Back to Journals » Journal of Blood Medicine » Volume 14
Real-World Amount of Clotting Factor Concentrates Dispensed and Annual Medical Expenditures for Japanese Patients with Hemophilia B
Authors Fukutake K, Togo K , Xu L , Markson LE, Alvir JMJ, Winburn I, Karumori T
Received 16 May 2023
Accepted for publication 11 December 2023
Published 20 December 2023 Volume 2023:14 Pages 649—661
DOI https://doi.org/10.2147/JBM.S418818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Katsuyuki Fukutake,1,2 Kanae Togo,3 Linghua Xu,3 Leona E Markson,4 José Maria Jimenez Alvir,5 Ian Winburn,6 Toshiyuki Karumori3
1Laboratory Medicine, Tokyo Medical University, Shinjuku, Tokyo, Japan; 2Department of Blood Coagulation Diseases, Ogikubo Hospital, Suginami, Tokyo, Japan; 3Pfizer Japan Inc, Shibuya-ku, Tokyo, Japan; 4Pfizer Inc, Collegeville, PA, USA; 5Pfizer Inc, New York, NY, USA; 6Pfizer Ltd, Surrey, UK
Correspondence: Toshiyuki Karumori, Pfizer Japan Inc, Shinjuku Bunka Quint Bldg, 3-22-7, Yoyogi, Shibuya-ku, Tokyo, Japan, Tel +81-80-4152-4243, Email [email protected]
Introduction: Until extended half-life (EHL) factor IX (FIX) concentrates became available in Japan in 2010, patients with hemophilia B received intravenous FIX replacement therapy with standard half-life (SHL) FIX concentrates.
Purpose: To investigate the amount of factor dispensed and the associated medical expenditures for the treatment of hemophilia B in the real-world clinical setting in Japan.
Methods: This retrospective study comprised patients with hemophilia B (N=197) who had filled prescriptions for FIX concentrates reported in Japan’s Medical Data Vision database from 2015 to 2019. Patients were included if they had 2 or more prescriptions for the same FIX concentrates within the first 6 months of the study period and the interval between prescriptions was at least 2 weeks.
Results: Since 2015, there was a decrease in the proportion of patients using SHL FIX concentrates and a corresponding increase in international units of dispensed EHL FIX concentrates. Median annualized dispensed dosages (IU/kg body weight) of EHL FIX concentrates were lower than for SHL concentrates for outpatient use only. Annual total health care expenditures per patient and annual expenditures for prescribed FIX concentrates increased each year during the study period. Following a switch from an SHL to an EHL concentrate, the median amount of prescribed FIX concentrate decreased slightly, although median total health care expenditures and FIX concentrate expenditures increased.
Conclusion: In the real-world setting in Japan, medical expenditures and the proportion of patients prescribed EHL FIX concentrates for the treatment of hemophilia B have increased.
Keywords: blood coagulation factors, drug labeling, factor IX, health care administrative claims, health care costs
Introduction
Hemophilia B is an inherited clotting disorder caused by missing or defective factor IX (FIX), a serine protease of the coagulation cascade intrinsic pathway.1 The World Federation of Hemophilia found that in 2021 there were 1252 patients with hemophilia B in Japan, as reported by Japan Foundation for AIDS Prevention.2 The main treatment approach for these patients is intravenous FIX replacement therapy.
In Japan, in addition to the plasma-derived and recombinant FIX concentrates with a standard half-life (SHL), several extended half-life (EHL) concentrates have become available since 2010.3,4 Eftrenonacog alfa was launched in September 2014, followed by albutrepenonacog alfa in November 2016 and nonacog beta pegol in November 2018.5–7 The SHL concentrate nonacog gamma became available in May 2016. With the development of gene therapy and other novel nonfactor products for management of hemophilia B, it is likely that the treatment paradigm will diversify further.8,9
FIX concentrate dosing and administration regimens vary widely from patient to patient, are often based on the physician’s treatment decision, and are governed by the medication’s label (ie, whether the medicine is to be used prophylactically, on demand, for surgery, or for immune tolerance induction).3,10 Treatment with FIX concentrates is individually tailored according to the patient’s degree of bleeding tendency, level of physical activity, and pharmacokinetics, and therefore varies widely even when used according to the guidelines and label.10 In addition to hospital-based treatment, home therapy for hemophilia is permitted in Japan and is performed at the patient’s discretion.
