Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Protection of Proanthocyanidins Against HSP Serum-Induced Inflammation and Oxidative Stress on Human Umbilical Vein Endothelial Cells
Authors Liu L, Wang M , Guo M, Xian L , Xu J , Xian D, Zhong J
Received 14 October 2023
Accepted for publication 16 March 2024
Published 23 March 2024 Volume 2024:17 Pages 731—743
DOI https://doi.org/10.2147/CCID.S440399
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Rungsima Wanitphakdeedecha
Lumei Liu,1,* Meng Wang,1,* Menglu Guo,2,* Li Xian,3,* Jixiang Xu,1 Dehai Xian,4 Jianqiao Zhong1
1Department of Dermatology, the Affiliated Hospital, Southwest Medical University, Luzhou, 646000, People’s Republic of China; 2Department of Dermatology, the People’s Hospital of Leshan, Southwest Medical University, Leshan, 614003, People’s Republic of China; 3Department of Emergency, the Affiliated Hospital of Southwest Medical University, Luzhou, 646000, People’s Republic of China; 4Department of Neurobiology, Southwest Medical University, Luzhou, 646000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianqiao Zhong, Department of Dermatology, the Affiliated Hospital, Southwest Medical University, No. 25 Tai Ping Jie, Luzhou, Sichuan, 646000, People’s Republic of China, Tel +86-15082088598, Fax +86-830-3198173, Email [email protected] Dehai Xian, Department of Neurobiology, Southwest Medical University, Luzhou, 646000, People’s Republic of China, Tel +86-18008203056, Email [email protected]
Background: Immune-mediated inflammation and oxidative stress play pivotal roles in Henoch-Schonlein purpura (HSP), primarily through the TLR4/MyD88/NF-κB pathway. Proanthocyanidins (PCs) exert anti-inflammatory and antioxidant effects by regulating some signals like TLR4/MyD88/NF-κB. Previous research uncovered that PCs could alleviate purpura-like lesions and pathological changes on rats likely through attenuating inflammation and OS damage. The mechanism of PCs on HSP deserves further investigation.
Objective: To clarify the potential mechanism of PCs to HUVECs induced by the serum of HSP patients.
Methods: HUVECs were randomly divided into blank, control, model, and low-, medium-, and high-concentration PCs group. Then, 25% HSP serum was assigned to the latter four groups, while 25% serum from healthy subjects to control group and serum-free culture medium to blank one. The last three groups separately received different concentrations of PCs. In addition, TAK-242, a TLR4 inhibitor, was applied to investigate the effect of TLR4-related signals in PCs against HSP serum-induced damage. Finally, inflammatory and OS-related parameters were detected by using cytological/molecular-biological techniques.
Results: Treated with HSP serum later, the levels of immuno-inflammatory and oxidative indicators obviously went up (P < 0.05), and those of antioxidants remarkably went down (P < 0.05). PCs, however, reversed above phenomena (P < 0.05). Moreover, TLR4, MyD88 and NF-κB proteins/genes highly expressed in the model group; but significantly fell off in the presence of PCs (P < 0.05). Amazingly, all of above indicators showed no significant difference among the groups of different PCs concentrations (P > 0.05). These alterations likewise occurred after TAK-242 pretreatment with or without PCs, ie a notable drop of TLR4, MyD88 and NF-κB appeared in TAK-242 presence, few differences existing when compared to the PCs groups.
Conclusion: PCs effectively protect HUVECs from inflammatory and OS damage provoked by HSP serum via blocking TLR4/MyD88/NF-κB signals.
Keywords: proanthocyanidins, Henoch-schönlein purpura, TLR4/MyD88/NF-κB, inflammation, oxidative stress
Introduction
Henoch-Schönlein purpura (HSP) is a common, immune-mediated, systemic small vessel vasculitis, frequently occurring in childhood with a high recurrence rate. In histopathology, it typically manifests as leukocytoclastic vasculitis and deposition of capillary immunoglobulin (Ig)A immune complexes.1,2 HSP causes various symptoms in different organs, especially preferring the skin, joints, gastrointestinal tract and kidneys, which tends to prejudice the pediatric well-being.3,4 At present, many therapeutic vehicles are available for HSP, such as vitamin C, calcium, corticosteroids, immunosuppressant (eg mycophenolate mofetil or cyclosporine), and so on; but most of them stand still due to their transient efficacy or undesirable reactions or recurrent troubles or high cost.5,6 So far, few treatments seem satisfactory for this condition. Consequently, a safe, effective and affordable approach is urgently needed.
Although the pathogenesis of HSP have not been fully elucidated, it is currently considered that immune inflammation and oxidative stress (OS) get deeply involved in it, especially the activation of toll-like receptor 4 (TLR4)/myeloid differentiation factor 88 (MyD88)/nuclear transcription factor-kappa B (NF-κB) signaling way.7–10 TLR4 is a key transmembrane protein mediating the inflammatory and OS reaction. It binds to the cytoplasmic adapter molecule MyD88 to activate the NF-κB signal,11–13 which triggers the release of pro-inflammatory factors and chemokines, and the appearance of abnormal oxidative/antioxidant indicators, eg, increased reactive oxygen species (ROS), malondialdehyde (MDA) and nitric oxide (NO), as well as decreased catalase (CAT), glutathione (GSH) and superoxide dismutase (SOD).14–18 Above alterations initiate the inflammatory response and OS, further facilitating more inflammatory cytokines release and amplifying the inflammatory response cascade, thereby creating a positive feedback loop that ultimately causes the damage to endothelial cell and the enhancement of vascular permeability.14,15,19,20 Thus, targeted suppression of TLR4/MyD88/NF-κB pathway is vital in management of HSP through alleviating inflammation and OS.
