Back to Journals » Clinical Ophthalmology » Volume 18
Quantification of Metamorphopsia in Resolved Idiopathic Central Serous Chorioretinopathy: An Analysis Using M-CHARTS, Amsler Grid, and Optical Coherence Tomography
Authors Suwal B , Khadka D , Shrestha A, Suwal R , Khatri B
Received 24 December 2023
Accepted for publication 16 March 2024
Published 27 March 2024 Volume 2024:18 Pages 937—942
DOI https://doi.org/10.2147/OPTH.S456556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr John B Miller
Barsha Suwal,1 Deepak Khadka,1 Arjun Shrestha,1 Rinkal Suwal,2 Bijay Khatri3
1Department of Ophthalmology, BP Eye Foundation, Hospital for Children, Eye, ENT, and Rehabilitation Services, Bhaktapur, Nepal; 2Department of Optometry, BP Eye Foundation, Hospital for Children, Eye, ENT, and Rehabilitation Services, Bhaktapur, Nepal; 3Academic and Research Department, BP Eye Foundation, Hospital for Children, Eye, ENT, and Rehabilitation Services, Bhaktapur, Nepal
Correspondence: Barsha Suwal, Department of Ophthalmology, BP Eye Foundation, Hospital for Children, Eye, ENT, and Rehabilitation Services, Bhaktapur, Nepal, Tel +977-9803056313, Email [email protected]
Purpose: To quantify metamorphopsia in patients with resolved idiopathic Central Serous Chorioretinopathy (CSCR) using M-CHARTS and compare the results with the traditional Amsler grid and optical coherence tomography (OCT).
Patients and Methods: For the purpose of this study, all consecutive cases of patients with resolved CSCR were evaluated for metamorphopsia (using the standard Amsler grid and M-CHARTS) and spectral domain OCT. The OCT images were analyzed for the following five parameters: central macular thickness, pigment epithelial detachment, retinal pigment epithelial bumps, discontinuation in the inner segment/outer segment junction or the external limiting membrane, fibrinous exudates in the subretinal space, and hyperreflective dots in the intraretinal and/or subretinal layer. Binary logistic regression was used to find the association between metamorphopsia and foveal morphology. Cohen’s Kappa was used to determine the agreement between the M-CHARTS and Amsler grid for diagnosing metamorphopsia. The sensitivity, specificity, and accuracy in the diagnosis of metamorphopsia were calculated against the Amsler grid.
Results: Of 41 eyes, Amsler Grid detected metamorphopsia in 39.02%, and M-CHARTS detected metamorphopsia in 53.66%. The agreement rate of detection between the two tests was moderate (Kappa=0.52). M-CHARTS had a sensitivity of 87.50%, a specificity of 68.00%, a positive predictive value of 63.64%; and a negative predictive value of 89.47% for the diagnosis of metamorphopsia compared to the Amsler grid. The presence of PED in OCT was significantly associated with metamorphopsia.
Conclusion: M-CHARTS can be a useful ancillary test to detect and quantify metamorphopsia even after fluid resolution in CSCR. Structural changes in macular morphology as observed with OCT can predict the likelihood of metamorphopsia.