In patients receiving regular prophylaxis, the use of EHL FIX concentrates can facilitate longer dosing intervals (up to 21 days) versus SHL FIX concentrates (single-dose administration every 3 days or twice per week in Japan).10–14 However, expenditures for the same dosage (in international units) of an EHL concentrate may exceed the expenditures for an SHL concentrate.15 Therefore, it is necessary to understand the actual usage of FIX concentrates in clinical practice and their associated expenditures. Given the need for long-term prophylaxis, expenditure is an important consideration in hemophilia care, and several studies have analyzed direct health care expenditures for different treatment regimens in several countries.3,16–18 Two analyses of the Truven Health MarketScan US pharmacy claims databases found that switching to an EHL concentrate was associated with increased median factor expenditures during the 12 months following the switch.15,19
Currently, no published evidence is available regarding real-world treatment patterns for the use of FIX concentrates and hemophilia B treatment expenditures in Japan. The current study analyzed real-world data from a Japanese hospital-based administrative database. The main objective was to investigate treatment of hemophilia B, including the amounts of FIX concentrate in international units (IU) that were dispensed and the medical expenditures for patients who had consistent prescription fills of FIX concentrates and were without factor inhibitors. The prevalence of the two major complications of hemophilia, bleeding during hospitalization and joint damage,20 was also analyzed.
Materials and Methods
Ethical Conduct
This study was conducted in accordance with legal and regulatory requirements in Japan, and included anonymized claims data, with no personal patient data. Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects do not apply to studies that use anonymized secondary data; consequently, this study was not reviewed by any institutional review board or ethics committee.
Study Design
This was a retrospective study of patients with hemophilia B included in the Medical Data Vision Co., Ltd (MDV) database, a hospital-based database containing inpatient and outpatient hospital data and prescription data collected after a hospital visit, along with health claims and laboratory data. As of May 2020, the MDV was the largest administrative database in Japan, with data from 413 facilities and more than 31 million patients, covering hospitals from all prefectures. The study period included data from January 1, 2015, through December 31, 2019. The index date was defined as the date of the first prescription fill of the FIX concentrate during the study period, with the disease name recorded as hemophilia B. The patients in the study population could have records pertaining to prescriptions for several FIX concentrates that met this inclusion criterion in the patient’s medical history. Treatment duration with each FIX concentrate was defined as the period from the first prescription fill of the FIX concentrate to the day before a switch to a different concentrate, or it was censored at the last prescription of the same concentrate if no switch occurred during the study period.
Study Population
Patients who met all of the following criteria were included: (1) evidence of visiting a health care facility in the MDV database during the study period; (2) diagnosis of hemophilia B (ICD-10 code D67, excluding hypothrombinemia) during the study period, excluding suspected diagnosis; and (3) ≥2 prescriptions of the same FIX concentrate within the first 6 months of the study period and ≥2 weeks apart from each other during the study period, excluding during hospitalization. Exclusion criteria included patients who (1) were diagnosed with a related disease name (“congenital hemophilia with FIX inhibitor”, Japanese claims code 845703) or (2) received bypassing agents (treatment for hemophilia B with inhibitors) after the index date.
Variables
Prescriptions for both SHL and EHL FIX concentrates were included in this study. For hemophilia B, SHL FIX concentrates have half-lives of approximately 18–40 hours.21 The half-life of EHL FIX concentrates is extended by up to five times compared with SHL concentrates.21 The following SHL concentrates were included: nonacog alfa, nonacog gamma, and plasma-derived FIX. The following EHL concentrates were included: eftrenonacog alfa, albutrepenonacog alfa, and nonacog beta pegol.