Proanthocyanidins (PCs), one of the flavanol polyphenol compounds extracted from grape seeds, possess a variety of pharmacological effects, eg, anti-inflammation, antioxidation, immunomodulation, etc.21–25 It is confirmed that PCs could inhibit the production of inflammatory mediators and regulate redox equilibrium.26,27 They powerfully take effect in the inflammatory or oxidative disorders through blocking TLR4/MyD88/NF-κB signaling pathway to mitigate the expression of interleukin (IL)-4, tumor necrosis factor-alpha (TNF-α), and other inflammatory factors as well as MDA and ROS, and to elevate the activity of CAT, SOD and GSH.11,28,29 Given that the functions of PCs and the pathogenesis of HSP, PCs would be a promising treatment for HSP.30 However, reports about PCs treating HSP scarcely exist. In the previous study, we initially revealed that PCs obviously relieved the clinical and pathological lesions of HSP-like rats, decreased the levels of inflammatory factors, ROS and MDA, and increased those of CAT, SOD and GSH, possibly involving the attenuation of inflammatory reaction and oxidative damage (as a separate paper to publish). So further to illumine the specific mechanism of PCs against HSP, the present study was carried out in an in-vitro cell model.
Materials and Methods
Patients
Following the informed consent of subjects or their parents and the approval of Ethics Committee of Affiliated Hospital of Southwest Medical University, serum samples were collected from 20 HSP patients aged 5–30 years, who were admitted to Dermatology Department of Affiliated Hospital of Southwest Medical University from January to December in 2020; meanwhile, serum from 20 age-and sex-matched healthy individuals were gathered at the same period. After standing for 2h, serum was separated at 4000rpm/10min (4°C centrifuge), and aspirated in an ultra-clean bench, dispensed in sterile freezing tubes and frozen at −80°C.
Cell Culture
HUVECs (Manassas, VA, USA) were cultured in a fresh complete medium, consisting of 89% Dulbecco’s modified Eagle’s medium (Gibco, USA), 10% fetal bovine serum and 1% penicillin-streptomycin, at 37°C, 5% CO2 in a humidified atmosphere. These cells were routinely subcultured to 2–3 passages for the next experiments.
Medicine and Reagents
PC powder (>95% purity) was obtained from Beijing Solabo Science and Technology Co., Ltd. (Beijing, China) and dissolved in fresh complete medium before application. TAK-242 (TLR4 inhibitor) solution (10 mM*1 mL in DMSO) was purchased from Shanghai Lanmu Chemical Co., Ltd. (Shanghai, China). Assay kits for NO, MDA, CAT, GSH, and SOD came from Nanjing Jiancheng Technology Co., Ltd. (Nanjing, China), whereas those for IgA, IL-4, IL-8, IL-17, TNF-α, iNOS and ROS from Andy Gene Biotechnology Co., Ltd. (Beijing, China). Primary anti-bodies against TLR4, MyD88, NF-κB and β-actin were all from Abcam, UK. In addition, IgG secondary antibody was got from beinglay, China.
Determination of Suitable Safe Concentration of PCs
To investigate the safe concentration of PCs, the Cell Counting Kit-8 (CCK-8) assay (Solarbio, Beijing) was employed. Namely, HUVECs were inoculated in 96-well plates at a density of 4 × 103 cells/well, and underwent a 24-hour starvation culture; after removal of the original media, they were respectively treated with eight concentrations of PCs (2, 4, 8, 16, 32, 64, 128, and 256 μg/mL); twenty-four hours later, the CCK-8 kit was applied and performed. At last, the 50% inhibitory concentration (IC50) values of PCs on HUVECs were calculated by using GraphPad Prism 9, thereby the suitable concentration being determined for the subsequent experiments.
Construction and Identification of the Cell Models
HUVECs cells were inoculated in 6-well plates at a density of 1×106 /well and then starved for 24 hours. Afterwards, they were supplemented with medium containing 25% HSP serum instead of the original medium for another 12-hour culture to construct the in-vitro cell model. Finally, above cells and supernatants were harvested for the detection of IgA, IL-4, IL-8, IL-17, TNF-α, ROS, MDA, TLR4, MyD88, NF-κB, CAT, SOD and GSH.
Intervention of PCs to the HSP Serum-Induced Cell Models
HUVECs, at the density of 1×106 cells/well, were seeded in 6-well plates and randomly divided into six groups: blank group (serum-free culture medium), control group (25% healthy serum), model group (25% HSP serum), low-concentration PCs group (25% HSP serum + 30μg/mL PCs), medium-concentration PCs group (25% HSP serum + 40μg/mL PCs) and high-concentration PCs group (25% HSP serum + 50μg/mL PCs). The original medium was aspirated following a 24-hour starving culture. Subsequently, the latter three groups respectively received 2mL/well medium containing 25% HSP serum and different concentrations of PCs (30 μg/mL, 40 μg/mL and 50 μg/mL), while the model group was administrated with an equal-volume medium containing 25% HSP serum, the control group with one containing 25% healthy serum, and the blank group only with equal-volume serum-free medium. After that, all above cells continued to be incubate for 12 hours. Finally, the cells and supernatants were collected for further detection.
