Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Reactive Arthritis Triggered by Adalimumab and Leflunomide in a Patient with Ankylosing Spondylitis
Authors Liu Y, He J , Jiang J, Wang Y, Liu T
Received 28 September 2022
Accepted for publication 24 November 2022
Published 2 December 2022 Volume 2022:15 Pages 2601—2605
DOI https://doi.org/10.2147/CCID.S390918
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ying Liu, Jia He, Jingjing Jiang, Yujuan Wang, Ting Liu
Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, Nanchong, People’s Republic of China
Correspondence: Ting Liu, Department of Dermatology, Affiliated Hospital of North Sichuan Medical College, No. 1 Maoyuan South Road, Shunqing District, Nanchong, Sichuan Province, 637000, People’s Republic of China, Tel +8615082780013, Email [email protected]
Abstract: Reactive arthritis (ReA) is uncommon. The present case is a Chinese man who has been treated with adalimumab and leflunomide to control ankylosing spondylitis (AS). During the treatment, the patient developed a range of symptoms, including fever, fatigue, pustular rash, suppurative urethritis, genital ulcers, oral ulcers, bilateral uveitis, heel pain and swelling and pain of the knee and ankle joints. The laboratory studies revealed the presence of HLA-B27, and urethral secretions were positive for Ureaplasma urealyticum. The patient was eventually diagnosed with ReA. The development of ReA may be related to the combination of adalimumab and leflunomide, which reduces immune function and triggers activation of potential U. urealyticum. The patient received 3 weeks of antibiotics, corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), resulting in a significant improvement. The dose of corticosteroids was gradually reduced, and adalimumab was reintroduced. The patient was followed up for 3 months without recurrence.
Keywords: reactive arthritis, Reiter syndrome, ankylosing spondylitis, urethritis, arthritis, uveitis, HLA-B27
Introduction
Reactive arthritis (ReA), also formerly known as Reiter syndrome (RS), is a systemic disease triggered by a bacterial infection, particularly of the genitourinary system (Chlamydia trachomatis and Ureaplasma urealyticum) or gastrointestinal tract (Salmonella, Shigella and Yersinia).1 The occurrence of ReA is thought to be genetically determined in the host response to a specific infection. The specific bacteria can reach the joints as a complete form or as fragments causing a sterile synovitis. Additionally, inheritance of the HLA-B27 allele is one of the risk factors for its occurrence, and the incidence of ReA in HLA-B27-positive individuals is approximately 50 times higher than that in healthy individuals.2 The present study retrospectively analyzed a case of ReA that occurred in a patient with HLA-B27-positive ankylosing spondylitis (AS) to determine the potential causes of ReA.
Case Report
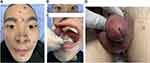
This study presents a 28-year-old male patient who started developing lumbosacral pain and stiffness 4 years ago, and he gradually developed left shoulder joint pain and limited spinal movement and humpback deformity 1 year ago. He was diagnosed with AS 3 months ago and is currently taking adalimumab (40 mg every 2 weeks) and leflunomide (20 mg/d) to control his condition, which has significantly relieved his symptoms of AS. Five days after the four adalimumab injections, the patient began to develop a fever, fatigue and pustular rash followed by urethral purulent discharge with urinary pain, glans annular erythema and oral ulcers. He came to our hospital because the treatment with systemic antibiotics (cephalosporins and clindamycin) was ineffective. Seven days prior to the hospital visit, the patient’s condition was further aggravated by purulent discharge in both eyes with blurred vision, swelling and pain in the knee and ankle joints, heel pain and genital redness, swelling and ulceration. The patient had a history of high-risk sexual contact before 4 months. The clinical examination revealed the following symptoms: pustular rashes on the face (Figure 1A), back and perineum; bilateral uveitis (Figure 1B); swollen and painful knee and ankle joints; enthesopathy; Achilles tendonitis; erosions and ulcers on the buccal mucosa (Figure 1C); purulent urethritis accompanied by genital redness, swelling and ulceration (Figure 1D). Laboratory studies showed elevated C-reactive protein (133.4 mg/L), increased erythrocyte sedimentation rate (46 mm/h), HLA-B27 antigen positivity, negative rheumatoid factor and negative antinuclear antibodies (ANAs). The urinalysis showed leukocytes, erythrocytes and protein. Microbiological examination of the pustular rash, oral ulcer surface, bilateral secretions, feces and blood samples were sterile. Urethral secretions were positive for U. urealyticum (PCR). The urethral secretions were positive for U. urealyticum (PCR) and negative for Neisseria gonorrhoeae (PCR), Chlamydia trachomatis (PCR) and Candida spp (culture). In addition, syphilis, HIV, hepatitis B virus and hepatitis C virus were negative. Total spine X-ray and bilateral sacroiliac joint computed tomography (CT) examination were consistent with AS. The chest CT, electrocardiography and echocardiography were normal. Both knee joint and ankle joint color Doppler ultrasound showed joint cavity effusion. The patient was eventually diagnosed with ReA and AS. Adalimumab and leflunomide were discontinued, and treatment with minocycline (200 mg/d), methylprednisolone (24 mg/d) and aceclofenac tablets (100 mg/d) was started. The local pustular rash was treated with fusidic acid cream. Uveitis in both eyes was treated with tobramycin dexamethasone eye drops and tropicamide eye drops. Povidone iodine gargle is used for the treatment of oral ulcers. Genital ulcers were washed with saline and fusidic acid cream for external use. The patient was discharged from the hospital (Figure 2A–D) after 3 weeks of treatment. After discharge, the patient continued to take minocycline and aceclofenac tablets, and the dosage of methylprednisolone was gradually reduced to zero followed by reintroduction of adalimumab. There was no recurrence in the patient’s condition after 3 months of follow-up.