Variables included treatment duration, weight, joint damage, bleeding, and hospitalization. Although the treatment duration could be censored, the summary of treatment duration was used for supporting interpretation of the annualized FIX dosage, described below. Weight was calculated based on the statistical average weight of Japanese individuals in the specified age groups.22,23 Joint damage and bleeding during hospitalization were evaluated from inpatient records, regardless of whether joint damage or bleeding were the primary cause of hospitalization. A determination of joint damage and/or bleeding was based on diagnostic information in physicians recorded clinical assessments.
Outcome Measures
The percentage of patients prescribed each FIX concentrate and the annualized dosage (factor IU/kg body weight) of each FIX concentrate were analyzed in an outpatient setting. The prevalence of joint damage and bleeding, expenditures for prescribed FIX concentrates, and expenditures for total health care were measured for inpatients and outpatients.
Annualized dosage was based on reported FIX concentrate prescriptions and the statistical average weight of Japanese individuals in the specified age groups. Median annual expenditures for prescribed FIX concentrates and median annual total health care expenditures were summarized in patients receiving treatment with regular replacement therapy. For the calculation of annual expenditures, regular replacement therapy was defined as the use of FIX concentrates that met each of the following criteria: total treatment period of the FIX concentrates of ≥1 year; prescription of any FIX concentrate ≥2 times on different dates during the year, and no interval >6 months between prescriptions. Expenditures per FIX concentrate were derived from the Japanese drug price standard.24 Expenditures in USD were calculated using a conversion of 109 yen/USD.25
The subpopulation of patients (both inpatients and outpatients) for whom data were available for ≥3 months before and ≥3 months after switching from an SHL concentrate to an EHL concentrate (ie, the first occurrence of a switch to an EHL concentrate) and those who filled ≥2 prescriptions for an EHL concentrate were included in the analyses for switching. The IU/kg of FIX concentrate dispensed in the 4 quarters before the switch, as well as expenditures for prescribed FIX concentrates and total health care expenditures after the switch, were recorded.
Data Analysis
Data were summarized and analyzed for the overall study period and by year using descriptive statistics (patient and treatment setting characteristics; bleeding during hospitalization). All analyses were conducted for the age subgroups 0–11, 12–19, 20–64, and ≥65 years. The prevalence rate of joint damage, estimated with a 95% confidence interval, was calculated as the number of patients with joint damage during the study period divided by the total follow-up duration of all patients (per 100 patient-years). For the switching analysis, summary statistics for annualized FIX concentrate dosage dispensed in the 4 quarters (3 months, 4–6 months, 7–9 months, and 10–12 months) before and after switching were reported. Incomplete quarters were not included. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study Population
A total of 197 patients with hemophilia B (median age, 25 years; range: <1–87 years) with ≥2 prescriptions filled for FIX concentrates within the first 6 months of the study period at intervals of >2 weeks and no prescriptions for bypass products were included in the analysis (Figure 1). Patient age and comorbidities at baseline are presented in Table 1. Of note, at baseline, 41.6% of the patients had viral hepatitis and 38.6% had chronic viral hepatitis C after sustained virologic response by antiviral therapy. The average follow-up duration for each patient was 3.3 years (median [first quartile to third quartile], 34.5 [30.2–57.8] months). Patients visited university hospitals (44.7% of patients), local public hospitals (33.5%), national hospitals (18.3%), and private hospitals (3.6%), in that order. The majority (71.1%) of the hospitals had more than 500 beds.