Application of TLR4 Inhibitor (TAK-242) to the HSP Serum-Induced Cells
To elucidate the specific mechanism of PCs on HSP, HSP serum-induced cells experienced the pretreatment of TLR4 inhibitor (TAK-242) in a separate experiment. HUVECs induced by HSP serum, in this additional experiment, were divided into four groups: HSP group, HSP+PCs group, HSP+TAK-242 group and HSP+PCs+TAK-242 group. Briefly, cells in the latter two groups were pretreated with 2mL medium containing 20μM TAK-242 plus 25% HSP serum for 4 hours in 6-well plates, while those in the former two with equal-volume medium containing DMSO and 25% HSP serum. Then, 30μg/mL PCs was added to HSP+PCs and HSP+TAK-242+PCs groups. All above groups were further cultured for 12 hours. Lastly, the cells and supernatants were collected for indicators determination.
CCK-8 Assay for Proliferative Activity of Cells
The cellular proliferation was measured by the CCK-8 assay. The amount of water-soluble formazan, in proportion to the number of living cells, was measured by Microplate Reader (Bio-Rad Laboratories, Inc. USA) monitoring the absorbance at 450 nm. All the procedures were carried out following the manufacturer’s guidelines.
ELISA Assay for Indicators of Inflammation and OS
To determine the levels of inflammatory and oxidative biomarkers in the supernatant of different groups, including IgA, IL-4, IL-8, IL-17, TNF-α, iNOS, ROS, NO, CAT, SOD, GSH and MDA, enzyme-linked immunosorbent assay (ELISA) was introduced. All the steps of these assay kits were performed according to the manufacturer’s instructions. Optical density (OD) values were read by Microplate Reader and calculated from standard curves.
Western Blotting Analysis for the Proteins of TLR4, MyD88 and NF-κB
To measure the protein levels of TLR4, MyD88 and NF-κB, cell samples of each group were collected for Western blotting (WB) analysis. Briefly, cell precipitation were treated with lysate (RIPA: PMSF=100:1 preparation) and protein loading buffers to extract total protein. After detection of total protein concentration by Pierce bicinchoninic acid (BCA) assay, the SDS-PAGE Gel system was applied. Membranes, then, were blocked with 5% skimmed milk in tris-buffered saline and tween (TBST) and incubated with primary antibodies, the primary antibodies against phosphorylated TLR4 (p-TLR4, 1:1000 v/v, rabbit anti-human, anti-p-TLR4 monoclonal antibody, R&D, USA), p-MyD88 (1:1000 v/v, rabbit anti-human, anti-p-MyD88 monoclonal antibody, abcam, UK) and p-NF-κB (1:1000 v/v, rabbit anti-human, anti-p-NF-κB monoclonal antibody, abcam, UK) at 4°C overnight followed by secondary antibody (horseradish peroxidase labeled goat anti-rabbit IgG, 1:10,000 v/v, beinglay, China;). Eventually, signals were detected and analyzed by enhanced chemiluminescence system (Bio-Rad Laboratories, Inc., CA, USA). As an internal control, besides, anti-β-actin monoclonal antibody (1:5000 v/v, mouse anti-human, beinglay, China) was used.
Quantitative Real-Time PCR Analysis for TLR4, MyD88 and NF-κB Genes
To determine the gene levels of TLR4, MyD88 and NF-κB, quantitative real-time PCR (qRT-PCR) analysis was performed to measure mRNA levels. Firstly, total RNA was extracted from cells in each group by TRIzol RNA extraction kit following the instructions (Ambion, USA). qRT-PCR, then, was performed on iCycler iQ instrument (BioRad, USA). Lastly, the evaluation of relative gene expression was carried out by using the ΔΔCT method. The gene primers were shown as following (Table 1). The expression of target genes was determined relative to that of the internal control, β-actin.
 |
Table 1 The Primers of Inflammatory/Oxidative Genes |
Statistical Analysis
Software SPSS 26.0 was applied in statistical analysis. The data were expressed as mean ± SD and evaluated by using analysis of variance (ANOVA) or Student’s t-test. A value of P < 0.05 indicated statistically significant differences among groups.
Results
Effect of PCs on Normal HUVECs
The normal HUVECs displayed a spindle-shaped or polygonal morphology and an adherent-growth pattern. Once aggregation and fusion into a piece, they presented as the paving stone-like appearance (Figure 1). The IC50 of PCs was determined as 52.33 μg/mL (Figure 2), according to which the appropriate concentration of PCs was finally chosen at low concentration (30 μg/mL), medium concentration (40 μg/mL) and high concentration (50 μg/mL) for further experiments.
 |
Figure 1 Morphology of normal HUVECs. |
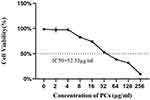 |
Figure 2 Influence of different-concentration PCs on normal HUVECs viability. |
Characteristics of HSP Serum-Induced Cell Models
After twelve hours of HSP serum induction, cells exhibited similar characteristics to normal ones, namely spindle-shaped appearance and adherent-growth mode (Figure 3). However, the levels of immuno-inflammatory and oxidative indicators, including IgA, IL-4, IL-8, IL-17, TNF-α, NO, iNOS, MDA and ROS, significantly went up in the model group compared with those in the blank and control groups (P < 0.05), whereas the expression of CAT, SOD and GSH greatly went down (P < 0.01).
 |
Figure 3 Morphology of HUVECs in the presence of HSP serum. |
PCs Attenuated the Inflammatory Reaction of HSP Serum-Induced Cells
HSP serum remarkably enhanced the levels of IgA, IL-4, IL-8, IL-17 and TNF-α of HUVECs (P < 0.05) (Figure 4). These inflammatory indicators induced by HSP serum, however, exhibited an opposite trend in the presence of PCs (P < 0.05); that was, PCs prominently cut down the expression of IgA, IL-4, IL-8, IL-17 and TNF-α in the different-concentration PCs groups (P < 0.01) (Figure 4); nevertheless, little significant difference was found among the different-concentration PCs groups (P > 0.05).
