Back to Journals » Research and Reports in Urology » Volume 16
Prognostic Biomarkers and AKI: Potential to Enhance the Identification of Post-Operative Patients at Risk of Loss of Renal Function
Authors Singh R, Watchorn JC, Zarbock A, Forni LG
Received 30 August 2023
Accepted for publication 29 February 2024
Published 5 March 2024 Volume 2024:16 Pages 65—78
DOI https://doi.org/10.2147/RRU.S385856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Rishabh Singh,1 James C Watchorn,2 Alexander Zarbock,3 Lui G Forni4,5
1Department of Surgery, Royal Surrey Hospital, Guildford, Surrey, UK; 2Intensive Care Unit, Royal Berkshire NHS Foundation Trust, Reading, Berkshire, UK; 3Department of Anesthesiology, Intensive Care and Pain Medicine, University Hospital Münster, Münster, Germany; 4Critical Care Unit, Royal Surrey Hospital, Guildford, Surrey, UK; 5School of Medicine, Kate Granger Building, University of Surrey, Guildford, UK
Correspondence: Lui G Forni, Email [email protected]
Abstract: Acute kidney injury (AKI) is a common complication after surgery and the more complex the surgery, the greater the risk. During surgery, patients are exposed to a combination of factors all of which are associated with the development of AKI. These include hypotension and hypovolaemia, sepsis, systemic inflammation, the use of nephrotoxic agents, tissue injury, the infusion of blood or blood products, ischaemia, oxidative stress and reperfusion injury. Given the risks of AKI, it would seem logical to conclude that early identification of patients at risk of AKI would translate into benefit. The conventional markers of AKI, namely serum creatinine and urine output are the mainstay of defining chronic kidney disease but are less suited to the acute phase. Such concerns are compounded in surgical patients given they often have significantly reduced mobility, suboptimal levels of nutrition and reduced muscle bulk. Many patients may also have misleadingly low serum creatinine and high urine output due to aggressive fluid resuscitation, particularly in intensive care units. Over the last two decades, considerable information has accrued with regard to the performance of what was termed “novel” biomarkers of AKI, and here, we discuss the most examined molecules and performance in surgical settings. We also discuss the application of biomarkers to guide patients’ postoperative care.
Plain Language Summary: Kidney damage is common after major surgery with a recent study showing almost 1 in 5 patients suffer kidney damage. The usual tests for measuring kidney function are excellent in the outpatient but not so good in acute scenario’s. Therefore, there has been a lot of interest in new markers of kidney damage (so-called novel biomarkers) which perform well acutely and allow earlier detection of damage allowing treatment to be started earlier. This article summarises the currently available biomarkers for use post-operatively and points out the different information that can be achieved by using them routinely.
Keywords: acute kidney injury, biomarkers, DKK-3, dickkopf-3, suPAR, soluble urokinase plasminogen activator receptor, IGFBP-7, insulin growth factor binding protein-7 and TIMP-2, tissue inhibitor of metalloproteinases-2, PENK, proenkephalin A 119-159, NGAL, neutrophil gelatinase-associated lipocalin, KIM-1, kidney injury molecule-1, CCL14, chemokine 14
Introduction
Acute kidney injury (AKI) is an abrupt deterioration in renal function resulting in derangement of metabolic, electrolyte and fluid homeostasis which occurs within hours or days following insult.1 Prior to the early 2000’s more than 35 different criteria existed for defining AKI, or acute renal failure as it was then labelled, but in 2004, the Acute Dialysis Quality Initiative (ADQI) published the first consensus definition of AKI, the RIFLE criteria, and these were refined further to form the current widely adopted Kidney Disease Improve Global Outcomes (KDIGO) classification (Table 1).2,3 Limitations on this classification remain, however, as the definitions rely on relative changes in creatinine from a baseline value which may be unknown, whilst serum creatinine is insensitive and urine output is non-specific for defining AKI.
 |
Table 1 Adapted KDIGO Criteria for AKI Diagnosis |
The Significance of AKI in Surgical Patients
Each year, over 300 million patients undergo major surgery globally and although considered a significant risk factor for AKI, the exact incidence of post-operative AKI was unknown given the retrospective nature of most studies involving surgical patients.4 Indeed, this is reflected in the literature, with widely differing post-operative AKI incidence rates after major abdominal surgery of between 1.8% and 39.3% quoted.5–8 Similarly, in patients undergoing cardiac surgery, rates of between 3.1% and 39.9% have been reported.9,10 However, recently, the epidemiology of surgery associated acute kidney injury (EPIS-AKI) study has been published.4 This was an international, prospective, observational study performed in over 30 countries where the primary endpoint was the development of post-operative AKI (PO-AKI) within 72 hours of major surgery, defined as having a duration of over 2 hours and requiring intensive care unit (ICU) or high dependency unit (HDU) facilities post-operatively. The KDIGO classification of AKI was used and, importantly, both serum creatinine (SCr) and urine output (UO) criteria were collected. This is relevant in that most of the retrospective studies examining PO-AKI often lack accurate, or indeed any, urine output data. Urine output is essential for diagnosing AKI in that a lack of these data result in underestimating the incidence of PO-AKI. Also, the presence of both SCr and UO criteria for AKI definition predicts worse outcomes compared to AKI based on the creatinine criterion alone.11 In the EPIS-AKI study, the final cohort consisted of 10,568 patients of which 18.4% developed AKI. Stage 1 AKI was the most common and accounted for 63.5% of all patients developing PO-AKI, 25.7% developed stage 2, and 10.07% stage 3. In 8.7% of patients with PO-AKI, renal replacement therapy was used. Unsurprisingly, the development of PO-AKI was associated with an increased