Back to Journals » Veterinary Medicine: Research and Reports » Volume 15
Rate of Beta-Lactam Resistance and Epidemiological Features of S. Aureus-Associated Bovine Mastitis in Cross-Bred Ethiopian Cows: Systematic Review
Authors Dagnaw M , Bazezew M, Mengistu B, Anagaw B, Mebratu AS
Received 28 April 2023
Accepted for publication 8 February 2024
Published 27 February 2024 Volume 2024:15 Pages 39—55
DOI https://doi.org/10.2147/VMRR.S415339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Young Lyoo
Melkie Dagnaw,1 Marshet Bazezew,2 Bemrew Mengistu,3 Birhan Anagaw,4 Atsede Solomon Mebratu5
1Department of Veterinary Clinical Medicine, College of Veterinary Medicine and Animal Science, University of Gondar, Gondar, Ethiopia; 2Department of Epidemiology, College of Veterinary Medicine and Animal Science, University of Gondar, Gondar, Ethiopia; 3Department of Biomedical Sciences, College of Veterinary Medicine and Animal Science, University of Gondar, Gondar, Ethiopia; 4Department of Pathology, College of Veterinary Medicine and Animal Science, University of Gondar, Gondar, Ethiopia; 5Department of Pharmacy, College of Veterinary Medicine and Animal Science, University of Gondar, Gondar, Ethiopia
Correspondence: Melkie Dagnaw, College of Veterinary Medicine and Animal Science, University of Gondar, Gondar, Ethiopia, P.O.box 196, Tel +251904573289, Email [email protected]
Background: Dairy cows get mastitis from a common infection called Staphylococcus aureus. Because of its broad distribution across diverse populations and capacity to acquire antibiotic resistance, this particular bacterial strain presents a serious threat to public health. The main goals of this study were to determine the beta-lactam resistance profile of S. aureus in Ethiopian dairy cows and to offer thorough epidemiological data.
Methods: We employed manual searches, Web of Science, PubMed Central, and Google Scholar HINARI for electronic bibliographic data.
Results: Twenty-six epidemiological studies were included in this systematic review. Of these studies, 12 articles in Oromia, 4 articles in Addis Ababa, 4 articles in Southern Nations, Nationalities, and People’s (SNNPRS), 3 articles in Tigray, and 3 articles in Amhara region. The average prevalence S. aureus were 34.3% in Oromia, 40.2% in Amhara, 39.5 in AA, 40% in Tigray and 21% in SNNPRS. The antimicrobial resistance rate of S. aureus, specifically in relation to beta-lactam drugs, exhibited an average estimation. Notably, penicillin resistance reached a rate of 75%, while amoxicillin resistance stood at 67%. Furthermore, it was determined that, when treating S. aureus, the resistance rates to ampicillin and cephalosporin were 50% and 57%, respectively.
Conclusion: The results of this analysis have demonstrated a considerable rise in S. aureus prevalence and beta-lactam resistance within the Ethiopian geographic environment. This emphasizes the critical need for alternate therapeutic approaches and preventative measures in order to successfully lessen the disease’s extensive spread and detrimental effects across the nation.
Keywords: antimicrobial resistance, beta-lactam, bovine mastitis, S. aureus, systematic review, veterinary epidemiology
Introduction
Bovine mastitis, a grave and substantial ailment, poses a considerable challenge to dairy production.1,2 Particularly in low-income countries like Ethiopia, it demands special attention from farmers. Numerous factors have been linked to mastitis, including lower milk production, milk that is judged unsafe for human consumption due to antibiotic residues, veterinary expenses, and the eventual culling of long-term sick cows.3 The categorization of bovine mastitis primarily revolves around the distinction between subclinical and clinical cases, as determined by the severity level.
Over 90% of Ethiopia’s total milk production loss is attributed to subclinical mastitis, according to study made by scholars.4,5 In addition, cross-breed dairy cows are predicted to cost 38 US dollars per lactation. Furthermore, it has an effect on public health since dairy products can shed zoonotic pathogens and their toxin, which poses a major risk to human health.6 Mastitis, can result from a variety of illnesses caused by bacteria, fungi, and viruses. In domestic animals, mastitis can be caused by at least 137 etiological agents, with bacteria being the most common cause.7 One of the most infectious bacteria that cause mastitis in cows and spoiling of milk is S. aureus.8
There is proof of the careless use of antimicrobial agents by medical professionals, unskilled practitioners, and others engaged in drug usage and animal husbandry in Ethiopia. Antibiotic resistance is also greatly influenced by elements related to the delivery of medication itself, such as an unfavorable length of treatment and mode of administration. Both in humans and animals, the incidence of staphylococcal infections has been steadily rising. The reason behind this trend is the increment in multidrug-resistant types of these illnesses, which has made treating them more difficult. There are several different processes underlying the emergence of antimicrobial resistance (AMR) in S. aureus. The most well-known resistance mechanisms include: antibiotics being inactivated by enzymes, changing the targets of antibiotics to reduce their affinity, antibiotics being trapped, efflux, pumps, and limited drug uptake through biofilm formation.7–11
It is essential to comprehend the complex interactions among these multifactorial and toxin-specific virulence variables in order to create successful treatment plans to combat S. aureus infections. By focusing on these elements, it may be possible to design new intervention strategies and preventative measures to counteract the pathogenicity of these adaptable bacteria. Future studies in this area will probably provide more information about the mechanisms behind S. aureus virulence and help develop mitigation methods for its harmful effects on human health.10,11
The continuous expression of several virulence factors in response to environmental stimuli during infections suggests the presence of global regulators, in which the expression of numerous unrelated target genes is controlled by a single regulatory determinant.12 She and Sek genes are detected in isolates and cause subclinical mastitis(SCM), while the CC479 strains of S. aureus are connected to severe clinical mastitis in cattle.13 Mean while, sed and sej isolates are mainly associated with continual mastitis.14 These regulators produce chemicals that aid in the survival of bacteria, allowing them to adapt to harsh settings. Drug resistance is a major public health risk associated with S. aureus. The death rate for people with drug-sensitive S. aureus infections is 64% lower than that of people with methicillin-resistant infections.