PCs Mitigated OS Damage of HSP Serum-Induced Cells
After HSP serum induction, the levels of SOD, CAT and GSH obviously dropped, but those of NO, iNOS, MDA and ROS apparently ascended (P < 0.05) (Table 2). However, PCs in varying degrees enhanced the expression of SOD, CAT and GSH, and lowered that of MDA and ROS, which was statistically significant in comparison with those in the model group (P < 0.01); yet, statistical differences rarely existed in groups with different-concentration PCs.
 |
Table 2 Levels of Oxidative/Anti-Oxidative Indicators in Different Groups (Mean ± SD) |
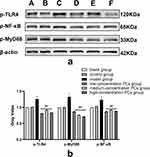
PCs Modulated the Expression of Inflammatory Proteins and Genes in HSP Serum-Induced Cells
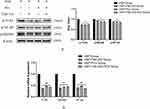
Following the induction of HSP serum, the phosphorylated protein and mRNA levels of TLR4, MyD88 and NF-κB in HUVECs was greatly upwards. Nevertheless, this condition went towards the opposite direction in the presence of PCs (P < 0.01); Compared with the model group, the application of different concentrations of PCs significantly reduced the expression levels of phosphorylated proteins TLR4, MyD88, and NF-κB (Figure 5) (P < 0.01). The gene levels of TLR4, MyD88, and NF-κB were similarly downregulated by PCs (Figure 6) (P < 0.01). However, little statistical significance, regardless of above proteins or genes, appeared among different-concentration PCs groups (P > 0.05).
 |
Figure 6 Relative mRNA levels of TLR4, MyD88 and NF-κB genes in different groups. Notes: Compared with the model group, **P < 0.01. |
PCs Suppressed TLR4 Signaling Pathway
To elucidate the mechanisms of PCs against HSP serum-induced cells, the TLR4 signaling pathway was investigated through targeting inhibition of TLR4. PCs treatment later, cells exhibited an evident reduction in inflammatory parameters (like IgA, IL-4, IL-8, IL-17, TNF-α, p-TLR4, p-MyD88 and p-NF-κB), and OS-related indicators (containing NO, iNOS, MDA and ROS), along with an obvious enhancement in CAT, SOD, and GSH. After pretreatment with TLR4 inhibitor (TAK-242), regardless of with or without PCs, similar alterations still appeared; notably, levels of IgA, IL-4, IL-8, IL-17, and TNF-α decreased (Figure 7) (P<0.01). Additionally, there was a decline in NO, iNOS, MDA, and ROS levels (Table 3) accompanied by a decrease in p-TLR4, p-MyD88 and p-NF-κB, and the mRNA expression levels of these three genes mirrored this trend (Figure 8). In contrast, CAT, SOD, and GSH levels displayed varying degrees of increase (Table 3) (P < 0.05). Little statistical difference was found between the PCs group and TAK-242 inhibitor group (P > 0.05).
 |
Table 3 Levels of OS-Related Indexes After Intervention with TLR4 Inhibitor and PCs (Mean ± SD) |
Discussion
The current study revealed the mechanism underlying the role of PCs against the HSP serum-induced HUVECs, which probably involved the arrest of inflammatory response and OS damage through blocking TLR4/MyD88/NF-κB signaling pathway. Firstly, the HSP serum-induced cell model was successfully constructed in vitro by using serum from HSP patients, along with increased inflammatory/oxidative parameters and decreased antioxidant index. Then in the presence of PCs, these inflammatory/oxidative mediators reduced, whereas antioxidant enzymes enhanced. Finally, after intervention with TLR4 inhibitor TAK-242, regardless of PCs treatment or not, HSP serum-induced cells showed similar changes as above, with low expression of TLR4, MyD88 and NF-κB proteins as well inflammatory/oxidative parameters, and high expression of antioxidants. These results suggest that PCs have an excellent ability to alleviate inflammation and OS damage to experimental HSP via inhibition of TLR4/MyD88/NF-κB pathway.
Natural plant extracts have widely been used in numerous clinical studies owing to their low toxicity, safety and affordability.31,32 PCs, a class of natural plant compounds, serve as an important player in the mitigation of inflammatory response and OS damage.33,34 They extensively exist in multiple vegetables, leaves, seeds, flowers and fruits23,35 with available, affordable, safe and effective advantages, which apply to both the elderly and young children, even pregnant women.36,37 Despite the good safety and low toxicity of PCs having being confirmed,33,34,36,37 it requires to guarantee their safe concentration when studying PCs pharmacological effects. In this safety scope, PCs would rarely impair normal cells. For determination of safe range, we firstly applied different-concentration PCs in the normal HUVECs. Our outcomes revealed that IC50 of PCs was set at 52.33 μg/mL, which concurred with the previously reported findings;38,39 basing on the IC50 value, the concentrations of 30μg/mL, 40μg/mL and 50μg/mL were perfect and selected for next experiments.