length of stay in both hospital and ICU as well as increased mortality rates with higher mortality rates in the ICU (6.3% [95% CI 5.2–7.4%] vs 0.7% [95% CI 0.5–0.9%]) and in hospital (8.5% [95% CI 7.3–9.8%] vs 1.3% [95% CI 1.1–1.6%]) compared to non-PO-AKI patients (p < 0.001). Endpoint rates increased with increasing severity of PO-AKI and mortality rates were highest in KDIGO stage 3 patients meeting both SCr and UO criteria of the AKI definition (Figure 1). This study highlights the significance of PO-AKI given that almost 1 in 5 patients undergoing major surgery develop AKI and postoperative individuals who develop PO-AKI are more likely to develop fluid overload, surgical site infection, pulmonary and urinary infections, and cardiac events. Long term, post- operative AKI confers an increased risk of chronic kidney disease and overall mortality.12 Furthermore, a diagnosis of AKI renders patients with an almost nine-fold increase in the risk of developing CKD.13
 |
Figure 1 Outline of the results from the EPIS-AKI trial.4 aPercentage of patients undergoing that surgery type who developed PO-AKI. bPatients who developed PO-AKI after 72 h and were therefore classified in the “No PO-AKI” group and received RRT (Renal Replacement Therapy). |
The Limitations of Traditional Diagnostic Methods for Detecting AKI
Under normal conditions, SCr is freely filtered by the glomeruli, secreted by renal tubules, and undergoes extrarenal secretion by the intestine but importantly is released at a constant rate, making it a suitable biomarker for chronic kidney disease.14 However, SCr levels are influenced by several factors including muscle mass, age, sex, diet, fluid status, concurrent medication use as well as the presence of sepsis. As a consequence, SCr is an imperfect marker of filtration during acute illness.15 As an indicator of tubular function, SCr lacks sensitivity, and tubular injury may occur without elevated SCr levels and indeed, drugs may block tubular secretion, increasing plasma concentration, but without renal injury.16 Although in the perioperative period such confounders are less of an issue, they still may play a role, as well as the influence of fluid administration throughout the perioperative period.17 Changes in UO occur more rapidly than that of SCr, and where the urinary bladder is catheterised, such as in the immediate post-operative period, easy to measure. UO changes occur before alterations in SCr and a sustained reduction for >6 hours in UO is associated with renal injury.18 However, it must be remembered that oliguria does not always equate to renal injury.
Physiological adaptation to hypovolaemia alters sodium and water handling under the influence of antidiuretic hormone and the renin-angiotensin-aldosterone pathways reflected in a fall in UO which may also be observed with sympathetic stimulation as well as painful stimuli, situations which are often encountered in the post-operative period. Consequently, a reduction in urine output is not specific for AKI, whilst a normal or increased urine output does not necessarily indicate normal function as reduced GFR in the presence of tubular injury may occur without oliguria.19 Thus, assessment of the postoperative patient should consider that multiple causes may reduce the predictive power of urine output for AKI, including volume status, intrinsic antidiuretic hormone levels, obstruction and diuretics.20 Moreover, outside of the ICU or HDU measurements of urine output are often unreliable, especially where the urinary bladder is not catheterised.
Novel Biomarkers of AKI
Given the limitations of UO and SCR for the detection of AKI, new approaches have been considered, with the development of candidate molecules to enable:
- The rapid identification and diagnosis of AKI more promptly than conventional techniques.
- The identification of “sub-clinical” AKI: this describes a state where SCr increase or UO thresholds have not been met but renal damage has occurred.
- Biomarker guided intervention: where rapid identification of potential renal injury leads to the use of various therapeutic options in a shorter timeframe.
- Identification of individuals at highest risk of developing PO-AKI.
Several biomarkers correlating with renal injury have been identified and should accurately diagnose tubular injury, the most common form of AKI in the hospital setting and aid with risk stratification as well as the duration of injury.21 In general, biomarkers of AKI may be categorised as markers of glomerular filtration, markers associated with damage and stress.22 In the surgical setting, particularly in the non-emergent setting, the timing of potential renal insult is known which allows for further biomarkers to be studied and allows risk stratification. As well as biomarkers associated with acute injury, further candidate molecules have been examined with the aim of assessing the persistence of renal injury.
Types and Mechanisms of AKI Biomarkers
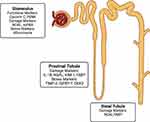
Biomarkers may be considered according to the mechanism behind their release including functional markers, those associated with injury, markers of renal stress and repair. Table 2 outlines some of the available biomarkers and, although not exhaustive, includes those that have been studied in surgical patients. Figure 2 outlines the markers discussed here in relation to their proposed sites of action/release. The mechanisms leading to the identification of such biomarkers accumulating in plasma and urine reflect different pathophysiological processes occurring during the development of AKI. This may occur through increased tubular epithelial synthesis in different parts of the nephron and/or as a consequence of impaired reabsorption in the proximal tubule. Moreover, secretion from activated immune cells migrating into the tubules may also contribute to the increase in biomarker concentrations. Some biomarkers may also be generated through increased synthesis in extra-renal tissue which not only increases circulating biomarker levels but will be further amplified through any reduction in GFR. Although these increases may reflect a physiological response to other insults such as sepsis even in the absence of AKI.
