanA, epithelial membrane antigen (EMA), SMA, Calponin, CD34, PR, CEA, CgA and Syn (Figure 3D and E). Besides, the lesion was detected to have small foci of positivity dispersedly on PAS stain but negativity on AB stain.
Regional lymph node examination, chest X-ray, abdomen B-ultrasound and blood tumor marker test were performed subsequently on the patient and no evidence of nodal metastasis or distant metastasis was found. Considering the large size of the tumor and its invasion of the periorbital tissue, we suggested the patient undergo enlarged resection and if necessary removal of orbital contents. However, the patient was unable to accept this proposal and chose to transfer to another hospital for further treatment. Unfortunately, we reached the patient’s family and learned that the patient had died due to the distant metastasis of SC 3 years later, though second surgery and multiple radiotherapy had been performed.
Discussion
SC is a rare cutaneous malignant neoplasm which accounting for less than 5.5% of all eyelid malignancies in Whites but much higher proportion in Asians (even over 30%).3,7,8 The published literature reported that about 75% SCs occur in the periocular region while the remaining 25% appear in extraocular areas.1,2 However, periocular SCs rarely originate from glands of the eyebrows, which causing it difficult to take SC into consideration promptly when the tumor occurred in the eyebrow region.3,9
As the clinical presentation of SC has no specific features and commonly masquerades as a variety of conditions both inflammatory and neoplastic, it frequently causes misdiagnosis or delayed diagnosis which resulting in inappropriate treatments and increasing mortality.1,9 Thus, ophthalmologists should be familiar with the differential diagnosis of SC which aiding in making the timely diagnosis. Given the fact that the majority of SCs present with upper eyelid involvement, its differential diagnosis usually includes atypical or recurrent chalazion, thickening of the eyelids (nodular or diffuse), non-treatment responsive blepharoconjunctivitis and persistent or unilateral blepharitis.1,2 However, when SC rarely occurs in the eyebrow region, being in the form of subcutaneous nodules usually, mainly other periorbital masses appearing in the same region are considered as the differential diagnosis, such as pyogenic granuloma, epidermoid cyst, etc. Malignant tumors like BCC, squamous cell carcinoma (SCC) and malignant melanoma can be masqueraded by SC and should be taken into consideration as well.
SC has characteristic imaging findings, so imaging examinations like orbital CT and MRI should be routinely performed when the tumor is suspected to be malignant to aid in the diagnosis and differential diagnosis of SC. MRI is the preferred imaging examination for SC which has a much higher resolution in soft tissue imaging than CT and can avoid the radiation damage of the lens and cornea.10,11 MRI typically exhibits eyelid SCs as nodular, “cauliflower-like”, ring-shaped or irregularly soft tissue mass shadows of the eyelid. The lesions are hypointense on T1WI and hyperintense on T2WI, and unevenly enhanced in the enhanced MRI scan. On CT, lesions usually present as soft tissue density masses in the shape of ring strip and nodule with unclear boundaries. The density is either homogenous, or heterogeneous with gas sign, patchy high-density shadow caused by hemorrhage or low-density shadow due to liquefaction necrosis.