The cellular sources for the construction of HSP-like cell models in vitro mainly involve primary HUVECs and HUVEC strains. The HUVECs strains possess the same biological characteristics as the primary ones. Unlike the tough harvest of primary HUVECs from neonatal umbilical cords, HUVECs strains have the superiorities of easy acquirement, excellent survival and high vitality,40,41 Hence, HUVECs strains were chosen as ideal cells in the present study to ensure the success of subsequent experiments. Additionally, we employed HSP serum to establish an in vitro HSP-like model; this modeling approach has been frequently used in the investigation of HSP pathogenesis and pharmaceutical effects.42
Once HUVECs stimulated with HSP serum, IgA in the serum could induce large amounts of inflammation and OS-related factors that further promote inflammatory response and OS generation, thereby in large part simulating the pathogenesis of HSP.43,44 Therefore, considering the evidence presented above, we established an in-vitro cell model mimicking HSP conditions using 25% HSP serum to stimulate HUVECs. Interestingly, we observed minimal alterations in cell morphology and quantity after a twelve-hour modeling period. This leads us to speculate that this modeling approach primarily impacts the cellular microenvironment rather than significantly affecting cellular morphology and quantity. To verify this conjecture, we then determined the expression of inflammatory /oxidative indicators; as a result, it showed that the levels of IgA, IL-4, IL-8, IL-17, TNF-α, ROS and MDA significantly increased, while those of CAT, SOD and GSH remarkably decreased after HSP serum stimulation. Above outcomes was in line with the reports from Bo and Cuiet al who observed that cells scarcely changed in morphology and quantity but an enhancement of inflammatory/oxidative parameters and a drop of antioxidants after HSP serum induction.42,45 Our findings indicated the successful establishment of HSP-like cell model in the present study and the pathogenesis of HSP involving inflammation and OS, offering a foundation for further experiments.
A serial of studies has shown that PCs actively exert in many diseases through inhibiting inflammatory factors production and arresting OS damage.46–49 More importantly, our previous experiment tentatively uncovered that PCs could effectively alleviate cutaneous and renal injuries in HSP-like rats probably via suppression of inflammatory responses and OS insults,50 but the exact mechanism of PCs against HSP still kept unclear. Thus, to clarify the therapeutic mechanism of PCs, we applied different-concentration PCs to interfere with the HSP-like cell model basing on the determination of drug safe concentrations and the establishment of cell model. Our outcomes exhibited that PCs greatly lowered the expressions of IgA, IL-4, IL-8, IL-17, TNF-α, NO, MDA and ROS, while markedly enhanced those of SOD, CAT and GSH, which was similar to the results from Wang and Chen, et al;47,48,51–53 they discovered that PCs powerfully facilitated the decrease of inflammatory factors and oxidative substance, and the increase of antioxidants. Based on our experimental results, there does not appear to be a significant difference between the PCs groups at different concentrations when compared to the model group. This suggests that the intervention of PCs in HUVECs treated with serum from HSP patients does not exhibit an evident dose-dependent relationship. However, these results also revealed that PCs effectively inhibited the inflammatory response and OS damage in the in vitro HSP cell model. These findings align with our previous animal experiments, affirming the beneficial effects of PCs in experimental HSP. Nevertheless, how actually did PCs work?
Several documents have reported that the inflammatory response and OS are crucial contributors to HSP pathogenesis,9,54–56 in which the abnormal activation of TLR4 signal get closely involved.8,19,57 When microorganisms (bacteria, viruses, etc.) invade and despoil the body’s barriers, TLR4, as one of key pattern recognition receptors, specifically recognizes infection/damage-associated molecules to initiate innate immunity. Then TLR4, by binding to MyD88, excites downstream NF-κB signaling pathway to encourage inflammatory factors release and ROS production, further irritating inflammatory responses and OS occurrence.30,58 Above molecular events in turn exacerbate vascular endothelial cells injury and vascular permeability, eventually lead to the onset of HSP.45,57,59 These confirm the importance of TLR4/MyD88 /NF-κB in HSP pathogenesis. So to elucidate the specific mechanism of PCs on HSP, the key components of this pathway, comprising TLR4, MyD88 and NF-κB, were investigated after PCs treatment with or without TLR4 inhibitor.
Results from our experiment showed that the protein and gene expressions of TLR4, MyD88 and NF-κB obviously elevated following HSP serum induction, which were consistent with the previous reports7,8,10 and reconfirmed TLR4/MyD88/NF-κB pathway quite critical to HSP. Instead, the phosphorylated protein and mRNA levels of TLR4, MyD88 and NF-κB greatly dropped after intervention with different-concentration PCs; this outcome coincided with what was reported by Manna K and Wang, et al,60–63 who demonstrated that PCs could reduce the expression of TLR4, MyD88 and NF-κB to inhibit inflammatory mediators release and deplete oxides, thereby curbing inflammation and OS. The current study, therefore, uncovered that PCs against experimental HSP was possibly through inhibiting the TLR4/MyD88/NF-κB pathway, TLR4 in particular. Further to identify the role of TLR4 in PCs treatment, TLR4 inhibitor was utilized to antagonize this signaling pathway. The results indicated that in the presence of the TLR4 inhibitor, the expressions of IgA, TLR4, MyD88, NF-κB, and inflammatory factors, as well as OS-related mediators, exhibited a noticeable decrease. Simultaneously, the levels of antioxidant substances increased, irrespective of PCs intervention. However, these alterations did not exhibit substantial differences when compared with those observed in the presence of PCs alone. The findings above imply that suppression of TLR4 pathway is indeed responsible for PCs treating HSP. Accordingly, it verifies that PCs reduced MyD88/NF-κB expression largely via inhibition of TLR4/MyD88/NF-κB, thereby mitigating inflammatory response and OS damage, preventing IgA deposition and vascular endothelial cells injury, and eventually controlling HSP.
Conclusion
In summary, our results reveal that PCs effectively serve in relieving the inflammatory response and OS damage to the HSP serum-induced HUVECs, the underlying mechanism of PCs against experimental HSP mostly involving the suppression of TLR4/MyD88/NF-κB signaling pathway. Therefore, blocking TLR4/MyD88/NF-κB signals would favor enhancing the effect of PCs against HSP, indicating a great potential value of PCs in management of HSP. Cell models, however, fail to afford the real condition of human HSP fully. Moreover, they are unable to reveal the effect of PCs on inflammatory cells through the current model. Hence, the establishment of three-dimensional model in vitro as well the co-culture of inflammatory cells and vascular endothelial cells in further studies should be needed to validate the role of PCs in controlling human HSP.