 |
Table 1 Patient Age and Comorbidities |
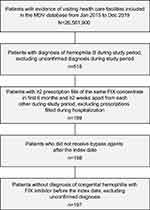 |
Figure 1 Study cohort selection. Abbreviations: FIX, factor IX; MDV, Medical Data Vision Co., Ltd. |
Percentage of Patients Prescribed Each FIX Concentrate in an Outpatient Setting
The percentage of patients for whom an SHL concentrate (ie, nonacog alfa, nonacog gamma, and plasma-derived FIX) was dispensed showed a continual decrease from 2015 onward, whereas the overall percentage of patients for whom an EHL concentrate (ie, eftrenonacog alfa, albutrepenonacog alfa, and nonacog beta pegol) was dispensed increased (Figure 2). The percentage of patients prescribed the EHL FIX concentrate albutrepenonacog alfa, launched in 2016, increased across all age categories over the analysis period (2015–2019) (Figure 2 and Supplementary Figures S1–S4).
 |
Figure 2 Outpatient use of FIX concentrates. Abbreviation: FIX, factor IX. |
Annualized Dosage for Each FIX Concentrate in an Outpatient Setting
Median annualized dosages (factor IU/kg body weight) of dispensed EHL FIX concentrates were lower (medians ranging from 1507.4 to 2447.4 IU/kg) than median annualized dosages of SHL FIX concentrates (medians ranging from 2092.9 to 3181.4 IU/kg) for outpatient use only (Table 2), both overall and according to age group (Supplementary Table S1). The treatment duration for each FIX concentrate varied.
 |
Table 2 Observed Treatment Duration and Annualized FIX Concentrate Dosage for Outpatients (All Ages) |
Prevalence of Joint Damage During the Study Period and Bleeding During Hospitalization
Overall, 58.9% of patients had documented joint damage (Table 3), which was highest (76.3%) for those aged 12 to 19 years (Supplementary Table S2). The most frequent type of joint damage was hemophilic arthritis, based on physicians’ recorded clinical assessment (Table 3). A total of 30 (15.2%) patients experienced a bleeding event while hospitalized (ie, hospitalization with bleeding when bleeding was or was not the primary cause of hospitalization) (Table 4).
 |
Table 3 Prevalence of Joint Damage in Diseases with a Frequency ≥1% Among the Study Population |
 |
Table 4 Bleeding During Hospitalization |
Expenditures for FIX Concentrate Treatment
Median annual total health care expenditures per patient with regular replacement therapy increased yearly during the study period, from ¥10,365,761 ($95,099 USD, conversion of 109 yen/USD)25 in 2015 to ¥28,714,530 ($263,436 USD) in 2019. Median annual expenditures for prescribed FIX concentrates per patient also increased, from ¥9,686,448 ($88,866 USD) to ¥28,484,282 ($261,324 USD) (Table 5). Median annual total health care and median annual prescribed FIX concentrate expenditures for each age category trended similarly (Supplementary Table S3). In those aged 20 to 64 years, total health care expenditures increased from ¥9,057,510 ($83,096 USD) in 2015 to ¥31,898,870 ($292,650 USD) in 2019. In the same age group, prescribed FIX concentrate expenditures increased from ¥8,673,434 ($79,573 USD) in 2015 to ¥30,718,086 ($281,817 USD) in 2019. The expenditures for prescribed FIX concentrates accounted for the majority of the annual total health care expenditures. The data indicate a trend for lower annual total health care expenditures for patients without versus with joint damage. Health care expenditures were not evaluated in the subgroups of patient with or without joint damage because of the small number of patients.
 |
Table 5 Medical Expenditures in Patients Receiving Regular Replacement FIX Therapy by Study Year |
Switch from SHL to EHL FIX Concentrate
The pattern of switching for patients who converted from an SHL to an EHL FIX concentrate is shown in Table 6. Overall, 25.9% (n=51/197) of patients switched from an SHL to an EHL concentrate during the study period, with the highest proportion of patients switching from nonacog alfa to either albutrepenonacog alfa (n=23/51 [45.1%]) or eftrenonacog alfa (n=14/51 [27.5%]). The median amount of prescribed FIX IU decreased slightly (2%) after the switch (Figure 3). However, median total health care (Figure 4) and FIX concentrate (Figure 5) expenditures increased shortly thereafter.