 |
Table 2 Significant Papers Investigating Biomarkers of AKI in Surgical Patients |
Prediction of Post-Operative AKI Using Biomarkers
Predicting AKI in vulnerable cohorts such as those undergoing surgery has considerable potential to improve outcomes. Much work has focussed on predictive modelling not only in surgical cases whereby routinely collected data is used to develop predictive algorithms.37,38 More recently, the application of machine learning techniques and advancements in artificial intelligence have resulted in an explosion of literature examining this approach. However, this approach is outside the scope of this review, and the reader is directed elsewhere.39–41
Urinary Albumin Quantification
Albuminuria occurs due to a failure of the permselective function of the glomerular basement membrane (GBM) and acts as a marker of dysfunction of both the GBM and proximal tubule, unable to retain and reabsorb this 66kDa anion.42 Urinary albumin is easy to measure, cheap and is an independent indicator of RRT risk and death following ICU admission.43,44 Like soluble urokinase plasminogen activator receptor (suPAR) and Dickkopf-3 (DKK3) described below, its preoperative levels predict the probability of AKI, whilst de novo albuminuria post injury increases the likelihood of progression to CKD.45 The TRIBE-AKI consortium demonstrated preoperative proteinuria provided a graded risk of death following cardiac surgery in a prospective study of 1199 patients(adjusted hazard ratio 2.85).46 Another study demonstrated patients with detectable preoperative urinary albumin were more likely to suffer post-operative AKI and require RRT.47 In combination with urinary NGAL, it had the greatest predictive power of all the markers assessed in the DAMAGE study, which followed 257 ICU admissions and predicted the risk of developing KDIGO stage 3 AKI (AUROC 0.87).48
Soluble Urokinase Plasminogen Activator Receptor (suPAR)
The ability to predict the risk of AKI before a renal insult could potentially transform the management of PO-AKI. Most current therapies are purely supportive in nature; however, the translation of promising experimental therapies to the bedside where administration before the insult occurs is needed and would be attainable if accurate prediction was possible.49
The ability of suPAR to predict AKI has been demonstrated both experimentally and in cardiac and coronary angiography cohorts.50 Plasma levels of suPAR were measured prior to coronary angiography as well as patients undergoing cardiac surgery and the risk of AKI at 7 days selected as the primary outcome. Among the 250 patients who underwent cardiac surgery, 27% developed postoperative AKI, 21% of which developed severe (stage 2 or 3) AKI with 12% of those requiring RRT. The risk of AKI increased steadily with increasing suPAR quartiles, the incidence of AKI being 40% in the highest suPAR quartile and 16% in the lowest quartile. A recent meta-analysis of 7 small trials examined the predictive role of suPAR demonstrating a combined sensitivity of suPAR in predicting AKI of 0.77 (95% CI 0.67–0.84); the specificity was 0.64 (95% CI 0.53–0.75); the odds ratio of diagnosis was 6 (95% CI 3–10); the pooled positive likelihood ratio was 2.2 (95% CI 1.6–2.9); the pooled negative likelihood ratio was 0.36 (95% CI 0.26–0.52); and the area under the summary receiver-operating characteristic (SROC) curve was 0.77 (95% CI 0.12~0.99).51 The authors concluded that suPAR is a valuable biomarker for the prediction of AKI and should be considered in the development of effective predictive tools for AKI.
Dickkopf-3 (DKK3)
DKK-3 is a stress induced, renal tubular epithelial-derived, secreted glycoprotein involved in the development of interstitial fibrosis and has primarily been assessed as a marker of fibrosis in chronic kidney disease (CKD).52,53 However, like suPAR, pre-insult levels may also be able to predict those who develop injury post-insult. The association between the ratio of preoperative urinary concentrations of DKK3 to creatinine (DKK3:creatinine) and postoperative AKI, defined according to the Kidney Disease Improving Global Outcomes criteria, and subsequent kidney function loss, as determined by estimated glomerular filtration rate, was assessed in patients undergoing cardiac surgery.36 Compared with clinical and other laboratory measurements, DKK3 improved AKI prediction (net reclassification improvement 0.32, 95% CI 0·23-0·42, p<0.0001) and high levels were independently associated with reduced kidney function at hospital discharge. Again, it was proposed that urinary DKK3 might aid in the identification of patients where preventative treatment strategies may be effective.
Functional Biomarkers
Cystatin C
Cystatin C is a proteinase inhibitor that is produced by all nucleated cells. It is freely filtered by the glomerulus and completely reabsorbed by tubules and is not present in healthy human urine. It is a marker of function similar to SCr but plasma concentrations are less affected by muscle mass, sex and race but are altered with thyroid disorders and corticosteroid administration.54 Therefore, blood levels are an accurate marker of filtration, whereas when present in the urine it is a marker of tubular injury through a failure of proximal tubule reabsorption.55 As such, it has unique features in that measurement may provide information regarding both filtration and tubular function. However, a wide range of performance in surgical patients has been documented with several meta-analyses quoting area under the receiver operator curve (AUC) of between 0.63 and 0.88 in predicting AKI.56,57
Proenkephalin a 119-159 (PENK)
Endogenous opioids, enkephalins, act primarily on delta opioid receptors and outside of the central nervous system the greatest concentration of these receptors is within the kidney. PENK is stable with a long in vivo half-life and is not affected by age or gender and is entirely filtered by the glomerulus with plasma levels correlating closely with measured GFR by inulin or iohexol clearance at steady state58 Given there is no renal tubular handling of PENK, levels do not reflect tubular function and are not associated with tubular biomarkers, adding to the specificity and highlights its potential role to provide assessment of filtration.59 As a marker of AKI in ICU admissions, PENK performs similarly to other novel biomarkers with an AUROC of approximately 0.8 depending on the patient population.60 Fewer studies have been performed in surgical cohorts although performance in patients undergoing cardiac and transplantation surgery has been reported.61,62