Methicillin resistance15 is caused by the acquisition of the mecA gene and is typified by the synthesis of PBP2A, an alternative penicillin-binding protein. There is a limited affinity of this protein for beta-lactam antibiotics.16 When it comes to dairy animals, Staphylococcus aureus usually affects the integument, nasal mucosa, and skull. But during milking time, an infected udder quarter serves as the main source of infection for non-infected animals. Depending on the developed standard state of the nation, this can happen through contaminated milkers hands or milking machines17 separating S. aureus from other species by coagulase formation, mannitol fermentation, and trehalose usage is part of the identification and isolation procedures.18 Staphylococcus aureus is the most common infectious and zoonotic pathogen in Ethiopia that causes mastitis and significantly lowers output. Numerous studies, undertaken in different places and times, have examined the prevalence of S. aureus infection in bovine mastitis, accounting for prevalence at the cow, herd, and quarter levels. Recent studies show that between 66.07% and 10% of both asymptomatic and symptomatic instances of bovine mastitis have S. aureus present.19–21
The purpose of this systematic review is aimed to offer useful information about S. aureus, a pathogen spread through milk, to experts in the fields of animal, human, and environmental health. It will function as a thorough information source for putting preventative and control measures, such as infection prevention, resistance reduction, and surveillance design, into practice on dairy farms. In both human and veterinary medicine, systematic reviews are frequently used to compile the results of several research studies. The combined proportion of the epidemiological distribution and the beta-lactam resistance profile of the S. aureus infection among Ethiopian dairy cows was, thus, the purpose of this review.
Methodology
The STROBIE (Preferred Reporting Items for Systematic Reviews and Meta-analysis) checklist (Supplementary file-1 STROBIE checklist for included cross-sectional studies) served as the basis for the systematic review. Based on the highlighted protocols, the checklists were utilized to verify that pertinent data from the chosen publications had been included.
Search Engine/Strategy
The systematic review was based on the STROBIE (Preferred Reporting Items for Systematic Reviews and Meta-analysis) criteria (Supplementary file-1 STROBIE checklist for included cross-sectional studies). The checklists used to confirm that the relevant data from the selected publications was incorporated in compliance with the protocols that were indicated. The literature search was conducted between November 20, 2021, and December 30, 2022. A comprehensive search strategy was independently devised by two authors (M.B. and B.M.) to locate included studies. Both manual methods and databases, such as PubMed, Web of Science, Google Scholar, and HINARI, were used for the literature searches. What is the prevalence or percentage of S. aureus infection among the bacteria that cause mastitis in Ethiopian dairy cows that are milked? How does this affect both clinical and subclinical mastitis? The CoCoPop (Condition, Context, and Population) paradigm was applied in order to locate relevant articles. The context was Ethiopia (Co), the condition was staphylococcus infection (Co), and the population was cows (Pop).
The search approach used a range of crucial keywords and Medical Subject Heading (MeSH) terms. At this point, epidemiology, mastitis/infection of the mammary gland, bovine/lactating cows, cross-sectional study, staphylococcus aureus, and staphylococcus infection are the MeSH terms used. Subsequently, pertinent terms and expressions were integrated, and the Boolean operator “AND /OR” was employed to do an online search. The search procedures that were used included Staphylococcus OR Staphylococcus infection OR Staphylococcus aureus AND (occurrence OR prevalence OR infection rate) AND (cows OR dairy cows OR) AND mastitis OR AND Ethiopia. The only language that could be published in was English. All identified studies were imported into End-Note 20 to remove duplicates.
Criteria for Selection of Articles
The following points were important considerations when selecting relevant studies such as all studies should have observational study designs, there should be precise estimations at the quarter- and cow-levels of the percentage of S. aureus infection in mastitis among bacterial pathogens; and studies was selected based on the occurrence of S. aureus in bovine species. All positive cases are chosen for SCM based on the primary clinical indicators and CMT. In the subsequent selection of all positive cases, the specimens that were cultivated, papers dictating S. aureus isolation and identification using conventional bacteriology techniques, the research was published in English, and the study was carried out in Ethiopia, were considered in their review work. Indexed and published papers that met the aforementioned inclusion requirements were chosen. Exclusion criteria included cohort and case control studies, case series, conventional reviews, experimental (clinical) research, and outbreak reports. Lastly, the data extraction process employed these inclusion and exclusion criteria (study screening strategy and reasons for exclusion). (Figure 1).
 |
Figure 1 PRISMA flow chart for selection of included and excluded of studies. Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. Creative Commons.22 |
Data Extraction Tools
The form contained studies from which M.D. and B.M. separately extracted the following data: the first author’s name, the year the study was conducted, the study design, the study regions, the geographic allocation, the number of animals, the number of CMT positive and culture positive quarters, and the actual prevalence of Staphylococcus aureus infection in cattle mastitis.
Quality Assessment and Evaluation of Study
The AMSTAR-2 (Supplementary file-2) quality evaluation was used in this review to evaluate the research protocols methodological quality and evidence strength in relation to the review question. The quality evaluation of the included studies in this systematic review and meta-analysis is what determines the outcomes (https://drive.google.com/file/d/186it7bH)
Data Synthesis and Statistical Analysis
Based on the papers that were examined, an estimate of the average prevalence of S. aureus in both clinical and subclinical instances of bovine mastitis was made using the SPSS software version 25. To ascertain the average prevalence of S. aureus, a subgroup calculation was carried out, taking into account the degree of mastitis, the “study year” and the study location. Three groups (earlier, middle, and latest) were created for the research year in order to track the evolution of S. aureus prevalence in cows during the course of the study. Furthermore, depending on the pooled papers, research year categories were created, including groups like (≤2012), (2013–2017), and (≥2018).
Results
Search results for Quarter Wise Prevalence Studies
Figure 1 illustrates how 458 articles were read using a variety of extra techniques and electronic resources. Using duplicate removal and eligibility tagging, a total of fifty-six articles were eliminated. After the titles and abstracts were screened, a total of 252 papers were disqualified. 49 of the 150 objects that were reported were retrieved once eligibility was established. In the end, only 26 full-text publications for the synthesis of S. aureus could be found.