 |
Figure 1 Clinical feature. (A) The pustular rash on the face. (B) Edema and congestion in both eyes. (C) Oral ulcer. (D) Purulent urethritis accompanied by genital redness, swelling and ulceration. |
Discussion
ReA is recognized as a syndrome of arthritis, genitourinary lesions, ocular lesions and mucocutaneous lesions, and it is a rare condition that is easily overlooked in the clinic due to the clinical polymorphism and multisystem involvement of the disease. ReA often has an acute onset with systemic manifestations, such as fever, fatigue and weight loss. Urethritis is the most common symptom of urogenital system and may be a manifestation of an underlying infection (U. urealyticum) or an extra-articular manifestation of reactive arthritis. Arthritis is characterized by acute asymmetrical oligoarthritis of the large joints of the lower extremities and is often accompanied by joint swelling and pain.3 Ocular damage is most common with conjunctivitis and uveitis, which occur in half of male patients with ReA caused by urinary tract infections.4 Conjunctivitis and uveitis can cause edema, congestion, pain and irritation or blurred vision; in severe cases, irreversible damage to vision can occur. Mucocutaneous lesions are varied, among which the most observed are keratoderma blennorrhagicum, circinate balanitis and oral ulcers.5 Other symptoms include erythema, pustular rash, ulcerative vulvitis, onycholysis and pitting.3,6 It has been reported that skin manifestations can mimic pustular psoriasis, atopic dermatitis, Behcet’s disease and contact dermatitis.7
The present case was a 28-year-old man with HLAB27-positive AS. The patient was treated with adalimumab and leflunomide, which significantly improved his articular manifestations. During the treatment, U. urealyticum infection was confirmed due to bacterial examination of urethral purulent discharge. Moreover, the patient developed a pustular rash, acute suppurative urethritis, oral ulcers, genital ulcers, peripheral arthritis and bilateral uveitis leading to the diagnosis of ReA. Although these symptoms can also occur in AS, it is less likely that AS is active after the use of adalimumab. Therefore, we believe that our case is a mix of AS, reactive arthritis, and sexually transmitted disease. The present case was also differentiated from Behcet’s disease. Behcet’s syndrome is characterized by iridocyclitis in the eye, aphthous stomatitis in the mouth and hard and painful ulcers in the genitals, but urethritis is generally not present in Behcet’s syndrome.
ReA and AS are members of seronegative spondyloarthritis, and the cause of their concurrent occurrence is not clear and may be related to the common mechanism of immune damage. Some studies have reported that ReA can progress to chronic arthritis, presenting as AS, especially in patients with HLA-B27 positivity.3,8,9 In the present case, the patient developed AS first and was complicated with acute manifestations of ReA during treatment, which is rare in the clinic. Only one similar case has been previously reported, in which the occurrence of ReA is thought to be related to the patient’s long-term use of adalimumab and leflunomide, which induced latent infection.10 As a TNF-α antagonist, adalimumab is widely used in the treatment of rheumatoid arthritis, AS, psoriasis, Crohn’s disease and other autoimmune diseases through specific and targeted antagonism of TNF-α. However, adalimumab also inhibits the normal physiological functions that require TNF-α involvement, especially anti-infection immunity. It has been reported that TNF-α antagonism may increase susceptibility to new infections and activation of potential infections.10 In the present case, the combination of adalimumab and immunosuppressive agent (leflunomide) had an additive effect on the immune function of the body, which may have been an important factor in inducing U. urealyticum infection. Additionally, it has been reported that TNF-α antagonists can also cause a variety of skin adverse reactions, such as psoriasis and psoriasiform rash, autoimmune skin diseases, vasculitis, granulomatous reaction, and skin infection.12 However, we do not believe that our patient developed a pustular rash directly related to the TNF-α antagonist because the rash did not recur after readministration of adalimumab.