Keywords: agreement, negative predictive value, positive predictive value, sensitivity, specificity
Introduction
Central serous chorioretinopathy (CSCR) is defined as the presence of serous detachment of the neurosensory retina affecting the macula. Metamorphopsia, a common and often overlooked symptom in patients with macular diseases, is defined as a distortion of perceived straight lines. It is reported to have an incidence of up to 67.7% in active cases when determined using the Amsler grid1 and 45.5% cases when determined using M-CHARTS.2 Various theories on its development have been suggested. The main consensus has been on the theory of retinal layer displacement leading to distorted vision. Recently, it has been suggested that cortical reorganization, in addition to retinal changes, plays a role in its development.3
For the assessment of metamorphopsia in macular disorders, the Amsler grid has been the gold standard of investigation in use since 1947.4 Although it is very economical and is easily understood by the patient, studies have shown that it produces a high false negative rate.5 Moreover, it does not allow for quantification of the severity of metamorphopsia. For this purpose, M-CHARTS (Inami Co., Tokyo, Japan) was developed as a diagnostic tool to quantify the degree of metamorphopsia by Matsumoto.6 Its usefulness has already been demonstrated in patients with epiretinal membranes (ERM),7,8 macular holes,9 branch retinal vein occlusion,10 Age-related macular degeneration,11 CSCR,2 and Diabetic macular edema.12
Our earlier study showed that baseline macular morphologic changes in OCT can help us predict the visual prognosis and clinical course in patients with CSCR.13 However, best-corrected visual acuity (BCVA) alone does not reflect structural changes in the neurosensory retina outside the foveal region.14 Therefore, our study strengthened the recommendation that other visual functions, such as metamorphopsia, should be taken into account even after recovering visual acuity. There is a limited understanding of the clinical parameters related to metamorphopsia after resolution of the subretinal fluid (SRF) in patients with CSCR. Therefore, this study aims to bridge this gap, quantify metamorphopsia using M-CHARTS, and compare the results with the traditional Amsler grid and the OCT results in patients with resolved CSCR.
Materials and Methods
This cross-sectional study was conducted in the Vitreo-retina Clinic of the Department of Ophthalmology at the Hospital for Children, Eye, ENT and Rehabilitation Services, Bhaktapur, Nepal, from July 2021 to March 2022. In order to participate in the study, all consecutive cases of patients with resolved CSCR, diagnosed clinically and confirmed by OCT, were included. Patients with active CSCR, CSCR patients who had received interventional treatment (laser or intravitreal injection), and eyes with retinal disorders other than CSCR were excluded.
The study adhered to the principles of the Declaration of Helsinki. Ethical approval was obtained from the Ethics Review Board (Protocol No. 423–2021) of the Nepal Health Research Council. The participants were explained about the nature of the study and written informed consent was obtained from each participant prior to enrollment.
Ocular examination included the BCVA test using Snellen charts, slit lamp examination, applanation tonometry, dilated fundoscopy, and spectral domain OCT (Topcon 3D, version 8.20.003.04). The evaluation of metamorphopsia by an optometrist included the standard Amsler grid (black grid on a white background) and M-CHARTS Ver 2.0 (Inami Co, Tokyo, Japan) on the same day, with near-correction and undilated pupils. The Amsler test was considered positive if the patient reported blurred lines.
In patients with metamorphopsia, a straight line projected onto the retina is recognized as an irregular line. When a dotted line is used and the dot interval changes from fine to coarse, the metamorphopsia decreases and finally disappears.7 If the patient recognizes the dotted line as straight, its visual angle is considered to be the metamorphopsia score. The M-CHARTS examination is performed for vertical lines, resulting in a vertical M score, and then rotated 90 degrees to test for horizontal lines, resulting in a horizontal M score. The mean summation score is calculated as the sum of the horizontal and vertical M-CHARTS scores. Matsumoto et al,7 previously found that the M score was 0 in all normal eyes and that the intra-individual variation in the M score in patients with ERM was within one line (± 0.1). Therefore, metamorphopsia was considered present if the sum of horizontal and vertical M-CHARTS scores was 0.3 or greater.15 Based on this, the included patients were categorized into two groups: Metamorphopsia group (the sum of the horizontal and vertical M-CHARTS scores ≥ 0.3) and non-metamorphopsia group (the sum of the horizontal and vertical M-CHARTS scores < 0.3).