Characteristics of Included Studies
Publicly available reports of staphylococcal infection in bovine mastitis in cross-breed, nursing cows from Ethiopia are included in this review. The complete study consists of twenty-six (26) relevant studies that address S. aureus infection in both clinical and subclinical bovine mastitis. All the studies included in this review, with one exception, used a cross-sectional methodology. This review includes studies conducted in different regions of Ethiopia between 2008 and 2022. For every study included in this evaluation, the minimum sample size was 4119, and the largest was 1502.23
All of the included investigations used standard microbiological techniques to isolate S. aureus. These techniques are detailed by NMC.1 Furthermore, the usual procedures for bacterial isolation and identification, bacteriological culture, and CMT for subclinical mastitis were used. In order to assess the pooled proportion of S. aureus infection in Ethiopia, 8441 cows total and 10,346 milk samples at the quarter level were used. The apparent prevalence of S. aureus in bovine mastitis ranged from 10 to 66 point six percent. The detailed elements of the investigation are presented in Table 1 and Figure 2. According to Hailemelekot et al (2018), 66% of S. aureus cases were detected in the Amhara area.24 However, according to Balemi et al,19 the Oromia regional state had the lowest frequency (10%) in 2018.
 |
Figure 2 Prevalence of S. aureus in the respect of the study year. Notes: The horizontal -axis represents the publication year listed while the y-axis is average prevalence of S. aureus. |
 |
Table 1 Characteristics of Included Studies (n=26) |
The extracted data pertaining to the primary components of the Materials and Methods sections of the reviewed papers is compiled in Table 2. The aspects that were most thoroughly explained were sample size calculations, study designs, ethical issues, and suitable explanations of sampling, environments, and correct laboratory practices (isolation techniques). On the other hand, the farm’s management system, housing arrangement, hygienic methods, and statistical management of the results were all either incompletely explained. In relation to the study animals descriptions (eg g. Risk factors), it was determined that this element lacked clarity.
 |
Table 2 Studies Distributed According to Clarity Scores of the Elements of the Materials and Methods Sections |
Geographical Distribution of Bovine Mastitis Associated S. Aureus in Ethiopia
According to Figure 3 and Table 3, the Amhara and Tigray regions had the highest average prevalence of S. aureus (40%), followed by AA (39 point 5%), Oromia (34 point 3%), and SNNPRS (21%). Twelve of the 26 investigations were conducted in the region of Tigray, three in Amhara, four in AA, four in SNNPRS, and three in Oromia.
 |
Figure 3 Geographical distribution of S. aureus in Ethiopia. |
 |
Table 3 Mean Prevalence of the S. aureus with Number of Studies in Regions of Ethiopia |
Prevalence of S. aureus in Different Study Year Categories
>2018 (43%) was the study year category with the greatest average proportion of S. aureus in mastitis, followed by <2012 (32%), and 2013–2017 (34%). Figure 4 illustrates how S. aureus infections in mastitis became more common over time.
 |
Figure 4 Prevalence of S. aureus in different study year categories in Ethiopia. |
The Average Prevalence of S. Aureus in Clinical and Subclinical Mastitis
As depicted in Figure 5, the staphylococcal infection rate in subclinical mastitis was 84%, but it was only 16% in clinical mastitis. Table 4 demonstrates that most research employed the California Mastitis Test to identify subclinical mastitis and clinical observation to identify clinical mastitis. Abebe et al,21 discovered that the highest percentage / prevalence (48%) of S. aureus in subclinical mastitis, and Zeryehun and Abera,30 discovered that the prevalence of clinical mastitis was the lowest at 1%.
 |
Figure 5 Average prevalence of S. aureus in clinical and subclinical mastitis in Ethiopia. |
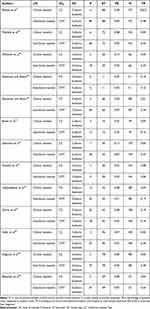 |
Table 4 Average Prevalence of Bovine Mastitis Associated S. Aureus in the Level of Mastitis |
Beta Lactam Antimicrobial Resistance Rate of S. aureus
Antibiotics are the sole known treatment for bovine mastitis in Ethiopia. The most often used class of antibiotics is specifically beta-lactams, which include ceftriaxone, cefotaxime, ampicillin, penicillin, and amoxicillin. The percentage of glands that experience bacteriological cure following antimicrobial therapy for clinical mastitis has been observed to vary widely.44
The pace of recovery is affected by bacterial factors, such as antibiotic resistance and strain, management variables, and cow-specific factors. These variables also affect the proportion of treatments that are successful.25,45–47 The existence of plasmids of various sizes has been connected to a number of antimicrobial resistances. It has been discovered that S. aureus possesses two main plasmid types, which may be involved in the pathogenicity and/or antibiotic resistance of the bacteria. Agar disc-diffusion studies, resistance-encoding gene identification, and broth dilution testing are just a few of the methods that can be used to assess antimicrobial resistance.
The beta-lactam antibiotic resistance rate of S. aureus linked to bovine mastitis is the subject of 14 investigations (Table 5) in total (containing ampicillin, cephalosporin resistance, penicillin, and amoxicillin). Most of the included papers offer a comprehensive account of the whole pathogen isolation procedure in cases of both clinical and subclinical bovine mastitis, together with the results of many antibiotic susceptibility testing. A minimum of one S. aureus isolate has been reported from the selected publications, and they ought to have included one beta-lactam antibiotic. There is only one paper that appears more than once in this systematic review.
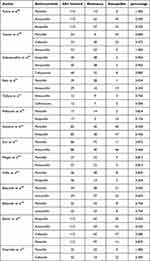 |
Table 5 Summary of Various Beta-Lactam Resistance for S. aureus in the Treatment of Bovine Mastitis (n=14) |
Among the 14 included trials, the resistance rate of S. aureus to various beta lactam antibiotics ranged from 0% to 100%.
Of the total studies (14), two of those48,57 found that 100% of the patients had penicillin resistance when treating S. aureus mastitis in cattle. Nonetheless, a study of 52 found that penicillin was 100% successful in getting rid of S. aureus. This study includes cefoxitin, ceftriaxone, and cefotaxime among other cephalosporins. Cefotaxime and cefoxitin showed the highest resistance rates (80%) among the three cephalosporins. When treated with penicillin, S. aureus had the highest average resistance rate (75%) to that of amoxicillin (67%), cephalosporin (57%), and ampicillin (50%), in that order (Figure 6).