For management of ReA caused by urinary tract infection, antibiotics are recommended.8 Given that U. urealyticum can be transmitted through sexual contact, antibiotic therapy is recommended for patients and their partners. NSAIDs are still the most commonly used drug to reduce joint symptoms, and systemic glucocorticoids are used in patients with poor response to NSAIDs or multiple joint involvement.3 Furthermore, anti-rheumatic drugs should be considered if there is a poor response to the above measures or in chronic forms of ReA.3,8,13 The present case responded well to antibiotics, corticosteroids and NSAIDs, and adalimumab was reintroduced to this patient. With this treatment regimen, no disease recurrence in this patient was observed during 3 months of follow-up.
Conclusion
We observed one patient with AS who developed ReA after infection, possibly triggered by concomitant immunosuppression. With the widespread use of TNF-α antagonists and immunosuppressants in patients with AS, more attention should be focused on the risk of infection, and additional measures should be taken to detect and treat occult infections in these patients.
Consent Statement
The images and patient data have been published with the consent of the patient. The need for ethics committee approval was waived, as this is a case report.
Acknowledgments
The authors are grateful to the patient and would also like to thank Professor Zhengzhong Zhang for his guidance regarding the diagnosis and treatment of the disease.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. García-Kutzbach A, Chacón-Súchite J, García-Ferrer H, et al. Reactive arthritis: update 2018. Clin Rheumatol. 2018;37(4):869–874. doi:10.1007/s10067-018-4022-5
2. Pennisi M, Perdue J, Roulston T, et al. An overview of reactive arthritis. JAAPA. 2019;32(7):25–28. doi:10.1097/01.JAA.0000558320.47868.2f
3. Jubber A, Moorthy A. Reactive arthritis: a clinical review. J R Coll Physicians Edinb. 2021;51(3):288–297. doi:10.4997/jrcpe.2021.319
4. Selmi C, Gershwin ME. Diagnosis and classification of reactive arthritis. Autoimmun Rev. 2014;13(4–5):546–549. doi:10.1016/j.autrev.2014.01.005
5. Bentaleb I, Abdelghani KB, Rostom S, et al. Reactive arthritis: update. Curr Clin Microbiol Rep. 2020;7(4):124–132. doi:10.1007/s40588-020-00152-6
6. Chudomirova K, Abadjieva TS, Yankova R. Clinical tetrad of arthritis, urethritis, conjunctivitis, and mucocutaneous lesions (HLA-B27-associated spondyloarthropathy, Reiter syndrome): report of a case. Dermatol Online J. 2008;14(12):4.
7. Wu IB, Schwartz RA. Reiter’s syndrome: the classic triad and more. J Am Acad Dermatol. 2008;59(1):113–121. doi:10.1016/j.jaad.2008.02.047
8. Schmitt SK. Reactive arthritis. Infect Dis Clin North Am. 2017;31(2):265–277. doi:10.1016/j.idc.2017.01.002
9. Wendling D, Prati C, Chouk M, et al. Reactive arthritis: treatment challenges and future perspectives. Curr Rheumatol Rep. 2020;22(7):29. doi:10.1007/s11926-020-00904-9
10. Thielen AM, Barde C, Janer V, et al. Reiter syndrome triggered by Adalimumab (Humira) and leflunomide (Arava) in a patient with ankylosing spondyloarthropathy and Crohn disease. Br J Dermatol. 2007;156(1):188–189. doi:10.1111/j.1365-2133.2006.07586.x
11. Ma Z, Liu X, Xu X, et al. Safety of tumor necrosis factor-alpha inhibitors for treatment of ankylosing spondylitis: a meta-analysis. Medicine. 2017;96(25):e7145. doi:10.1097/MD.0000000000007145
12. Aikaterini-Evaggelia M, Matekovits A, Dessinioti C, et al. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009;61(3):486–504. doi:10.1016/j.jaad.2008.10.060
13. Lucchino B, Spinelli FR, Perricone C, et al. Reactive arthritis: current treatment challenges and future perspectives. Clin Exp Rheumato. 2019;37(6):1065–1076.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.