Damage Biomarkers
Neutrophil Gelatinase-Associated Lipocalin (NGAL)
NGAL is a 25kDa protein that exists in monomeric, homodimeric and heterodimeric forms and is produced by epithelial cells and has been implicated in a variety of processes at a cellular level: the immune response, tumorigenesis and cell survival.63,64 NGAL is found in trace amounts of the epithelia of many organs, including the kidney with serum and urinary concentrations increasing significantly in response to renal tubular injury.65 It functions as a siderophore, scavenging labile iron that is released by cell structures as a result of renal ischaemia. In doing so, it is thought to reduce apoptosis in and promote proliferation of renal tubular cells.66 Given the wide range of functions associated with NGAL, fears have often been raised as to a lack of specificity to renal injury for it to be used diagnostically.67 The TRIBE study assessed the predictive ability of urinary and plasma NGAL after cardiac surgery in approximately 1000 adults and 300 children. In children, NGAL levels peaked within 6 hours whereas serum creatinine continued to peak until 48 hours. Higher urinary NGAL concentrations were associated with a five-fold increase in the odds of developing severe AKI. Plasma NGAL was weakly associated with mild AKI and displayed no association with severe AKI with an AUC of 0.71 and 0.56, respectively.68,69 In adults, performance was similar with an AUC of 0.67 for urinary NGAL, and 0.7 for plasma NGAL. A 2015 meta-analysis calculated the AUC for urinary NGAL as 0.72 although the lack of specificity towards purely renal damage should be borne in mind.56
Kidney Injury Molecule-1 (KIM-1)
KIM-1 is a type 1 transmembrane glycoprotein, also known as T-cell immunoglobulin and mucin domain 1 and Hepatitis A virus cellular receptor 1. It has intracellular and cytoplasmic components forming part of the regulation of innate and adaptive immune responses and has been implicated in atopy.70 The expression of KIM-1 is upregulated on proximal renal tubular epithelial cells in response to ischaemic injury and in AKI urinary concentration of KIM-1 increases significantly. The extracellular domain is shed and is readily measurable in the urine. KIM-1 has a role in the remodelling of kidneys, signalling renal tubular cells that have undergone apoptosis, facilitating their subsequent phagocytosis.71,72 A meta-analysis of 11 studies (4 involving cardiac surgery) arrived at an AUC of 0.86 with sensitivity peaked at 2 to 6 hours and decreased by 12 hours, the biomarker performing better in paediatric patients. The AUC in a 2015 meta-analysis focussing on adult surgery was 0.72.56 Increased urinary KIM-1 has also been shown to be associated with a higher risk of mortality.73,74
Interleukin-18 (Il-18)
IL-18 is a pro-inflammatory cytokine. Like NGAL, it is produced in the renal tubular cells in ischaemic injury, promoting tubular necrosis and becoming detectable in urine.71 Several studies have shown that IL-18 levels are raised 4–6 hours following cardiac surgery and that it may also predict the severity of AKI.68,69,75 A meta-analysis of 11 papers demonstrated an AUC of 0.77. Of those, 4 assessed cardiac surgery and 1 liver transplantation.76 Another meta-analysis of 6 studies that focussed on cardiac surgery calculated the AUC at 0.66.56 Raised levels are also known to correlate with cardiac and lung injury, and urinary tract infection,77 probably reflecting systemic inflammation.
Fatty Acid Binding Protein (FABP)
L-FABP is a fatty acid binding protein highly expressed in the liver and present in other organs including the kidneys. It facilitates long chain fatty acid transport and reduces oxidative stress. L-FABP has a reno-protective role and is not detectable in healthy human urine. Reabsorption of L-FABP is reduced in the proximal tubule after ischaemia-reperfusion injury.69,71
Urinary L-FABP is strongly correlated with renal ischaemic time in transplant procedures. A meta-analysis of 15 studies, 6 of which assessed cardiac surgery and 1 liver transplantation concluded that L-FABP is a useful tool for the diagnosis of AKI in the early postoperative period and that it may also predict mortality.56 FABP1 has also shown promise in combination with urinary IL-18 in children undergoing cardiac surgery with an AUROC of 0.78 achieved.78 In the largest study to-date, poor predictive ability was demonstrated with an AUROC of 0.61.78 Moreover, FABP1 was not found to be predictive of AKI in general ICU admissions but has demonstrated excellent discriminatory power in predicting ICU RRT initiation in a German multicentre study of 120 patients.79,80
Renal Stress Biomarkers
IGFBP-7 (Insulin Growth Factor Binding Protein-7) and TIMP-2 (Tissue Inhibitor of Metalloproteinases −2)
IGFBP-7 belongs to the insulin growth factor binding protein superfamily, peptides that have autocrine, endocrine and paracrine functions involved in cell proliferation, differentiation and growth. IGFBP-7 is expressed by a wide variety of tissues including the renal tubular epithelium. The exact function of IGFBP-7 is unclear, as when bound to IGFBP-1. It promotes growth, but in isolation, it can inhibit cell proliferation.81,82 Matrix metalloproteinases degrade the extracellular matrix and are involved in healing, angiogenesis and metastasis. These enzymes are regulated by tissue inhibitors of metalloproteinases including TIMP-2.83 IGFBP-7 and TIMP-2 are implicated in G1 cell cycle arrest as a response to sepsis or ischaemia induced AKI.84 Initial studies on the ability of TIMP-2/IGFBP-7 to predict AKI in a range of critically ill patients were published in 2013.29 Subsequent analysis on high risk surgical patients including major trauma and cardiac surgery demonstrated an AUC of 0.84 for prediction of stage 2 and 3 AKI.30 A further study assessed the test in 107 trauma, transplant, hepatobiliary and vascular patients, demonstrating an AUC of 0.85 and also found that higher levels of the biomarkers were correlated with the need for renal replacement therapy and mortality.31 A meta-analysis of 10 studies in 2016 gave an overall AUC at 0.88.85 These encouraging data has led to the use of these biomarkers being advocated in enhanced recovery programmes for cardiac surgery.86