 |
Figure 6 Beta lactam antimicrobial resistance rate of S. aureus in the treatment of bovine mastitis. |
Discussion
These 26 studies provide an average prevalence of 35.4% (95% confidence interval: 0.31–0.41) among bacterial pathogens, with an emphasis on S. aureus in Ethiopia. Girma et al28 in Ethiopia’s Tigray regional state reported similar results. The overall percentage of S. aureus linked to bovine mastitis was lower than the results of several separate investigations carried out in Ethiopia, including 51% by Abebe et al,21 47% by Fessha.33 66% by Hailemelekot,24 and 45% by Birhanu et al.5 The total percentage of the pathogen in mastitis in domestic cows, however, was higher than in several of the included studies, among which Tesfaye et al (24%),27,28 Zerihun et al (21%), and Abebe et al30 reported 21%.20
In cases of both clinical and subclinical mastitis, the sub-total occurrence of S. aureus infection varied depending on the study year: 32% (>2012), 34% (2013–2017), and 43% (> 2018) for each study year group. This systematic review found that the rate of S. aureus infection in bovine mastitis increased when the included publications were categorized based on the study year. It is estimated that approximately 50% of S. aureus-induced mastitis generate β-lactamase, which may be due to increased antibiotic resistance via a variety of mechanisms.47 The proliferation of resistant strains in people, animals, and the environment may be related to this. Contributing causes could also include Ethiopia’s restricted access to therapeutic treatment and the underuse of antibiotics in veterinary medicine. The development of S. aureus biofilms in cow mastitis is likewise associated with a reduction in antibiotic sensitivity. This is explained by a decrease in the metabolic activity of the bacteria within biofilms and a reduction in the amount of antibiotics that pass through the biofilm matrix.58 There are other circumstances as well, like sub-inhibitory antibiotic concentrations. Moreover, lactose and proteases found in milk promote the growth of S. aureus biofilms inside the mammary gland of cows.59,60
The Amhara Region had the highest percentage of S. aureus infections (40.2%) among the study areas, while the SNNPRS region had the lowest (21%). This may have to do with the average annual relative humidity, the cleanliness of the farming system, and the prudent use of antibiotics.
Moreover, the production and consumption of raw milk and other dairy products often take place in unhygienic conditions in Ethiopia.61 Consequently, there is an extremely high chance of coming into contact with S. aureus as a result of consuming dairy products.62 Research conducted in Ethiopia has demonstrated that S. aureus is present in milk at several points throughout the milk value chain. This phenomenon may be explained by contamination from mastitis-affected cows, cross-infection of milk at collection sites from contaminated milk from infected farms, improper handling practices, and use of unclean equipment.63
Within the global context, this particular study found that the mean incidence of S. aureus-associated bovine mastitis (35.4%) was almost identical to the percentage found in a study conducted in China (36.23%) by Wang et al64 The estimated percentages for the US, Kenya, and Canada are as follows: 20.8–23.3% in the US, 20–22% in Canada, and 15.7% in Kenya.65 However, the result was lower than Elhaig and Selim’s claimed 56.7%, with66 in Nigeria.
During milking, S. aureus spreads among cows; thus, it is necessary to work together to minimize the spread of the infection to healthy animals. S. aureus isolates from cows are also a major source of foodborne illnesses, and raw milk and bulk tank products are crucial conduits for the spread of infection to people. Researchers in Ethiopia have shown that adult milkers between the ages of 30 and 40 had higher prevalence of S. aureus on their hands. These factors included inadequate udder cleaning, not washing hands before milking, using a shared towel, not dipping the teat after milking, routinely disinfecting the milking area, and lacking knowledge about the contagious nature of S. aureus and MRSA. Additionally, farms with semi-intensive management systems, inadequate barn drainage systems, and cows who had previously been exposed to mastitis showed greater prevalence of the organism.67
This systematic study revealed that, in relation to the degree of mastitis, the average occurrence of S. aureus infection was higher in subclinical mastitis (26%) than in clinical mastitis in cows (5%). As a result, our finding demonstrated that S. aureus infection has a significant economic impact on Ethiopia’s dairy industry, as subclinical mastitis is the main obstacle facing the industry in poor nations.68 Due to decreased milk quality and production, S. aureus is a very opportunistic infection that usually co-occurs with subclinical mastitis and results in large economic losses.69 It has been demonstrated that intramuscular infection by CC97 strains in nursing cows can lead to asymptomatic, subclinical, or chronic infections, making pathogen control in dairy herds more challenging.70
Despite significant variance in the overall rate of resistance among different types of antimicrobial drugs, S. aureus resistance to these agents poses a significant concern. According to the epidemiological estimate, 68% of Ethiopians were resistant to beta-lactam antibiotics overall. This rate is quite high, and may be caused by the production of beta-lactamase by S. aureus, which degrades the beta-lactam ring in the drug. Another possible reason could be a changed PBP with reduced affinity for most beta-lactam antibiotics.
S. aureus had a 75% beta-lactam resistance rate to penicillin, a 67% rate to amoxicillin, a 57% rate to cephalosporin, and a 50% rate to ampicillin. The penicillin resistance to S. aureus was the highest. This might be because penicillin is widely available and utilized. Even non-professionals (farmers, drug dealers, and animal owners) possess penicillin, which they self-administer without understanding the proper dosage or timing.71 Compared to other beta lactam medicines, S. aureus had the highest resistance to penicillin in the current investigation. As reported by Gianneechini et al,72 André et al,73 Turutoglu et al,74 Kalmus et al,75 Sakwinska et al,76 Peles et al,77 Jørgensen et al,78 Anderson et al,78 and Tenhagen et al,79 respectively, this percentage was higher than the values reported in Argentina (47.6%), Brazil (69.9%), Turkey (62%), Estonia (61%), France (30%), Hungary (30%), Germany (17%), the United States (10%), and Norway (6%), respectively. However, the percentage of resistance exhibited a lesser magnitude in comparison to the values that have been previously documented in, Tigray (90%),80 Addis Ababa (95.3%),81 Hawassa (100%),82 Iran (100%),83 India (74.7%)84 and 80% in Sweden.85
The results of the systematic review show that S. aureus had a 50% mean rate of ampicillin resistance. Our results, which are somewhat inferior to those of prior Ethiopian research on ampicillin resistance in Tigray (100%) and Hawassa (70.9%),86 it was shown that S. aureus had the lowest level of ampicillin resistance (50%), in this particular investigation. However, it has been noted that ampicillin resistance may increase to 76.1–89.7% in Japan.87 The majority of S. aureus isolates from bovine mastitis in Nepal showed total resistance (100%) to ampicillin (73.2%), whereas in Uganda (73.2%), India (74.42%), and Nepal (20.5%) showed resistance to ampicillin.88
In order to prevent antibiotic resistance and minimize the use of antibiotics, society must be empowered through education. The aforementioned therapies are required under the current standard guidelines for the use of antibiotics. The objective areas also include continuing professional development and pre- and in-service education for health professionals. The public and society have been empowered through the dissemination of mass media information regarding the usage of antibiotics and resistance. The system’s ability to manage the threats that AMR has introduced is still lacking.