 |
Table 6 Switching from an SHL to an EHL FIX Concentrate |
 |
Figure 4 Median total health care expenditures before and after patients switched from an SHL to an EHL FIX concentrate. Abbreviations: EHL, extended half-life; SHL, standard half-life. |
 |
Figure 5 Median expenditures for prescribed FIX concentrates before and after patients switched from an SHL to an EHL concentrate. Abbreviations: EHL, extended half-life; SHL, standard half-life. |
Discussion
The results of this real-world study show that dispensed factor doses of EHL FIX concentrates are increasing in Japan, with an accompanying increase in health care expenditures that is likely to be associated with switching from an SHL to an EHL concentrate. The number of hospitals in the MDV system also increased in since 2017 (291 sites in 2015 to 384 sites in 2019). The increase in median expenditures observed in the current analysis was similar to findings from other studies in smaller populations of patients with hemophilia B in the United States.15,19 A US study conducted by Tortella et al found a 238% increase in median health care expenditures after patients switched from an SHL to an EHL concentrate.19 Although the increase in drug expenditures is likely reflective of the increased uptake in EHL concentrates, it may be counterbalanced by improved treatment outcomes, such as quality of life,16 the assessment of which was beyond the scope of this study.
The current study highlighted a trend that indicates health care expenditures are higher for patients with joint damage compared with those with no joint damage. However, given the small number of patients hospitalized due to bleeding events, the hospital-related contribution to annual health care expenditures may be minimal. Further studies are necessary to fully elucidate this finding.
The retrospective design of the current analysis precludes more in-depth analysis of potential expenditure savings for various stakeholders, such as reduction in hospitalizations and the consequent decrease in associated hospital-related expenditures. In addition, this study was not designed to account for potential indirect expenditures incurred for bleeding events not requiring hospitalization that were treated in the community setting. Home therapy is allowed in Japan and is performed at the patient’s discretion, even in the case of bleeding. Nevertheless, these data highlight the majority of medical expenditures for patients with hemophilia involve drug acquisition. In Japan, publicly funded financial support is available for patients with hemophilia, limiting out-of-pocket expenditures for patients. Although hemophilia B is generally associated with high expenditures in Japan,26 as has been observed in other settings, such as the United States,27 prophylaxis is considered the optimal care for treatment of hemophilia. As such, prophylaxis is the standard treatment approach to prevent bleeding events and preserve joint function.
In the current study, switching from an SHL to an EHL FIX concentrate was associated with a 2% decrease in the median number of FIX IU during the first 3 months after switching to an EHL concentrate, suggesting that more EHL FIX IU were dispensed than expected in the first 3 months after switching. Despite the minimal change in FIX IU dispensed in the first 3 months after switching, expenditures dramatically increased for health care (249% increase) and FIX concentrate (256% increase) during this period. In comparisons from 1 year before switching to 1 year after switching, a slight decrease in the median number of FIX IU occurred, although the median for health care and FIX concentrate expenditures still increased more than 2-fold. The drug price for eftrenonacog alfa, the first EHL concentrate in Japan, was 5% greater than the cost incurred if SHL concentrates were the standard routine prophylaxis regimen, and other EHL concentrates were priced accordingly. As a result, the drug price per IU of eftrenonacog alfa is approximately double the price of nonacog alfa.