Biomarkers of AKI Persistence
Chemokine CCl14
More recently efforts have also focussed on biomarkers which may predict recovery from AKI. AKI recovery may occur as distinct phenotypes, early recovery, delayed recovery, relapsing and non-recovery; with the associated 1-year mortality ranging from <10% for early recovery but >60% for non-recovery.87 The availability of biomarkers to predict either recovery or persistence would enable clinicians to time therapy decisions and provide greater input to higher risk phenotypes such as those with persistent AKI and acute kidney disease (AKD), who are at greatest risk of progression.88 The RUBY study published in 2020 investigated multiple potential biomarkers for their performance at predicting persistent AKI. ICU patients with stage 2 or 3 AKI with the primary endpoint being AKI persisting beyond 72 hours were studied with candidate molecules with known involvement in apoptosis, endothelial injury or other inflammatory pathways measured in plasma or urine. Chemokine CCL14 demonstrated the greatest prediction for persistent AKI with an AUROC of 0.83.89 A further study demonstrated an AUROC of 0.81 for predicting persistent AKI in a secondary analysis of the SAPPHIRE dataset, although severe AKI only occurred in 28 patients, duration of persistence was proportional to CCL14 levels.90 A recently published prospective study measured CCL14 levels in 100 patients pre- and post-cardiac surgery demonstrating an AUROC of 0.92 for predicting persistence.91
Towards a New, More Precise Definition of AKI
Nowadays, the KDIGO definition is used to diagnose and stage AKI. This definition is based on changes of the serum creatinine and urine output. However, serum creatinine and urine output have a low sensitivity and specificity, respectively. Recent studies demonstrated that a kidney damage without a loss of kidney function is associated with a worse outcome. In a large study, the authors demonstrate that patients who have high NGAL levels (damage biomarker), but a normal serum creatinine, have an increased dialysis dependence as well as mortality rate. Based on this landmark study, the term “subclinical AKI” was introduced. However, not all AKI biomarkers can be used to diagnose “subclinical AKI”. The Acute Disease and Quality Initiative (ADQI) group recently suggested a new definition of AKI, combining functional markers with biomarkers to detect and stage AKI. Future trials have to evaluate the new definition of AKI.
Potential Role of Biomarkers in Guiding the Precision Medicine Approach
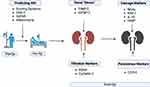
AKI following major surgery is a common complication and provides potentially large study cohorts when assessing the performance of new biomarkers. Furthermore, the kinetics of biomarkers can be accurately assessed, as particularly in elective surgery, the timing of the insult to the kidney is known. Patients often undergo detailed pre-operative assessment, enabling clinicians to not only determine baseline renal function and comorbidities but provide the opportunity to measure novel markers. Post-operatively, patients are monitored closely on HDU or ICU. Units, facilitating comparison of biomarkers with other clinical and biochemical parameters. To date, research has mainly focussed on patients undergoing cardiac surgery, and to a lesser extent, transplant and vascular surgery given the higher rates of AKI reported in these groups. The paediatric cardiac surgery population particularly lends itself to the discovery of new markers as patients are generally devoid of comorbidities. The ultimate goal underpinning the introduction of biomarkers is to facilitate early recognition of patients who have suffered a renal insult enabling early intervention. Or, as in the case of DKK-3 and suPAR, identify patients at risk prior to procedure and personalise treatment plans at that juncture.
One of the criticisms of biomarker use is that treatment of AKI is predominantly supportive and that little can be done to affect the outcome resulting in the “myth of inevitability” of AKI.92 However, this was shattered by the Prev-AKI study where biomarker positive patients were randomised to a kidney protective “bundle” of care in patients predominantly undergoing cardiac surgery where significantly lower rates of AKI were observed in the intervention group.93 These results were also seen in a multicentre study where rates of moderate and severe AKI were reduced on implementation of a KDIGO-derived treatment bundle.94 Similarly, in major abdominal, transplant and vascular surgery where patients were randomised to a biomarker guided intervention to prevent AKI, lower rates of AKI were observed in the intervention group.95 Therefore, integration of biomarkers into care plans may allow for precision treatment based on the risk of AKI (Figure 3). Currently, intervention is mainly supportive although analysis of the Prev-AKI data suggested that avoidance of even short periods of hypotension coupled with improvement of the cardiac index remain key to prevention.96 Indeed, the benefit of care bundles in the prevention of AKI has been further supported by meta-analysis.97
Conclusions
The potential for biomarkers to predict AKI in patients undergoing surgery is well established. Over the last decade, a variety of candidates have been described which will allow the earlier identification of AKI and thereby allow timely intervention. These include biomarkers to enhance risk prediction including the presence of albuminuria, suPAR and DKK3 where preoperative measurements may aid in detecting patients at higher risk of PO-AKI, particularly in those undergoing cardiac surgery. Many studies have confirmed the role of biomarkers in identifying PO-AKI early and the use of biomarkers associated with renal stress (TIMP-2 and IGFBP-7) have been studied extensively in general surgical patients as well as those undergoing cardiac surgery. In turn, this has already been translated into improved outcomes in patients not only undergoing cardiac surgery but also major abdominal surgery. Although hurdles exist regarding widespread adoption, these are founded predominantly on cost issues and a lack of familiarity. The latter will be addressed by more data and acceptance by the wider community. The former will also, we are sure, become evident with time.