Thus, continuous intervention for scaling up is necessary for both AMR containment and prevention. Empowerment and education remain crucial strategies for stopping and containing MRSA. MRSA containment and prevention require a coordinated and integrated approach at the global, institutional, national, and individual levels. A comprehensive, evidence-based response approach is necessary due to the multidimensional character of MRSA, which encompasses biological, behavioral, technical, economic, regulatory, and educational components. Preventive and control measures must be put into place in healthcare facilities as well as the general public in order to lessen the danger of infection and the need for antibiotics. This will help to stop the emergence of resistant strains, which need to be quickly found and contained to stop them from spreading to other areas. In order to effectively address the challenges posed by MRSA, foster stewardship practices, and drive innovation, collaboration among all sectors is important.
Conclusion
The thorough investigation’s findings demonstrate the widespread distribution of S. aureus, with particularly high sub-group average prevalence in the Amhara and Tigray regions. Furthermore, a rise in S. aureus prevalence in recent years has been shown by sub-group calculation depending on study year. The incidence of S. aureus in subclinical mastitis in dairy cows is five times higher than in clinical mastitis. In general, S. aureus exhibits a high rate of beta lactamase resistance; however, in cases of mastitis in local breed cattle, the rate of resistance to Penicillin is highest. The relevance for the economy and public health is shown by the prevalence of S. aureus in subclinical mastitis. Veterinarians, medical professionals, veterinary pharmacists, farm laborers, milkers, and consumers of raw milk must thus make it a top priority to control and prevent S. aureus in bovine mastitis while also lowering the incidence of beta-lactam resistance.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We would like to acknowledge these authors in the included studies in this systematic review.
Author Contributions
Whether it was through ideation, study design, execution, data acquisition, analysis, and interpretation, or all of these areas combined, all authors made a significant contribution to the work reported. They also agreed on the journal to which the article was submitted, helped draft, revise, or critically review the article, and approved the final version that was published.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Abebe R, Hatiya H, Abera M, Megersa B, Asmare K. Bovine mastitis: prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Vet Res. 2016;12:1–11. doi:10.1186/s12917-016-0905-3
2. Bardhan I, Lin S. Research note—business value of information technology: testing the interaction effect of IT and R&D on Tobin’s Q. Inform Syst Res. 2013;24(4):1147–1161.
3. Seegers H, Fourichon C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003;34(5):475–491. doi:10.1051/vetres:2003027
4. Mungube EO, Tenhagen B, Regassa F, Kyule MN, Shiferaw Y. Reduced milk production in udder quarters with subclinical mastitis and associated economic losses in crossbred dairy cows in Ethiopia. Trop Ani Health Produ. 2005;37(6):503–512. doi:10.1007/s11250-005-7049-y
5. Birhanu M, Leta S, Mamo G, Tesfaye S. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu. BMC Res Notes. 2017;10(1):1–6. doi:10.1186/s13104-016-2345-3
6. Cervinkova D, Vlkova H, Borodacova I, et al. Prevalence of mastitis pathogens in milk from clinically healthy cows. Ann Int Confer IEEE Eng Med Biol Soc. 2013;2013(11):567–575. doi:10.1109/EMBC.2013.6609563
7. Bradley AJ Bovine Mastitis: an Evolving Disease; 2002;116–128.
8. Al-majali AM, Al-qudah KM Risk factors associated with camel brucellosis in Jordan; 2008;193–200.
9. Holmes A, Ganner M, Mcguane S, et al. Staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and wales: frequency, characterization, and association with clinical disease staphylococcus aureus isolates carrying panton-valentine leucocidin genes in England and wales: frequency, characterization, and association with clinical disease; 2005.
10. Novick RP. MicroReview Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mole Microbiol. 2003;48(6):1429–1449. doi:10.1046/j.1365-2958.2003.03526.x
11. Cheung AL, Bayer AS, Zhang G, Gresham H, Xiong Y-Q. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol Med Microbiol. 2004;40(1):1–9. doi:10.1016/S0928-8244(03)00309-2
12. Hoekstra J, Zomer AL, Rutten VPMG, et al. Genomic analysis of European bovine Staphylococcus aureus from clinical versus subclinical mastitis. Sci Rep. 2020;10(1):1–11. doi:10.1038/s41598-019-56847-4
13. Hoekstra J, Rutten V, Sommeling L, et al. High Production of LukMF in Staphylococcus aureus field strains is associated with clinical bovine mastitis.:1–10.
14. Rantala L, Pyo S, Rantala L, Pyörälä S. Virulence genes of bovine Staphylococcus aureus from persistent and nonpersistent intramammary infections with different clinical characteristics. J Appl Microbiol. 2007;103(4):993–1000. doi:10.1111/j.1365-2672.2007.03356.x
15. Vanderhaeghen W, Hermans K, Haesebrouck F, Butaye P. Methicillin-resistant Staphylococcus aureus (MRSA) in food production animals. Epidemiol Infect. 2010;138(5):606–625. doi:10.1017/S0950268809991567
16. Unit RH, Place N, Sciences D A mathematical model of Staphylococcus aureus control in. 2002;397–416.
17. Zadoks RN, Allore HG, Hagenaars TJ, Barkema HW, Schukken YH. A mathematical model of Staphylococcus aureus control in dairy herds. Epidemiol Infect. 2002;129(2):397–416. doi:10.1017/S0950268802007483
18. Pal M, Kerorsa GB, Marami LM. Epidemiology, pathogenicity, animal infections, antibiotic resistance, public health significance, and economic impact of staphylococcus aureus: a comprehensive review. Am J Public Health Res. 2020;8(1):14–21.
19. Balemi A, Gumi B, Amenu K, Girma S, Gebru M, Tekle M. Prevalence of mastitis and antibiotic resistance of bacterial isolates from CMT positive milk samples obtained from dairy cows, camels, and goats in two pastoral Districts in Southern Ethiopia. Animals. 2021;11(6):1530. doi:10.3390/ani11061530
20. Mesele A, Belay E, Kassaye A, Yifat D, Kebede A, Desie S. Major causes of mastitis and associated risk factors in smallholder dairy cows in Shashemene, southern Ethiopia. J Agri Res. 2012;7(24):3513–3518.