Data Sharing Statement
The data used to support the findings of this study are included within the article.
Ethics Statement
This research was carried out following the ethical rules of the Declaration of Helsinki and was approved by the Clinical Trial Ethics Committee of the Affiliated Hospital of Southwest Medical University, with approval number KY2024088. All participants received detailed information about the study’s aims, methods, and potential benefits before joining.
Acknowledgments
We sincerely acknowledge the clinical medical research center of the Affiliated Hospital of Southwest Medical University for their support for our work. We highly appreciate all persons for their help in this manuscript.
Funding
This study was supported by the grants from Health Commission of Sichuan Province [grant number 18PJ411] and Luzhou Science and Technology Bureau [grant number 2023JYJ039].
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Oñate I, Ortiz M, Suso A, et al. IgA vasculitis with nephritis (Henoch-Schönlein purpura) after COVID-19: a case series and review of the literature. Nefrologia. 2022;42(4):481–489.
2. Hammad H, Krausz J, Barcan M, et al. Adult Henoch-Schönlein Purpura: comprehensive Assessment of Demographic, Clinical, and Histopathological Features as Predictors for Systemic Involvement. Dermatology. 2023;1–7.
3. Hetland LE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein Purpura: a Literature Review. Acta Derm Venereol. 2017;97(10):1160–1166. doi:10.2340/00015555-2733
4. Sestan M, Kifer N, Sozeri B, et al. Clinical features, treatment and outcome of pediatric patients with severe cutaneous manifestations in IgA vasculitis: multicenter international study. Semin Arthritis Rheum. 2023;61:152209. doi:10.1016/j.semarthrit.2023.152209
5. Reamy BV, Servey JT, Williams PM. Henoch-Schönlein Purpura (IgA Vasculitis): rapid Evidence Review. Am Fam Physician. 2020;102(4):229–233.
6. Hahn D, Hodson EM, Craig JC. Interventions for preventing and treating kidney disease in IgA vasculitis. Cochrane Database Syst Rev. 2023;2(2):Cd005128. doi:10.1002/14651858.CD005128.pub4
7. Zhu Y, Dong Y, Wu L, et al. Changes of inflammatory mediators and oxidative stress indicators in children with Henoch-Schönlein purpura and clinical effects of hemoperfusion in the treatment of severe Henoch-Schönlein purpura with gastrointestinal involvement in children. BMC Pediatr. 2019;19(1):409. doi:10.1186/s12887-019-1802-2
8. Xu H, Jiang G, Shen H, et al. Association of TLR4 gene polymorphisms with childhood Henoch-Schönlein purpura in a Chinese population. Rheumatol Int. 2017;37(11):1909–1915. doi:10.1007/s00296-017-3815-1
9. Soylemez K, Temiz F, Dalkiran T, et al. Evaluation of Oxidative Stress Biomarkers in Patients with Henoch-Schönlein Purpura. Folia Med. 2021;63(6):928–931. doi:10.3897/folmed.63.e59406
10. Liu X, Lu B, Fu J, et al. Amorphous silica nanoparticles induce inflammation via activation of NLRP3 inflammasome and HMGB1/TLR4/MYD88/NF-kb signaling pathway in HUVEC cells. J Hazard Mater. 2021;404(Pt B):124050. doi:10.1016/j.jhazmat.2020.124050
11. Yang H, Fang Z, Qu X, et al. Procyanidin Compound (PC) Suppresses Lipopolysaccharide-Induced Cervical Cancer Cell Proliferation Through Blocking the TLR4/NF-κB Pathway. Cancer Manag Res. 2020;12:497–509. doi:10.2147/CMAR.S226547
12. Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78(4):1233–1261. doi:10.1007/s00018-020-03656-y
13. Anthoney N, Foldi I, Hidalgo A. Toll and Toll-like receptor signalling in development. Development. 2018;145(9). doi:10.1242/dev.156018
14. Xuemei L, Qiu S, Chen G, et al. Myrtenol alleviates oxidative stress and inflammation in diabetic pregnant rats via TLR4/MyD88/NF-κB signaling pathway. J Biochem Mol Toxicol. 2021;35(11):e22904. doi:10.1002/jbt.22904
15. Heineke MH, Ballering AV, Jamin A, et al. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun Rev. 2017;16(12):1246–1253. doi:10.1016/j.autrev.2017.10.009
16. Omma A, Colak S, Can Sandikci S, et al. Serum neopterin and ischemia modified albumin levels are associated with the disease activity of adult immunoglobulin A vasculitis (Henoch-Schönlein purpura). Int J Rheum Dis. 2019;22(10):1920–1925. doi:10.1111/1756-185X.13673
17. Seif M, Deabes M, El-Askary A, et al. Ephedra sinica mitigates hepatic oxidative stress and inflammation via suppressing the TLR4/MyD88/NF-κB pathway in fipronil-treated rats. Environ Sci Pollut Res Int. 2021;28(44):62943–62958. doi:10.1007/s11356-021-15142-4
18. ElSayed MH, Atif HM, Eladl MA, et al. Betanin improves motor function and alleviates experimental Parkinsonism via downregulation of TLR4/MyD88/NF-κB pathway: molecular docking and biological investigations. Biomed Pharmacother. 2023;164:114917. doi:10.1016/j.biopha.2023.114917
19. Chang H, Zhang QY, Lin Y, et al. Correlation of TLR2 and TLR4 expressions in peripheral blood mononuclear cells to Th1- and Th2-type immune responses in children with henoch-schönlein purpura. Int J Clin Exp Med. 2015;8(8):13532–13539.