Disclosure
Dr Alexander Zarbock reports consulting and/or lecture fees from Baxter, BioMerieux, Guard Therapeutics, Bayer, Fresenius, Novartis, AM Pharma, Paion, during the conduct of the study. Professor Lui Forni reports grants note directly related to this work from Baxter, grants for biomarkers integrated as part of a multicentre research study from Biomerieux, personal fees for lectures on biomarkers from Ortho clinical diagnostics, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193–207. doi:10.1038/nrneph.2013.282
2. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute dialysis quality initiative w. acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the acute dialysis quality initiative (ADQI) group. Crit Care. 2004;8(4):R204–12. doi:10.1186/cc2872
3. Kellum JA, Lameire N; Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care. 2013;17(1):204. doi:10.1186/cc11454
4. Zarbock A, Weiss R, Albert F, et al. Epidemiology of surgery associated acute kidney injury (EPIS-AKI): a prospective international observational multi-center clinical study. Intensive Care Med. 2023;2023. doi:10.1007/s00134-023-07169-7
5. Murugan R, Kellum JA. Acute kidney injury: what’s the prognosis? Nat Rev Nephrol. 2011;7(4):209–217. doi:10.1038/nrneph.2011.13
6. Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. 2016;67(6):872–880. doi:10.1053/j.ajkd.2015.07.022
7. Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851–858. doi:10.1097/SLA.0b013e3181a40a0b
8. Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med. 2018;378(24):2263–2274. doi:10.1056/NEJMoa1801601
9. Brunelli SM, Waikar SS, Bateman BT, et al. Preoperative statin use and postoperative acute kidney injury. Am J Med. 2012;125(12):1195–1204.e3. doi:10.1016/j.amjmed.2012.06.021
10. Kim JY, Joung KW, Kim KM, et al. Relationship between a perioperative intravenous fluid administration strategy and acute kidney injury following off-pump coronary artery bypass surgery: an observational study. Crit Care. 2015;19:350. doi:10.1186/s13054-015-1065-8
11. Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26(9):2231–2238. doi:10.1681/asn.2014070724
12. Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756–766. doi:10.1016/S0140-6736(11)61454-2
13. Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81(5):442–448. doi:10.1038/ki.2011.379
14. Johnson RJ, Feehally J, Floege J. Comprehensive Clinical Nephrology E-Book. Elsevier Health Sciences; 2014.
15. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20(3):672–679. doi:10.1681/ASN.2008070669
16. Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23(1):13–21. doi:10.1681/ASN.2010111124
17. Prowle JR, Leitch A, Kirwan CJ, Forni LG. Positive fluid balance and AKI diagnosis: assessing the extent and duration of ‘creatinine dilution’. Intensive Care Med. 2015;41(1):160–161. doi:10.1007/s00134-014-3538-7
18. Macedo E, Malhotra R, Bouchard J, Wynn SK, Mehta RL. Oliguria is an early predictor of higher mortality in critically ill patients. Kidney Int. 2011;80(7):760–767. doi:10.1038/ki.2011.150
19. Legrand M, Payen D. Understanding urine output in critically ill patients. Ann Intensive Care. 2011;1(1):13. doi:10.1186/2110-5820-1-13
20. Ostermann M, Philips BJ, Forni LG. Clinical review: biomarkers of acute kidney injury: where are we now? Crit Care. 2012;16(5):233. doi:10.1186/cc11380
21. Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: perspectives on translation. Clin J Am Soc Nephrol. 2008;3(2):481–490. doi:10.2215/CJN.03520807
22. Malhotra R, Siew ED. Biomarkers for the early detection and prognosis of acute kidney injury. Clin J Am Soc Nephrol. 2017;12(1):149–173. doi:10.2215/CJN.01300216
23. Nisula S, Yang R, Kaukonen KM, et al. The urine protein NGAL predicts renal replacement therapy, but not acute kidney injury or 90-day mortality in critically ill adult patients. Anesth Analg. 2014;119(1):95–102. doi:10.1213/ANE.0000000000000243
24. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi:10.1016/S0140-6736(05)74811-X
25. Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823. doi:10.1681/ASN.2008070673
26. Bagshaw SM, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Med. 2010;36:452–461. doi:10.1007/s00134-009-1724-9
27. Koyner JL, Garg AX, Coca SG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905. doi:10.1681/ASN.2011090907
28. Au V, Feit J, Barasch J, Sladen RN, Wagener G. Urinary neutrophil gelatinase–associated lipocalin (NGAL) distinguishes sustained from transient acute kidney injury after general surgery. Kidney Int Rep. 2016;1(1):3–9. doi:10.1016/j.ekir.2016.04.003
29. Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. doi:10.1186/cc12503
30. Gunnerson KJ, Shaw AD, Chawla LS, et al. TIMP2*IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg. 2016;80(2):243–249. doi:10.1097/TA.0000000000000912
31. Gocze I, Koch M, Renner P, et al. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One. 2015;10(3):e0120863. doi:10.1371/journal.pone.0120863
32. Meersch M, Schmidt C, Van Aken H, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury after pediatric cardiac surgery. PLoS One. 2014;9(10):e110865. doi:10.1371/journal.pone.0110865
33. Koyner JL, Vaidya VS, Bennett MR, et al. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5(12):2154. doi:10.2215/CJN.00740110
34. Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246–255. doi:10.1016/j.jacc.2011.10.854
35. WK H, Wagener G, Zhu Y, Wang S, HT L. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4(5):873. doi:10.2215/CJN.04810908