21. Abebe R, Abera M, Denbarga Y, et al. Prevalence, risk factors and bacterial causes of bo- vine mastitis in southern Ethiopia. Ethiop Veter J. 2020;24(1):52–68. doi:10.4314/evj.v24i1.4
22. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021:
23. Girma S, Teshale S, Tadesse F, Beyene TJ. Study on prevalence of bovine mastitis and its major causative agents in West Hararghe zone, Doba district, Ethiopia. J Vet Med Anim Health. 2012;87(3):583–592. doi:10.3168/jds.S0022-0302(04)732002
24. Hailemelekot M. Clinical and subclinical bovine mastitis and associated risk factors in small-scale dairy farms in Bahir Dar and its envirion, Amhara region. 2021;1–16.
25. Mcdougall S, Agnew KE, Cursons R, Hou XX, Compton CRW. Parenteral treatment of clinical mastitis with tylosin base or penethamate hydriodide in dairy cattle. J Dairy Sci. 2007;90(2):779–789. doi:10.3168/jds.S0022-0302(07)
26. Mekibib B, Furgasa M, Abunna F, Megersa B, Regassa A. Clinical and subclinical bovine mastitis and associated risk factors in small-scale dairy farms in Bahir Dar and its envirion, Amhara region. Vet World. 2010;3(9):397–403. doi:10.5455/vetworld.2010.397-403
27. Tesfaye K, Gizaw Z, Haile AF. Prevalence of mastitis and phenotypic characterization of methicillin-resistant staphylococcus aureus in lactating dairy cows of selected dairy Farms in and Around AdamaTown, Central Ethiopia. Environ Health Insight. 2021;15:11786302211021297. doi:10.1177/11786302211021297
28. Wubshet AK, Tesema TS, Gebru M, Derib BT, Haile AF, Wedeabyezgi HA. Incidence of heifer mastitis and identification of major associated pathogens in dairy farms at wolaita soddo town, southern Ethiopia. J Dairy Vet Anim Res. 2017;5(5):169–176. doi:10.15406/jdvar.2017.05.00156
29. Yohannes K, Alemu B. Prevalence of bovine mastitis in lactating cows and associated risk factors in and around WolaytaSoddo, Southern Ethiopia. Int J Adv Res Biol Sci. 2018;5(12):60–69.
30. Zeryehun T, Abera G. Prevalence and bacterial isolates of mastitis in dairy farms in selected districts of eastern harrargheZone, Eastern Ethiopia. J Vet Med. 2017;2017:3. doi:10.1155/2017/6498618
31. Tekle Y, Berihe T. Bovine mastitis: prevalence, risk factors and major pathogens in the Sidamo Zone SNNPRS, Ethiopia. Euro J Biol Med Sci Res. 2016;4(5):27–43.
32. Bitew M, Tafere A, Tolosa T. Study on bovine mastitis in dairy farms of Bahir Dar and its environs. J Anim Vet Adv. 2010;9(23):2912–2917. doi:10.3923/javaa.2010.2912.2917
33. Fesseha H, Mathewos M, Aliye S, Wolde A. Study on prevalence of bovine mastitis and associated risk factors in dairy farms of Modjo town and suburbs, central Oromia, Ethiopia. Veter Med. 2021;8:271–283.
34. Duguma A, Tolosa T, Yohannes A. Prevalence of clinical and sub-clinical mastitis on cross bred dairy cows at Holleta Agricultural Research Center, Central Ethiopia. J Vet Med Anim Health. 2014;6(1):13–17. doi:10.5897/JVMAH2013.0259
35. Seid U, Zenebe T, Almaw G, et al. Prevalence, risk factors and major bacterial causes of Bovine mastitis in West Arsi Zone of Oromia Region, Southern Ethiopia. Nat Sci. 2015;13(8):19–27.
36. Mekonnen H, Tesfaye A. Prevalence and etiology of mastitis and related management factors in market oriented smallholder dairy farms in Adama, Ethiopia. Revue de Med Veterina. 2010;161:574–579.
37. Abera M, Belay E, Kassaye A, Yifat D, Kebede A, Desie S. Major causes of mastitis and associated risk factors in smallholder dairy cows in Shashemene, southern Ethiopia. Afr J Agric Res. 2012;7(24):3513–3518.
38. Belayneh R, Belihu K, Wubete A. Dairy cow’s mastitis survey in Adama town, Ethiopia. J Vet Med Anim Health. 2013;5(10):281–287.
39. Adane B, Bayissa B, Tuffa S, Tola T, Mekonnen S. Participatory impact assessment of ticks on cattle milk production in pastoral and agro-pastoral production systems of Borana Zone, Oromia Regional State, Southern Ethiopia. Ethiop Veter J. 2012;16(1):1–3. doi:10.4314/evj.v16i1.1
40. Bihon A, Syoum A, Assefa A. Assessment of risk factors and isolation of Staphylococcus aureus and Escherichia coli from bovine subclinical mastitic milk in and around Gondar, Northwest Ethiopia. Trop Anim Health Prod. 2019;1(51):939–948. doi:10.1007/s11250-018-1777-2
41. Abebe R, Abera M, Denbarga Y, et al. Prevalence, risk factors and bacterial causes of bovine mastitis in southern Ethiopia. Ethiop Veter J. 2020;24(1):1.
42. Zenebe N, Habtamu T, Endale B. Study on bovine mastitis and associated risk factors in Adigrat, Northern Ethiopia. Afr J Microbiol Res. 2014;8(4):327–331. doi:10.5897/AJMR2013.6483
43. Haftu R, Taddele H, Gugsa G, Kalayou S. Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop Anim Health Prod. 2012;44(7):1765–1771. doi:10.1007/s11250-012-0135-z
44. Roberson JR, Warnick LD, Moore G. Mild to moderate clinical mastitis: efficacy of intramammary amoxicillin, frequent milk-out, a combined intramammary amoxicillin, and frequent milk-out treatment versus no treatment. J Dairy Sci. 2004;87(3):583–592. doi:10.3168/jds.S0022-0302(04)73200-2
45. Schukken YH, Sampimon OC, Barkema HW, Schukken YH. Factors associated with cure after therapy of clinical mastitis caused by staphylococcus aureus. J Dairy Sci. 2000;83(2):278–284. doi:10.3168/jds.S0022-0302(00)74875-2
46. Taponen BS, Jantunen A, Pyörälä E, Pyörälä S. Efficacy of targeted 5-day combined parenteral and intramammary treatment of clinical mastitis caused by penicillin-susceptible or penicillin- resistant staphylococcus aureus. Acta Veterin Scandina. 2003;44(1):53–62. doi:10.1186/1751-0147-44-53
47. Bradley AJ, Green MJ. Factors affecting cure when treating bovine clinical mastitis with cephalosporin-based intramammary preparations. J Dairy Sci. 2009;92(5):1941–1953. doi:10.3168/jds.2008-1497
48. Ayana HW, Mekonnen BT, Bulle AS, Berecha MS. Isolation and identification of methicillin-resistantStaphlococcus aureus from mastitic dairy cows in Bishoftu town, Ethiopia. J Microbiol Res. 2017;11(44):1606–1613.