20. Zhang Z, Guo L, Yang F, et al. Adiponectin Attenuates Splenectomy-Induced Cognitive Deficits by Neuroinflammation and Oxidative Stress via TLR4/MyD88/NF-κb Signaling Pathway in Aged Rats. ACS Chem Neurosci. 2023;14(10):1799–1809. doi:10.1021/acschemneuro.2c00744
21. Liu HJ, Pan XX, Liu BQ, et al. Grape seed-derived procyanidins alleviate gout pain via NLRP3 inflammasome suppression. J Neuroinflammation. 2017;14(1):74. doi:10.1186/s12974-017-0849-y
22. Wang L, Fumoto T, Masumoto S, et al. Regression of atherosclerosis with apple procyanidins by activating the ATP-binding cassette subfamily A member 1 in a rabbit model. Atherosclerosis. 2017;258:56–64. doi:10.1016/j.atherosclerosis.2017.01.032
23. Yang L, Xian D, Xiong X, et al. Proanthocyanidins against Oxidative Stress: from Molecular Mechanisms to Clinical Applications. Biomed Res Int. 2018;2018:8584136. doi:10.1155/2018/8584136
24. Andersen-Civil AIS, Arora P, Williams AR. Regulation of Enteric Infection and Immunity by Dietary Proanthocyanidins. Front Immunol. 2021;12:637603. doi:10.3389/fimmu.2021.637603
25. González-Quilen C, Rodríguez-Gallego E, Beltrán-Debón R, et al. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients. 2020;12(1):130. doi:10.3390/nu12010130
26. Yang Y, Zhao Y, Lai R, et al. An Emerging Role of Proanthocyanidins on Psoriasis: evidence from a Psoriasis-Like Mouse Model. Oxid Med Cell Longev. 2022;2022:5800586. doi:10.1155/2022/5800586
27. Zhou Y, Lan H, Dong Z, et al. Dietary proanthocyanidins alleviated ovarian fibrosis in letrozole-induced polycystic ovary syndrome in rats. J Food Biochem. 2021;45(5):e13723. doi:10.1111/jfbc.13723
28. Han S, Gao H, Chen S, et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci Rep. 2019;9(1):15087. doi:10.1038/s41598-019-51614-x
29. Ferreira YAM, Jamar G, Estadella D, et al. Proanthocyanidins in grape seeds and their role in gut microbiota-white adipose tissue axis. Food Chem. 2023;404(Pt A):134405. doi:10.1016/j.foodchem.2022.134405
30. Xie Y, Deng Q, Guo M, et al. Proanthocyanidins: a novel approach to Henoch‑Schonlein purpura through balancing immunity and arresting oxidative stress via TLR4/MyD88/NF‑κB signaling pathway (Review). Exp Ther Med. 2023;25(6):300. doi:10.3892/etm.2023.11999
31. Sitarek P, Kowalczyk T, Wieczfinska J, et al. Plant Extracts as a Natural Source of Bioactive Compounds and Potential Remedy for the Treatment of Certain Skin Diseases. Curr Pharm Des. 2020;26(24):2859–2875. doi:10.2174/1381612826666200417160049
32. Piao M, Tu Y, Zhang N, et al. Advances in the Application of Phytogenic Extracts as Antioxidants and Their Potential Mechanisms in Ruminants. Antioxidants (Basel). 2023;12(4):879. doi:10.3390/antiox12040879
33. Zhang X, Song X, Hu X, et al. Health benefits of proanthocyanidins linking with gastrointestinal modulation: an updated review. Food Chem. 2023;404(Pt A):134596. doi:10.1016/j.foodchem.2022.134596
34. Zhao Y, Jiang C, Lu J, et al. Research progress of proanthocyanidins and anthocyanidins. Phytother Res. 2023;1–26.
35. El-Shitany NA, Eid B. Proanthocyanidin protects against cisplatin-induced oxidative liver damage through inhibition of inflammation and NF-κβ/TLR-4 pathway. Environ Toxicol. 2017;32(7):1952–1963. doi:10.1002/tox.22418
36. Lai R, Xian D, Xiong X, et al. Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. 2018;23(1):130–135. doi:10.1080/13510002.2018.1462027
37. Sano A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem Toxicol. 2017;108(Pt B):519–523. doi:10.1016/j.fct.2016.11.021
38. Zhao L, Jin X, Li Y, et al. Effects of A-type oligomer procyanidins on protein glycation using two glycation models coupled with spectroscopy, chromatography, and molecular docking. Food Res Int. 2022;155:111068. doi:10.1016/j.foodres.2022.111068
39. Rupasinghe HPV, Parmar I, Neir SV. Biotransformation of Cranberry Proanthocyanidins to Probiotic Metabolites by Lactobacillus rhamnosus Enhances Their Anticancer Activity in HepG2 Cells In Vitro. Oxid Med Cell Able Longev. 2019;2019:4750795.
40. Cai W, Liang L, Ji P, et al. Experimental study on culture method of human umbilical vein endothelial cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011;25(2):139–143.
41. Chen X, Chen B, Yang Y, et al. Isolation, culture and identification of human umbilical vein endothelial cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2016;32(3):328–331.