When routine prophylaxis is implemented according to the package insert of each FIX concentrate and Japanese Society for Thrombosis and Hemostasis guidelines,14 the switch would be expected to reduce the dispensed FIX IU volume by approximately 50%, and the drug expenditure for EHL concentrates would be almost the same as that for SHL concentrates. However, the results of the current study show that the dispensed EHL FIX IU volume was approximately 70% to 80% that of the SHL FIX concentrate, with a corresponding approximate 2-fold increase in expenditures, in the year after switching. Given that drug pricing takes into account the extended half-life of EHL FIX concentrates, higher treatment expenditures associated with switching from SHL FIX concentrates suggest higher and/or more frequent dosing than originally assumed, whereas SHL FIX concentrate prescribing trends remain relatively constant. Given that home therapy is allowed in Japan, it is possible that more EHL FIX concentrate prescriptions are dispensed than are actually administered, with the intention of stocking them at home after the switch, leading to an increase in medical costs. However, it is unlikely that this would have a significant impact on medical expenditures after switching.
The significant increase in expenditures reported in this study highlights the difference between actual prescribing trends in clinical practice and use of the standard treatment regimen as recommended in guidelines and package inserts. Although the clinical outcomes of switching have not been studied, the increased expenditure for treatment suggests that the intensity of treatment for each patient has increased or that previously undertreated patients are adequately treated after the switch. The standard regimen of regular prophylaxis treatment is stated in package inserts for EHL concentrates,5–7 not SHL concentrates. This difference may have improved prophylaxis treatment in patients with hemophilia B as treatment goals continue to advance. It is also possible that adherence to prophylaxis may have improved after switching.28 These issues warrant further study. Understanding the impact on quality of life for patients who switch from an SHL to an EHL FIX concentrate may further enable quantification of the clinical benefits of switching.16
The diagnostic information in MDV pertained only to patients who required in-hospital tests or treatment. Consequently, the presence of comorbidities such as chronic hepatitis as well as the prevalence of joint damage may not reflect a real-world clinical setting. Chronic viral hepatitis C was present in a relatively large percentage (38.6%) of the study population; it is likely that many of these patients had sustained virologic response by antiviral therapy. However, the trends in patient demographics and baseline characteristics in this study followed those of the Nationwide Survey of Blood Coagulation Disorders 2020,29 which reflects the overall picture of patients with hemophilia in Japan.
As stated, patients in the MDV database include only those receiving in-hospital treatment, which employs a per-diem payment system of diagnostic procedure combination. Characteristics such as the severity of comorbidities and/or joint damage may differ between patients managed within a hospital versus a clinic setting, thereby affecting treatment patterns and expenditures. In this study, weight was calculated based on the statistical average weight of Japanese individuals in the specified age groups, which could have led to an over- or under-estimate of annualized dosage of FIX concentrates. An additional limitation is potential underestimation of treatment-related and broader medical expenditures, as this analysis covers only expenditures for medications dispensed in the hospital setting, although patients do not typically receive prescriptions for FIX concentrates from multiple hospitals/clinics in the same time period. Each patient in the MDV database is assigned a unique identifier in a hospital; however, if a patient moves to another hospital also covered by the database, the patient would be recorded as two different patients. The evolution of treatment goals must be considered when interpreting differences in the estimated expenditures between SHL and EHL concentrates. However, information about the treatment goal is not included in the MDV database. Finally, patients with congenital hemophilia who had FIX inhibitors were excluded from this study; therefore, pre-switch and post-switch inhibitor rates were not evaluated. However, given the rarity of inhibitor in hemophilia B, a low incidence of FIX inhibitor would be expected.
Conclusions
This retrospective study found increased factor IU of EHL FIX concentrates dispensed for the treatment of Japanese patients with hemophilia B, along with an increase in associated medical expenditures for patients who switched from an SHL to an EHL FIX concentrate. The quality of prophylaxis treatment improved after EHL concentrates became available as treatment goals for hemophilia B continued to advance. Overall, these results provide insight into the treatment and expenditures of patients with hemophilia B in Japan and highlight the disease burden in patients requiring hospital care.
Data Sharing Statement
All the rights for the database used for the current study are reserved with Medical Data Vision. However, Pfizer Japan Inc. have a contract with Medical Data Vision to use this database and publish the results. The data may be made available upon request and are subject to a license agreement with Medical Data Vision.