49. Tassew A, Negash M, Demeke A, Feleke A, Tesfaye B, Sisay T. Isolation, identification and drug resistance patterns of methicillin resistant Staphylococcus aureus from mastitic cows milk from selected dairy farms in and around Kombolcha, Ethiopia. J Vet Med Anim Health. 2016;8(1):1. doi:10.5897/JVMAH2015.0422
50. Gebremedhin EZ, Ararso AB, Borana BM, et al. Isolation and identification of Staphylococcus aureus from milk and milk products, associated factors for contamination, and their antibiogram in Holeta, Central Ethiopia. Vet Med Int. 2022;6:202.
51. Reta MA, Bereda TW, Alemu AN. Bacterial contaminations of raw cow’s milk consumed at Jigjiga City of Somali Regional State, Eastern Ethiopia. Int J Food Contam. 2016;3(1):1–9. doi:10.1186/s40550-016-0027-5
52. Getahun K, Kelay B, Bekana M, Lobago F. Bovine mastitis and antibiotic resistance patterns in Selalle smallholder dairy farms, central Ethiopia. Trop Anim Health Prod. 2008;40(4):261–268. doi:10.1007/s11250-007-9090-5
53. Sori T, Hussien J, Bitew M. Prevalence and susceptibility assay of Staphylococcus aureus isolated from bovine mastitis in dairy farms of Jimma town, South WestEthiopia. J Anim Vet Adv. 2011;10(6):745–749. doi:10.3923/javaa.2011.745.749
54. Moges N, Asfaw Y, Belihu K, Tadesse A. Aantimicrobial susceptibility of mastitis pathogens from smallholder dairy herds in and around Gondar, Ethiopia. J Anim Vet Adv. 2011;10(12):1616–1622. doi:10.3923/javaa.2011.1616.1622
55. Fentaw S, Getahun M, Hussein M, et al. Microbial aetiology of gastroenteritis, antimicrobial resistance and associated factors among under five children in Addis Ababa, Ethiopia. Ethiop J Public Health Nutri. 2020;3:1. Nov 12.
56. Elemo KK, Sisay T, Shiferaw A, Fato MA. Prevalence, risk factors and multidrug resistance profile of Staphylococcus aureus isolated from bovine mastitis in selected dairy farms in and around Asella town, Arsi Zone, South EasternEthiopia.African. J Microbiol Res. 2017;11(45):1632–1642.
57. Overvliet MV. Antibiotic susceptibility of Staphylococcus aureus of bovine milk samples in Gondar and Bahir Dar region, Ethiopia; 2016.
58. Ster C, Lebeau V, Leclerc J, Fugère A, Veh KA, Roy JP. In vitro antibiotic susceptibility and biofilm production of Staphylococcus aureus isolates recovered from bovine intramammary infections that persisted or not following extended therapies with cephapirin, pirlimycin or ceftiofur. Vet Res. 2017;3:1–10.
59. Melchior MB, Graat RM, Duijkeren E, Mevius DJ. Biofilm formation and genotyping of Staphylococcus aureus bovine mastitis isolates: evidence for lack of penicillin-resistance in Agr -type II strains. Vet Microbiol. 2009;137(1–2):83–89. doi:10.1016/j.vetmic.2008.12.004
60. Fabres-klein MH, Junior M, Santos C, Klein RC, de Oliveira Barros Ribon A. An association between milk and slime increases biofilm production by bovine Staphylococcus aureus. BMC Vet Res. 2015;11(1):1–8. doi:10.1186/s12917-015-0319-7
61. Wubete A Bacteriological quality of bovine milk in small holder dairy farms in Debre Zeit, Ethiopia AAU.FVM.Msc Thesis. 2004.
62. Yilma Z, Guernebleich E, Sebsibe A A Review of the Ethiopian Dairy Sector. Rudolf Fombad. Addis Ababa: Food and Agriculture Organization of the United Nations, Sub Regional Office for Eastern Africa (FAO/SFE); 2011. Available from: http://www.fao.org/3/a-aq291e.pdf.
63. Desissa F Quantitative risk assessment of consuming milk contaminated with Staphylococcus aureus in Debre-Zeit. AAU, FVM,msc thesis; 2010.
64. Levison LJ, Miller-Cushon EK, Tucker AL, et al. Incidence rate of pathogen-specific clinical mastitis on conventional and organic Canadian dairy farms. J Dairy Sci. 2016;99(2):1341–1350. doi:10.3168/jds.2015-9809
65. Mbindyo CM, Gitao GC, Mulei CM. Prevalence, etiology, and risk factors of mastitis in dairy cattle in Embu and Kajiado Counties, Kenya. Vet Med Int. 2020;4:202.
66. Elhaig MM, Selim A. Molecular and bacteriological investigation of subclinical mastitis caused by Staphylococcus aureus and Streptococcus agalactiae in domestic bovids from Ismailia, Egypt. Trop Anim Health Prod. 2015;47(2):271–276. doi:10.1007/s11250-014-0715-1
67. Tibebu L, Belete Y, Tigabu E, Tsegaye W. Prevalence of Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus and Potential Risk Factors in Selected Dairy Farms at the Interface of Animal and Human in Bishoftu, Ethiopia. Vet Med. 2021;12:241–251. doi:10.2147/VMRR.S331968
68. Hagoss YT. Bovine Mastitis: prevalence, Risk Factors and Major Pathogens in Sidama Zone. 2020.
69. Gonçalves JL, Kamphuis C, Martins CMMR, Barreiro JR, Tomazi T, Gameiro AH. Bovine subclinical mastitis reduces milk yield and economic return. Livest Sci. 2018;210:25–32. doi:10.1016/j.livsci.2018.01.016
70. Hata E, Kobayashi H, Nakajima H, Shimizu Y, Eguchi M Epidemiological Analysis of Staphylococcus aureus Isolated from Cows and the Environment of a Dairy Farm in Japan. 2010.