42. Bo Y, Yuan LP, Zhang JJ, et al. Total flavonoids of Bidens bipinnata L. a traditional Chinese medicine inhibits the production of inflammatory cytokines of vessel endothelial cells stimulated by sera from Henoch-Schönlein purpura patients. J Pharm Pharmacol. 2012;64(6):882–887. doi:10.1111/j.2042-7158.2012.01480.x
43. Maritati F, Canzian A, Fenaroli P, et al. Adult-onset IgA vasculitis (Henoch-Schönlein): update on therapy. Presse Med. 2020;49(3):104035. doi:10.1016/j.lpm.2020.104035
44. González-Gay MA, López-Mejías R, Pina T, et al. IgA Vasculitis: genetics and Clinical and Therapeutic Management. Curr Rheumatol Rep. 2018;20(5):24. doi:10.1007/s11926-018-0735-3
45. Cui M, Liu J, Geng L, et al. Let-7a Targeting TNFAPI3 Promotes Vascular Endothelial Cell Apoptosis of Pediatric Patients with Henoch-Schönlein Purpura via NF-κB Signaling Pathway. J Healthc Eng. 2022;2022:3889318. doi:10.1155/2022/3889318
46. Gupta M, Dey S, Marbaniang D, et al. Grape seed extract: having a potential health benefits. J Food Sci Technol. 2020;57(4):1205–1215. doi:10.1007/s13197-019-04113-w
47. Wang ZS, Shu B, Han Q, et al. Effects of grape seed-derived proanthocyanidin B2 pretreatment on oxidative stress, endoplasmic reticulum stress and apoptosis of renal tubular epithelial cells in renal ischemia-reperfusion injury model of mice. Int Urol Nephrol. 2023;55:2599–2610. doi:10.1007/s11255-023-03494-4
48. Zhao Y, Xie Y, Li X, et al. The protective effect of proanthocyanidins on the psoriasis-like cell models via PI3K/AKT and HO-1. Redox Rep. 2022;27(1):200–211. doi:10.1080/13510002.2022.2123841
49. Baranowska M, Bartoszek A. Antioxidant and antimicrobial properties of bioactive phytochemicals from cranberry. Postepy Hig Med Dosw. 2016;70:1460–1468. doi:10.5604/17322693.1227896
50. Özdamar MY, Yurtçu M, Toy H, et al. Renal iskemi-reperfüzyon hasarında üzüm çekirdeği proantosiyanidin ekstresinin etkisi. Genel Tip Dergisi. 2010;20(1).
51. Chen WC, Hossen M, Liu W, et al. Grape Seed Proanthocyanidins Inhibit Replication of the Dengue Virus by Targeting NF-kB and MAPK-Mediated Cyclooxygenase-2 Expression. Viruses. 2023;15(4):884. doi:10.3390/v15040884
52. Mancini M, Cerny MEV, Cardoso NS, et al. Grape Seed Components as Protectors of Inflammation, DNA Damage, and Cancer. Curr Nutr Rep. 2023;12(1):141–150. doi:10.1007/s13668-023-00460-5
53. Kangwan N, Kongkarnka S, Pintha K, et al. Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model. Biomedicines. 2023;11(2):265. doi:10.3390/biomedicines11020265
54. Niknam N, Ha L, Gautam-Goyal P. Adult onset immunoglobulin A vasculitis (Henoch-Schonlein purpura) with alveolar hemorrhage. IDCases. 2018;12:47–48. doi:10.1016/j.idcr.2018.03.006
55. Xu L, Li Y, Wu X. IgA vasculitis update: epidemiology, pathogenesis, and biomarkers. Front Immunol. 2022;13:921864. doi:10.3389/fimmu.2022.921864
56. Oni L, Sampath S. Childhood IgA Vasculitis (Henoch Schonlein Purpura)-Advances and Knowledge Gaps. Front Pediatr. 2019;7:257. doi:10.3389/fped.2019.00257
57. Bergallo M, Loiacono E, Galliano I, et al. HERV-K and W expression in peripheral mononuclear cells of children with Henoch-Schönlein purpura and relation with TLR activation. Minerva Pediatr. 2022;74(4):421–427. doi:10.23736/S2724-5276.17.04717-X
58. Nikolaishvili M, Pazhava A, Di Lernia V. Viral Infections May Be Associated with Henoch-Schönlein Purpura. J Clin Med. 2023;12(2):697. doi:10.3390/jcm12020697
59. Jia X, Zhu H, Jiang Q, et al. Identification of key genes and imbalance of immune cell infiltration in immunoglobulin A associated vasculitis nephritis by integrated bioinformatic analysis. Front Immunol. 2023;14:1087293. doi:10.3389/fimmu.2023.1087293
60. Liu WZ, Ma ZJ, Kang JH, et al. Grape Seed Proanthocyanidins Exert a Neuroprotective Effect by Regulating Microglial M1/M2 Polarisation in Rats with Spinal Cord Injury. Mediators Inflamm. 2022;2022:2579003. doi:10.1155/2022/2579003
61. Wang H, Hao W, Yang L, et al. Preconditioning with procyanidin B2 protects MAC-T cells against heat exposure-induced mitochondrial dysfunction and inflammation. Mol Immunol. 2022;147:126–135. doi:10.1016/j.molimm.2022.05.001
62. Manna K, Khan ZS, Saha M, et al. Manjari Medika Grape Seed Extract Protects Methotrexate-Induced Hepatic Inflammation: involvement of NF-κB/NLRP3 and Nrf2/HO-1 Signaling System. J Inflamm Res. 2023;16:467–492. doi:10.2147/JIR.S338888
63. Kim SH, Bang J, Son CN, et al. Grape seed proanthocyanidin extract ameliorates murine autoimmune arthritis through regulation of TLR4/MyD88/NF-κB signaling pathway. Korean J Intern Med. 2018;33(3):612–621. doi:10.3904/kjim.2016.053
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.