Spectral domain-OCTs were analyzed for central macular thickness (CMT), presence of pigment epithelial detachment (PED), retinal pigment epithelial (RPE) bumps, discontinuation in the inner segment (IS)/outer segment (OS) junction or external limiting membrane (ELM), fibrinous exudates in the subretinal space and hyperreflective dots in the intraretinal and/or subretinal layer. Details on the acquisition of OCT images have been described in a previous study.13
The patient’s details were recorded in a proforma. The collected data were checked for completeness and entered into MS Excel 2019 (Microsoft Corp., Redmond, Washington, USA). Data were further cleaned and categorized using MS Excel 2019. All statistical analyses were performed with IBM SPSS 26.0 (0 (IBM Corp., Armonk, NY, USA). Continuous data was expressed as mean, standard deviation, and percentages. The Mann–Whitney U-test was used for comparison between baseline characteristics between groups. Binary logistic regression (chi-square test and Fisher’s exact test) was used to find the association between metamorphopsia and OCT findings. Cohen’s Kappa was used to determine the agreement between the M-CHARTS and Amsler grid for diagnosing metamorphopsia. The sensitivity, specificity, and accuracy in the diagnosis of metamorphopsia were calculated against the Amsler grid. A p-value less than 0.05 was considered statistically significant.
Results
In this study, 41 eyes of 38 patients were included. The mean age of the total of patients was 45.74 ± 8.13 years. Among them, 30 (73.2%) were men. The right eyes (n=25) were more commonly affected than the left eyes (n=16) (p=0.04). 22 eyes (53.66%) had metamorphopsia from the M-CHARTS analysis. The mean vertical and horizontal M-CHARTS scores were significantly higher in the metamorphopsia group. There was no statistical difference in mean scores of CMT, BCVA, and age in the two groups (See Table 1).
 |
Table 1 Baseline Characteristics of the Metamorphopsia and Non-Metamorphopsia Group |
Table 2 shows the association between metamorphopsia diagnosed by M-CHARTS and retinal morphology on OCT. The presence of PED was significantly associated with metamorphopsia; eyes having PED were nearly four times more likely to have metamorphopsia. PED was found in 16 eyes (39.0%), RPE bumps in 13 eyes (31.7%), hyperreflective dots in the intraretinal or subretinal layer in 10 eyes (24.45), and discontinuity in the IS/OS junction or ELM line in 8 eyes (19.5%). None of the eyes had fibrinous exudates in the subretinal space.
 |
Table 2 Association Between Metamorphopsia Diagnosed by M- CHARTS and OCT Parameters |
With reference to the Amsler grid, M-CHARTS had 75.61% accuracy in diagnosing metamorphopsia in patients with resolved idiopathic CSCR. There was moderate agreement between the M-CHARTS and the Amsler grid in diagnosing metamorphopsia, κ = 0.520, p < 0.001 (See Table 3).
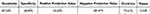 |
Table 3 Diagnostic Accuracy of M-CHARTS in the Detection of Metamorphopsia in Reference to Amsler Grid |
Discussion
Metamorphopsia is a major visual symptom in patients with CSCR, which often leads to poor visual quality. We investigated the clinical parameters associated with metamorphopsia in resolved CSCR. In the present study, 53.3% of patients had metamorphopsia even after fluid resolution when tested with M-CHARTS. This finding is similar to another study by Bae and Jin,15 where they reported it is present in 52.8% (19 eyes out of 36 eyes).
We found that the mean vertical and horizontal M scores were significantly higher in the metamorphopsia group. This suggests that these patients indeed have a distorted vision of some sort. However, we did not find a correlation with visual acuity. This finding aligns with that of Bae and Chae,2 and Matsumoto et al,7 who found that the M-scores did not correlate with BCVA. Likewise, CMT was not found to be statistically different between the two groups in the present study, in contrast to another study, where they found a thinner macula in the metamorphopsia group.15 Our study excluded eyes that had received an intervention such as laser or intravitreal injection, but the latter study15 included eyes with a history of photodynamic therapy and laser photocoagulation, which can impact the thickness of the retina. We know that chronic accumulation of central SRF has been known to cause retinal thinning.16 There was no correlation between the degree of metamorphopsia and CMT or visual acuity in AMD eyes.11
When comparing metamorphopsia with changes in OCT in active CSCR, a study has reported a higher incidence of PED in those with metamorphopsia.2 In line with this, we found that PED, when present even after SRF resolution, was significantly associated with metamorphopsia. This could be explained by the fact that focal RPE detachment distorts the retina at the site of detachment and can induce the development of metamorphopsia. Second, the location of PED when central may cause metamorphopsia and the noncentral location may cause otherwise. It is also possible that the duration of morbidity may play a role; it may be different between patients, which may influence the presence or disappearance of PED and, hence, metamorphopsia. Our observation is corroborated by the morphological changes in the OCT.