36. Schunk SJ, Zarbock A, Meersch M, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394(10197):488–496. doi:10.1016/S0140-6736(19)30769-X
37. Hodgson LE, Dimitrov BD, Roderick PJ, Venn R, Forni LG. Predicting AKI in emergency admissions: an external validation study of the acute kidney injury prediction score (APS). BMJ Open. 2017;7(3):e013511. doi:10.1136/bmjopen-2016-013511
38. Hodgson LE, Sarnowski A, Roderick PJ, Dimitrov BD, Venn RM, Forni LG. Systematic review of prognostic prediction models for acute kidney injury (AKI) in general hospital populations. BMJ Open. 2017;7(9):e016591. doi:10.1136/bmjopen-2017-016591
39. Li T, Yang Y, Huang J, et al. Machine learning to predict post-operative acute kidney injury stage 3 after heart transplantation. BMC Cardiovas Dis. 2022;22(1):288. doi:10.1186/s12872-022-02721-7
40. Song X, Liu X, Liu F, Wang C. Comparison of machine learning and logistic regression models in predicting acute kidney injury: a systematic review and meta-analysis. Int J Med Inform. 2021;151:104484. doi:10.1016/j.ijmedinf.2021.104484
41. Bellini V, Valente M, Bertorelli G, et al. Machine learning in perioperative medicine: a systematic review. J Anesth Analg Critl Care. 2022;2(1):2. doi:10.1186/s44158-022-00033-y
42. Benzing T, Salant D. Insights into glomerular filtration and albuminuria. N Engl J Med. 2021;384(15):1437–1446. doi:10.1056/NEJMra1808786
43. MacKinnon KL, Molnar Z, Lowe D, Watson ID, Shearer E. Use of microalbuminuria as a predictor of outcome in critically ill patients. Br J Anaesth. 2000;84(2):239–241. doi:10.1093/oxfordjournals.bja.a013409
44. Ralib AM, Pickering JW, Shaw GM, Than MP, George PM, Endre ZH. The clinical utility window for acute kidney injury biomarkers in the critically ill. Crit Care. 2014;18(6):601. doi:10.1186/s13054-014-0601-2
45. Levey AS, Grams ME, Inker LA. Uses of GFR and albuminuria level in acute and chronic kidney disease. N Engl J Med. 2022;386(22):2120–2128. doi:10.1056/NEJMra2201153
46. Coca SG, Garg AX, Thiessen-Philbrook H, et al. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014;25(5):1063–1071. doi:10.1681/ASN.2013070742
47. Coca SG, Jammalamadaka D, Sint K, et al. Preoperative proteinuria predicts acute kidney injury in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143(2):495–502. doi:10.1016/j.jtcvs.2011.09.023
48. McMahon BA, Galligan M, Redahan L, et al. Biomarker predictors of adverse acute kidney injury outcomes in critically ill patients: the Dublin Acute Biomarker Group Evaluation Study. Am J Nephrol. 2019;50(1):19–28. doi:10.1159/000500231
49. Faubel S. SuPAR: a potential predictive biomarker for acute kidney injury. Nat Rev Nephrol. 2020;16(7):375–376. doi:10.1038/s41581-020-0276-7
50. Hayek SS, Leaf DE, Samman Tahhan A, et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382(5):416–426. doi:10.1056/NEJMoa1911481
51. Huang Y, Huang S, Zhuo X, Lin M. Predictive value of suPAR in AKI: a systematic review and meta-analysis. Clin Exp Nephrol. 2023;27(1):1–11. doi:10.1007/s10157-022-02300-2
52. Federico G, Meister M, Mathow D, et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. JCI Insight. 2016;1(1):e84916. doi:10.1172/jci.insight.84916
53. Zewinger S, Rauen T, Rudnicki M, et al. Dickkopf-3 (DKK3) in urine identifies patients with short-term risk of eGFR loss. J Am Soc Nephrol. 2018;29(11):2722–2733. doi:10.1681/ASN.2018040405
54. Cavalcante C, Cavalcante MB, Castello Branco KMP, et al. Biomarkers of acute kidney injury in pediatric cardiac surgery. Pediatr Nephrol. 2022;37(1):61–78. doi:10.1007/s00467-021-05094-9
55. Baxmann AC, Ahmed MS, Marques NC, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–354. doi:10.2215/CJN.02870707
56. Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66(6):993–1005. doi:10.1053/j.ajkd.2015.06.018
57. Yong Z, Pei X, Zhu B, Yuan H, Zhao W. Predictive value of serum cystatin C for acute kidney injury in adults: a meta-analysis of prospective cohort trials. Sci Rep. 2017;7:41012. doi:10.1038/srep41012
58. Donato LJ, Meeusen JW, Lieske JC, Bergmann D, Sparwasser A, Jaffe AS. Analytical performance of an immunoassay to measure proenkephalin. Clin Biochem. 2018;58:72–77. doi:10.1016/j.clinbiochem.2018.05.010
59. Matsue Y, Ter Maaten JM, Struck J, et al. Clinical correlates and prognostic value of proenkephalin in acute and chronic heart failure. J Card Fail. 2017;23(3):231–239. doi:10.1016/j.cardfail.2016.09.007
60. Marino R, Struck J, Hartmann O, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol. 2015;28(6):717–724. doi:10.1007/s40620-014-0163-z
61. Khorashadi M, Beunders R, Pickkers P, Legrand M. Proenkephalin: a new biomarker for glomerular filtration rate and acute kidney injury. Nephron. 2020;144(12):655–661. doi:10.1159/000509352
62. Lima C, Gorab DL, Fernandes CR, Macedo E. Role of proenkephalin in the diagnosis of severe and subclinical acute kidney injury during the perioperative period of liver transplantation. Pract Laborat Med. 2022;31:e00278. doi:10.1016/j.plabm.2022.e00278
63. Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–1056. doi:10.1016/s1097-2765(02)00710-4
64. Devireddy LR, Teodoro JG, Richard FA, Green MR. Induction of apoptosis by a secreted lipocalin that is transcriptionally regulated by IL-3 deprivation. Science. 2001;293:829–34. doi:10.1126/science.1061075
65. Schmidt-Ott KM, Mori K, Li JY, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413. doi:10.1681/ASN.2006080882
66. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A; Group NM-aI. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. doi:10.1053/j.ajkd.2009.07.020
67. Martensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014;37(4):304–310. doi:10.1159/000364937
68. Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J Am Soc Nephrol. 2011;22(9):1737–1747. doi:10.1681/ASN.2010111163
69. Parikh CR, Moledina DG, Coca SG, Thiessen-Philbrook HR, Garg AX. Application of new acute kidney injury biomarkers in human randomized controlled trials. Kidney Int. 2016;89(6):1372–1379. doi:10.1016/j.kint.2016.02.027
70. Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–189. doi:10.1111/j.0105-2896.2010.00903.x
71. Gavric A, Kalisnik JM. Novel biomarkers for early diagnosis of acute kidney injury after cardiac surgery in adults. Kardiochir Torakochirurgia Pol. 2016;13(1):31–38. doi:10.5114/kitp.2016.58962
72. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290(2):F517–29. doi:10.1152/ajprenal.00291.2005
73. Ghatanatti R, Teli A, Tirkey SS, Bhattacharya S, Sengupta G, Mondal A. Role of renal biomarkers as predictors of acute kidney injury in cardiac surgery. Asian Cardiovasc Thorac Ann. 2014;22(2):234–241. doi:10.1177/0218492313502028
74. Koyner JL, Davison DL, Brasha-Mitchell E, et al. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol. 2015;26(8):2023–2031. doi:10.1681/asn.2014060535
75. Brazzelli M, Aucott L, Aceves-Martins M, et al. Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2022;26(7):1–286. doi:10.3310/UGEZ4120
76. Lin X, Yuan J, Zhao Y, Zha Y. Urine interleukin-18 in prediction of acute kidney injury: a systemic review and meta-analysis. J Nephrol. 2015;28(1):7–16. doi:10.1007/s40620-014-0113-9
77. Chang CH, Fan PC, Lin CY, et al. Elevation of interleukin-18 correlates with cardiovascular, cerebrovascular, and peripheral vascular events: a cohort study of hemodialysis patients. Medicine. 2015;94(42):e1836. doi:10.1097/md.0000000000001836
78. Parikh CR, Thiessen-Philbrook H, Garg AX, et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin J Am Soc Nephrol. 2013;8(7):1079–1088. doi:10.2215/cjn.10971012
79. Peerapornratana S, Priyanka P, Wang S, et al. Sepsis-associated acute kidney disease. Kidney Int Rep. 2020;5(6):839–850. doi:10.1016/j.ekir.2020.03.005
80. Dihazi H, Koziolek MJ, Datta RR, et al. FABP1 and FABP3 have high predictive values for renal replacement therapy in patients with acute kidney injury. Blood Purif. 2016;42(3):202–213. doi:10.1159/000447115
81. Allard JB, Duan C. IGF-binding proteins: why do they exist and why are there so many? Review. Front Endocrinol. 2018;9. doi:10.3389/fendo.2018.00117
82. Degeorges A, Wang F, Frierson HF, Seth A, Sikes RA. Distribution of IGFBP-rP1 in normal human tissues. J Histochem Cytochem. 2000;48(6):747–754. doi:10.1177/002215540004800603
83. Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi:10.1016/j.cardiores.2005.12.002
84. Wetz AJ, Richardt EM, Wand S, et al. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015;19(1):3. doi:10.1186/s13054-014-0717-4
85. Su Y, Gong Z, Wu Y, Tian Y, Liao X. Diagnostic value of urine tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 for acute kidney injury: a meta-analysis. PLoS One. 2017;12(1):e0170214. doi:10.1371/journal.pone.0170214
86. Engelman DT, Ben Ali W, Williams JB, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019;154(8):755–766. doi:10.1001/jamasurg.2019.1153
87. Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med. 2017;195(6):784–791. doi:10.1164/rccm.201604-0799OC
88. Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the acute disease quality initiative (ADQI) 16 workgroup. Nat Rev Nephrol. 2017;13(4):241–257. doi:10.1038/nrneph.2017.2
89. Hoste E, Bihorac A, Al-Khafaji A, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. 2020;46(5):943–953. doi:10.1007/s00134-019-05919-0
90. Bagshaw SM, Al-Khafaji A, Artigas A, et al. External validation of urinary C-C motif chemokine ligand 14 (CCL14) for prediction of persistent acute kidney injury. Crit Care. 2021;25(1):185. doi:10.1186/s13054-021-03618-1
91. Massoth C, Kullmar M, Enders D, et al. Comparison of C-C motif chemokine ligand 14 with other biomarkers for adverse kidney events after cardiac surgery. J Thorac Cardiovasc Surg. 2023;165(1):199–207 e2. doi:10.1016/j.jtcvs.2021.03.016
92. Kellum JA. Acute kidney injury: AKI: the myth of inevitability is finally shattered. Nat Rev Nephrol. 2017;13(3):140–141. doi:10.1038/nrneph.2017.11
93. Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43(11):1551–1561. doi:10.1007/s00134-016-4670-3
94. Zarbock A, Kullmar M, Ostermann M, et al. Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg. 2021;133(2):292–302. doi:10.1213/ANE.0000000000005458
95. Gocze I, Jauch D, Gotz M, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK study. Ann Surg. 2018;267(6):1013–1020. doi:10.1097/SLA.0000000000002485
96. von Groote TC, Ostermann M, Forni LG, Meersch-Dini M, Zarbock A. The AKI care bundle: all bundle components are created equal-are they? Intensive Care Med. 2022;48(2):242–245. doi:10.1007/s00134-021-06601-0
97. Schaubroeck HAI, Vargas D, Vandenberghe W, Hoste EAJ. Impact of AKI care bundles on kidney and patient outcomes in hospitalized patients: a systematic review and meta-analysis. BMC Nephrol. 2021;22(1):335. doi:10.1186/s12882-021-02534-4
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.