71. Ismael A. Epidemiology of bovine mastitis in Ethiopia. J Veter Med Health. 2018;2(1):1–779.
72. Gianneechini RE, Concha C, Franklin A. Antimicrobial susceptibility of udder pathogens isolated from dairy herds in the west littoral region of Uruguay. Acta Veterin Scandina. 2002;43(1):1–10. doi:10.1186/1751-0147-43-1
73. André MC, Campos MR, Borges LJ, Kipnis A, Pimenta FC, Serafini AB. Comparison of Staphylococcus aureus isolates from food handlers, raw bovine milk and Minas Frescal cheese by antibiogram and pulsed-field gel electrophoresis following SmaI digestion. Food Control. 2008;19(2):200–7007. doi:10.1016/j.foodcont.2007.03.010
74. Turutoglu HU, Ercelik S, Ozturk D. Antibiotic resistance of Staphylococcus aureus and coagulase-negative staphylococci isolated from bovine mastitis. Bulletin Veter Inst InPul. 2006;50(1):41.
75. Kalmus P, Aasmäe B, Kärssin A, Orro T, Kask K. Udder pathogens and their resistance to antimicrobial agents in dairy cows in Estonia. ActaVeterinariaScandinavica. 2011;53:1–7.
76. Sakwinska O, Morisset D, Madec JY, Waldvogel A, Moreillon P, Haenni M. Link between genotype and antimicrobial resistance in bovine mastitis-related Staphylococcus aureus strains, determined by comparing Swiss and French isolates from the Rhone Valley. Appl Environ Microbiol. 2011;77(10):3428–3432. doi:10.1128/AEM.02468-10
77. Peles F, Wagner M, Varga L, et al. Characterization of Staphylococcus aureus strains isolated from bovine milk in Hungary. Int J Food Microbiol. 2007;118(2):186–193. doi:10.1016/j.ijfoodmicro.2007.07.010
78. Jørgensen HJ, Mørk T, Caugant DA, Kearns A, Rørvik LM. Genetic variation among Staphylococcus aureus strains from Norwegian bulk milk. Appl Environ Microbiol. 2005;71(12):8352–8361. doi:10.1128/AEM.71.12.8352-8361.2005
79. Anderson KL, Lyman RL, Bodeis-Jones SM, White DG. Genetic diversity and antimicrobial susceptibility profiles among mastitis-causing Staphylococcus aureus isolated from bovine milk samples. Am J Vet Res. 2006;67(7):1185–1191. doi:10.2460/ajvr.67.7.1185
80. Kalayu AA, Woldetsadik DA, Woldeamanuel Y, Wang S-H, Gebreyes WA, Teferi T. Burden and antimicrobial resistance of S. aureus in dairy farms in Mekelle, Northern Ethiopia. BMC Vet Res. 2020;16(1):20. doi:10.1186/s12917-020-2235-8
81. Beyene T, Hayishe H, Gizaw F, et al. Prevalence and antimicrobial resistance profile of Staphylococcus in dairy farms, abattoir and humans in Addis Ababa, Ethiopia. BMC Res Notes. 2017;10(1):171. doi:10.1186/s13104-017-2487
82. Daka D, G/silassie S, Yihdego D. Antibiotic-resistance Staphylococcus aureus isolated from cow’s milk in the Hawassa area, South Ethiopia. Ann Clin Microbiol Antimicrob. 2012;11(1):26. doi:10.1186/1476-0711-11-2630
83. Massawe HF, Mdegela RH, Kurwijila LR. Antibiotic resistance of Staphylococcus aureus isolates from milk produced by smallholder dairy farmers in Mbeya Region, Tanzania. Int J Health. 2019;5(5):31–37.
84. Sudhanthiramani S, Swetha CS, Bharathy S, Veterinary SV. Prevalence of antibiotic resistant Staphylococcus aureus from raw milk samples collected from the local vendors in the region of Tirupathi, India. Vet World. 2015;8(4):478–481. doi:10.14202/vetworld.2015.478-481
85. Landin H. Treatment of mastitis in Swedish dairy production (in Swedish with English summary). Sven Veterinar. 2006;58:19–25.
86. Girmay W, Gugsa G, Taddele H, et al. Isolation and identification of methicillin-resistant Staphylococcus aureus (MRSA) from milk in Shire dairy farms, Tigray, Ethiopia. Vet Med Int. 2020;2020:e8833973. doi:10.1155/2020/8833973
87. Thongratsakul S, Usui M, Higuchi H, et al. Prevalence and characterization of Staphylococcus aureus isolated in raw milk from cows in Hokkaido, Japan. Trop Anim Health Prod. 2020;52(4):1631–1637. doi:10.1007/s11250-019-02169-6
88. Annamanedi M, Sheela P, Sundareshan S, et al. Molecular fingerprinting of bovine mastitis-associatedStaphylococcusaureusisolates from India. Sci Rep. 2021;11(1):15228. doi:10.1038/s41598-021-94760-x
89. Asiimwe BB, Baldan R, Trovato A, Cirillo DM. Prevalence and molecular characteristics of Staphylococcus aureus, including methicillin resistant strains, isolated from bulk can milk and raw milk products in pastoral communities of South-West Uganda. BMC Infect Dis. 2017;17(1):422. doi:10.1186/s12879-017-2524-4
90. Mullen KA, Sparks LG, Lyman RL, Washburn SP, Anderson KL. Comparisons of milk quality on North Carolina organic and conventional dairies. J Dairy Sci. 2013;96(10):6753–6762. doi:10.3168/jds.2012-651
91. Shrestha A, Bhattarai RK, Luitel H, Karki S, Basnet HB. Prevalence of methicillin-resistant Staphylococcus aureus and pattern of antimicrobial resistance in mastitis milk of cattle in Chitwan, Nepal. BMC Vet Res. 2021;17(1):239. doi:10.1186/s12917-021-02942-6
92. Wang K, Cha J, Liu K, et al. The prevalence of bovine mastitis-associated Staphylococcus aureus in China and its antimicrobial resistance rate: a meta-analysis. Front Veter Sci. 2022;2:9.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