Another study by Bae et al, found that the final integrity of ELM was the only independent structural parameter that affected the outcome of metamorphopsia in resolved CSCR, where they speculated that metamorphopsia in resolved CSCR could be attributed to damage to the photoreceptor layer extending toward the photoreceptor cell bodies, but not to the outer parts of the photoreceptor layer, such as the IS/OS junction.15 However, we did not find a statistical association of metamorphopsia with discontinuation in the IS/OS junction or the external limiting membrane (ELM) and disintegrated photoreceptors, which are represented by hyperreflective dots in retinal layers in OCT.17 Although hyperreflective dots have been shown to be related to poor visual outcomes in acute CSCR and the need for early intervention,18,19 we could not find statistically significant association with metamorphopsia in resolved cases.
In our study, we found that M-CHARTS detected metamorphopsia in 53.66% and Amsler Grid detected metamorphopsia in 39.02% in resolved cases of CSCR. This was lower compared to another study of 36 AMD patients, which showed that the rate of metamorphopsia detection was 89% with M charts and 69% with the Amsler test.11 However, our results were comparable with the detection rate of metamorphopsia in eyes with diabetic macular edema, which was 37% with the Amsler test examination and 50% with M-charts.20 The type of disease could affect the detection of metamorphopsia, which has also been suggested in another study that compared the detection of metamorphopsia in various macular disorders.21
Likewise, we found that M-CHARTS had a specificity of 68.00% in resolved CSCR, which was lower than in AMD cases where they found the specificity of the M-charts to be 100%.11 There was moderate agreement between the M-CHARTS and Amsler grid in the diagnosis of metamorphopsia in resolved CSCR in our study, similar to another study in active CSCR patients.21
M-CHARTS has the limitation of a subjective scoring system. This may not estimate the actual metamorphopsia scores. Moreover, the present result might be influenced by the measurements over different time periods. Scanning of a larger retinal area, not just a few scans centered on the fovea, could be necessary to evaluate the relationships with metamorphopsia. Further studies with larger sample sizes and serial follow-up are suggested.
Conclusion
To conclude, metamorphopsia was detected using M-CHARTS in about half of the patients (53.66%) with resolved CSCR. PED was more likely to be present in such patients. The agreement rate of detection between the Amsler grid and M-CHARTS was moderate.
Acknowledgments
The authors would like to thank Hospital Administration for their permission to conduct the study.
Funding
There is no funding to report.
Disclosure
The authors report that they have no conflicts of interest in this work.
References
1. Baran NV, Gürlü VP, Esgin H. Long-term macular function in eyes with central serous chorioretinopathy. Clin Exp Ophthalmol. 2005;33(4):369–372. doi:10.1111/j.1442-9071.2005.01027.x
2. Bae SW, Chae JB. Assessment of metamorphopsia in patients with central serous chorioretinopathy. Indian J Ophthalmol. 2013;61(4):172–175. doi:10.4103/0301-4738.112162
3. Midena E, Vujosevic S. Metamorphopsia: an overlooked visual symptom. Ophthalmic Res. 2015;55(1):26–36. doi:10.1159/000441033
4. Amsler M. L’examen qualitatif de la fonction maculaire [Qualitative examination of macular function]. Ophthalmologica. 1947;114(4–5):248–261. French. doi:10.1159/000300476
5. Schuchard RA. Validity and interpretation of Amsler grid reports. Arch Ophthalmol. 1993;111(6):776–780. doi:10.1001/archopht.1993.01090060064024