The definitive diagnosis of SC is established by histopathological examinations with IHC and special staining occasionally serving as ancillary tools to confirm the diagnosis and differentiate SC from other malignant tumors like BCC, SCC and malignant melanoma.12 Immunostaining for AR, EMA, Ber-EP4 and adipophilin (ADP) have been used as reliable markers of sebaceous differentiation and to differentiate SC from BCC and SCC.13,14 Generally, SCs were EMA+, ADP+, Ber-EP4– and AR+, SCCs were EMA+, ADP–/+, Ber-EP4–/+ and AR–, whereas BCCs were EMA–, ADP–, Ber-EP4+ and AR–/+.13–16 EMA is expressed primarily in both the cytoplasm and membrane of the sebocytes, while in SC the positive expression of EMA refers to the cytoplasmic positive rather than membranous positive, and corresponds to the vacuolated cytoplasm morphologically. Hence, in the present case the negative expression of EMA may resulted from the poor differentiation of tumor with tumor cells lacking the typical vacuolate-like morphology. Unfortunately, Ber-EP4 and ADP antibodies were not used in our case. The anti-cytokeratin antibodies are used in IHC detection of SC as well, however, the sensitivity and specificity of them are lower than those of other antibodies and the positive expressions of them are significant while the negative findings are not so valuable in diagnosing.17 CK5/6 and p63 are regarded as traditional immunochemical markers for the diagnosis of SCC and squamous differentiation, while they had been found to be positive in some SCs,12,18,19 for instance, Soares et al19 reported that 10 cases of SC originating from major salivary glands were positive for CK5, and half of them were also positive for p63 which were predominantly expressed in the immature sebocytes as well as in poorly-differentiated cells. CK5/6 and p63 were also positive in the poorly-differentiated SC in our case, which were consistent with Soares’s findings.19 CK7 showed positive reactions in approximately half cases of SC,17,20 yet was negative in the present case. Special staining of Oil Red O and Sudan IV are classical methods to confirm the presence of lipid in cytoplasmic vacuoles and can form the primary basis for a diagnosis of SC whose tumor cells usually had lipid-rich cytoplasm.2, 18 However, fresh excised samples in many cases were not kept to be stained with Oil Red O or Sudan IV in the intraoperative frozen pathological examination and similar condition arose in our case.14,18 We then used AB-PAS staining which can highlight and distinguish neutral mucins from acid mucins in the postoperative sections and the results showed the cytoplasmic vacuoles containing little glycogen. To our knowledge, AB-PAS staining was seldomly used in the diagnosis of SC, and PAS was found to be positive in partial area in a SC of submandibular gland while negative in another case of right palate.18,21
Surgical excision is the mainstream treatment for SC, while chemotherapy and radiotherapy can be employed as adjunctive therapies for metastatic and recurrent SC. According to the 8th edition American Joint Committee on Cancer (AJCC)/tumor‐node‐metastasis (TNM) staging system, the tumor in the present case was evaluated to be in T3N0M0 category and a surgical approach of extended resection plus orbital exenteration was immediately suggested.22 It has been revealed that clinicopathologic risk factors associated with poorer outcomes in SC included simultaneous involvement of both upper and lower eyelids, orbital invasion, pagetoid infiltration, large tumor size and tumor duration of 6 months or greater.23 The tumor in our case met the conditions pointing toward a dismal prognosis with the location of the superficial orbit and a duration over 6 months. The patient was not psychologically ready to accept the extended resection at that time. In fact, although he underwent the second surgery at another hospital after a while, the long delay in the treatment aggravated his condition rapidly and led to a fatal prognosis at final. This reminded us that properly and adequate communication regarding the condition of patients especially those with malignancy is an extremely important skill for clinicians. Clinicians should have a knowledge of the patients’ uncertainty and concerns of the condition, and try to persuade them to accept the treatments which are beneficial to improve the condition in a way that is understandable to them.
Conclusion
In summary, it is noteworthy that despite its rarity SC should be considered as a differential diagnosis of tumors located in the eyebrow region, and it may lead to poorer outcomes because of a great likelihood of orbital invasion and excessive tumor size. Histopathologic evaluation must be carried out to reach the definite diagnosis and treatments are supposed to be performed appropriately and timely by properly and adequate doctor-patient communication.
Ethics Approval and Consent for Publication
The case report adhered to the ethical principles outlined in the Declaration of Helsinki. A written informed consent was obtained from the patient’s family for publication of this study and any accompanying image. Institutional ethical approval was not required to publish this case details.
Acknowledgments
We thank the patient and physicians for participating in our study.
Funding
This study was supported by Hangzhou Municipal Health Science and Technology Plan General Project (Class A) (No. A20210032).
Disclosure
None of the authors have any potential conflict of interest to disclose.
References
1. Shields JA, Demirci H, Marr BP, et al. Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol. 2005;50:103–122.
2. Shields JA, Demirci H, Marr BP, et al. Sebaceous carcinoma of the eyelids: personal experience with 60 cases. Ophthalmology. 2004;111:2151–2157.
3. Chang K, Wileman JM, Gabbard RD, et al. Isolated primary sebaceous gland carcinoma of the bulbar conjunctiva. Am J Ophthalmol Case Rep. 2022;27:101675.