6. Matsumoto C. Quantitation of metamorphopsia. Method of evaluation. Rinsho Ganka. 1990;44:271–274.
7. Matsumoto C, Arimura E, Okuyama S, et al. Quantification of metamorphopsia in patients with epiretinal membranes. Invest Ophthalmol Vis Sci. 2003;44(9):4012–4016. doi:10.1167/iovs.03-0117
8. Arimura E, Matsumoto C, Okuyama S, et al. Retinal contraction and metamorphopsia scores in eyes with idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci. 2005;46(8):2961–2966. doi:10.1167/iovs.04-1104
9. Arimura E, Matsumoto C, Okuyama S, et al. Quantification of metamorphopsia in a macular hole patient using M-CHARTS™. Acta Ophthalmol Scand. 2007;85(1):55–59. doi:10.1111/j.1600-0420.2006.00729.x
10. Manabe K, Tsujikawa A, Osaka R, et al. Metamorphopsia associated with branch retinal vein occlusion. PLoS One. 2016;11(4):e0153817. doi:10.1371/journal.pone.0153817
11. Nowomiejska K, Oleszczuk A, Brzozowska A, et al. M-charts as a tool for quantifying metamorphopsia in age-related macular degeneration treated with the bevacizumab injections. BMC Ophthalmol. 2013;13:13. doi:10.1186/1471-2415-13-13
12. Achiron A, Lagstein O, Glick M, et al. Quantifying metamorphopsia in patients with diabetic macular oedema and other macular abnormalities. Acta Ophthalmol. 2015;93(8):e649–e653. doi:10.1111/aos.12735
13. Suwal B, Khadka D, Shrestha A, et al. Baseline predictive factors of visual outcome and persistence of subretinal fluid based on morphologic changes in spectral domain optical coherence tomography in patients with idiopathic central serous chorioretinopathy. Clin Ophthalmol. 2019;13:2439–2444. doi:10.2147/OPTH.S233273
14. Reibaldi M, Boscia F, Avitabile T, et al. Functional retinal changes measured by microperimetry in standard-fluence vs low-fluence photodynamic therapy in chronic central serous chorioretinopathy. Am J Ophthalmol. 2011;151(6):953–960.e2. doi:10.1016/j.ajo.2010.12.007
15. Bae S, Jin K, Kim H, et al. Clinical parameters related to metamorphopsia outcome in patients with resolved central serous chorioretinopathy using M-CHARTS: retrospective cohort study. BMC Ophthalmol. 2015;15(1):180. doi:10.1186/s12886-015-0170-4
16. Gawęcki M, Jaszczuk-Maciejewska A, Jurska-Jaśko A, et al. Impairment of visual acuity and retinal morphology following resolved chronic central serous chorioretinopathy. BMC Ophthalmol. 2019;19(1):160. doi:10.1186/s12886-019-1171-5
17. Shinojima A, Hirose T, Mori R, et al. Morphologic findings in acute central serous chorioretinopathy using spectral domain-optical coherence tomography with simultaneous angiography. Retina. 2010;30(2):193–202. doi:10.1097/IAE.0b013e3181c70203
18. Plateroti AM, Witmer MT, Kiss S, et al. Characteristics of intraretinal deposits in acute central serous chorioretinopathy. Clin Ophthalmol. 2014;8:673–676. doi:10.2147/OPTH.S48894
19. Lai WY, Tseng CL, Wu TT, Lin HS, Sheu SJ. Correlation between baseline retinal microstructures in spectral-domain optic coherence tomography and need for early intervention in central serous chorioretinopathy. BMJ Open Ophthalmol. 2017;2(1):e000054. doi:10.1136/bmjophth-2016-000054
20. Kalinowska A, Nowomiejska K, Brzozowska A, Maciejewski R, Rejdak R. Metamorphopsia score and central visual field outcomes in diabetic cystoid macular edema. Biomed Res Int. 2018;2018:1–10. doi:10.1155/2018/4954532
21. Imai A, Fukuyama H, Gomi F. Comparison of the detection of metamorphopsia between Amsler chart and M-CHARTS. Graefes Arch Clin Exp Ophthalmol. 2023;261(5):1503–1504. doi:10.1007/s00417-022-05930-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