4. Chao AN, Shields CL, Krema H, et al. Outcome of patients with periocular sebaceous gland carcinoma with and without conjunctival intraepithelial invasion. Ophthalmology. 2001;108:1877–1883.
5. Vittoria Cicinelli M, Kaliki S. Ocular sebaceous gland carcinoma: an update of the literature. Int Ophthalmol. 2018;39(5):1187–1197.
6. Zihan N, Xiaolin X, Bin L, et al. Prognostic factors of 354 cases for eyelid sebaceous gland carcinoma. Eye. 2022;31:104–108.
7. Xu XL, Li B, Sun XL, et al. Eyelid neoplasms in the Beijing Tongren eye centre between 1997 and 2006. Ophthalmic Surg Lasers Imaging. 2008;39:367–372.
8. Cook BE, Bartley GB. Epidemiologic characteristics and clinical course of patients with malignant eyelid tumors in an incidence cohort in Olmsted County. Minnesota Ophthalmol. 1999;106:746–750.
9. Buitrago W, Joseph AK. Sebaceous carcinoma: the great Masquerader. Dermatol Ther. 2008;21:459–466.
10. Shuang W, Wei C, Tao Z, et al. The CT and MRI Findings and Differential Diagnosis of Sebaceous Carcinoma of Eyelid and Basal Cell Carcinoma. CTTA. 2022;31(5):662–668.
11. Aiping K, Lixing W, Juan L. Radiographic findings of meibomian adenocarcinoma. HeiLongjiang Med J. 2014;38(9):1027–1028.
12. Sahni V, Cassarino DS. Sebaceous Carcinoma of the Penis: a Rare, Dangerous Clinical Entity and the Importance of Immunohistochemistry in Diagnosis. Case Rep Dermatol Med. 2023;2023:6944296.
13. Asadi-Amoli F, Khoshnevis F, Haeri H, et al. Comparative examination of androgen receptor reactivity for differential diagnosis of sebaceous carcinoma from squamous cell carcinoma and basal cell carcinoma. Am J Clin Pathol. 2010;134:22–26.
14. Mulay K, White VA, Shah SJ, et al. Sebaceous carcinoma: clinicopathologic features and diagnostic role of immunohistochemistry (including androgen receptor). Can J Ophthalmol. 2014;49(4):326–332.
15. Mulay K, Shah SJ, Aggarwal E, et al. Periocular sebaceous gland carcinoma: do androgen receptor (NR3C4) and nuclear survivin (BIRC5) have a prognostic significance? Acta Ophthalmol. 2014;92:e681–e687.
16. i AS, Takeichi H, Arase S, et al. Sebaceous carcinoma: an immunohistochemical reappraisal. Am J Dermatopath. 2011;33(6):579–587.
17. Ansai S, Arase S, Kawana S, et al. Immunohistochemical findings of sebaceous carcinoma and sebaceoma: retrieval of cytokeratin expression by a panel of anti-cytokeratin monoclonal antibodies. J Dermatol. 2011;38:951–958.
18. Qun L, Xiaoyue F, Huang Y. Sebaceous carcinoma of the right palate: case report and literature review. Gland Surg. 2021;10(5):1819–1825.
19. Soares CD, Morais TML, Carlos R, et al. Sebaceous adenocarcinomas of the major salivary glands: a clinicopathological analysis of 10 cases. Histopathology. 2018;73(4):585–592.
20. Plaza JA, Mackinnon A, Carrillo L, et al. Role of immunohistochemistry in the diagnosis of sebaceous carcinoma: a clinicopathologic and immunohistochemical study. Am J Dermatopath. 2015;37(11):809–821.
21. Wei J, Luo G, Shi Y, et al. Sebaceous Carcinoma of the Submandibular Gland a Case Report and Review of the Literature. Cancer Manag Res. 2023;15:123–130.
22. AlHammad F, Edward D, Alkatan HM, et al. Eyelid sebaceous gland carcinoma: an assessment of the T classification of the American Joint Committee of Cancer TNM staging system 8th versus. Eur J Ophthalmol. 2021;31(4):2055–2063.
23. Takahashi Y, Takahashi E, Nakakura S, et al. Risk factors for local recurrence or metastasis of eyelid sebaceous gland carcinoma after wide excision with paraffin section control. Am J Ophthalmol. 2016;171:67–74.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.