Ethics Approval
This study was conducted in accordance with legal and regulatory requirements in Japan, and included anonymized claims data, with no personal patient data. Japanese Ethical Guidelines for Medical and Biological Research Involving Human Subjects do not apply to studies that use anonymized secondary data; consequently, this study was not reviewed by any institutional review board or ethics committee.
Acknowledgments
Medical writing and editorial assistance were provided by Courtney M. Cameron, PhD, of Engage Scientific Solutions and by Mia DeFino, MS, ELS, and Michael Morren, RPh, MBA, of Peloton Advantage, an OPEN Health company, and funded by Pfizer.
The affiliation for Leona E Markson was Pfizer Inc, Collegeville, PA, USA at the time of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Pfizer.
Disclosure
Katsuyuki Fukutake has received honoraria from and/or served as a member of an advisory board or speakers’ bureau for Bayer Yakuhin, Ltd., Chugai Pharmaceutical Co., Ltd., Fujimoto Pharmaceutical Co., Pfizer Japan Inc., Sanofi S.A., CellGenTech, Inc. and Takeda Co., Ltd. Kanae Togo, Linghua Xu, and Toshiyuki Karumori are employees of Pfizer Japan Inc. and may own stock/options in the company. José Maria Jimenez Alvir is an employee of Pfizer Inc. and may own stock/options in the company. Leona Markson was employed at Pfizer at the time of this study and previously retired from Merck and may hold stock/options or receive other retiree benefits from these companies. Ian Winburn is an employee of Pfizer Ltd. and may own stock/options in the company.
References
1. Franchini M. Current management of hemophilia B: recommendations, complications and emerging issues. Expert Rev Hematol. 2014;7(5):573–581. doi:10.1586/17474086.2014.947955
2. World Federation of Hemophilia. Report on the annual global survey 2021; 2022; Available from: https://www1.wfh.org/publications/files/pdf-2324.pdf.
3. Mancuso ME, Santagostino E. Outcome of clinical trials with new extended half-life FVIII/IX concentrates. J Clin Med. 2017;6(4):E39. doi:10.3390/jcm6040039
4. Fukutake K, Taki M, Matsushita T, et al. Postmarketing safety and effectiveness of recombinant factor IX (nonacog alfa) in Japanese patients with haemophilia B. Haemophilia. 2019;25(4):e247–e256. doi:10.1111/hae.13783
5. Pharmaceuticals and Medical Devices Agency. Refixia (nonacog beta pegol) Japanese regulatory review: report on the deliberation results; 2018; Available from: https://www.pmda.go.jp/files/000238159.pdf.
6. Pharmaceuticals and Medical Devices Agency. Idelvion (albutrepenonacog alfa) Japanese regulatory review: report on deliberation results; 2016; Available from: https://www.pmda.go.jp/files/000221483.pdf.
7. Biogen. Alprolix (eftrenonacog alfa); 2016; Available from: https://www.pharmacodia.com/yaodu/html/v1/biologics/1e5e1435c95e420a1cd34d3202769c18.html.
8. Pelland-Marcotte MC, Carcao MD. Hemophilia in a changing treatment landscape. Hematol Oncol Clin North Am. 2019;33(3):409–423. doi:10.1016/j.hoc.2019.01.007
9. Mannucci PM. Hemophilia therapy: the future has begun. Haematologica. 2020;105(3):545–553. doi:10.3324/haematol.2019.232132
10. Collins PW. Personalized prophylaxis. Haemophilia. 2012;18(suppl 4):131–135. doi:10.1111/j.1365-2516.2012.02838.x
11. Kavakli K, Smith L, Kuliczkowski K, et al. Once-weekly prophylactic treatment versus on-demand treatment with nonacog alfa in patients with moderately severe to severe hemophilia B. Haemophilia. 2016;22(3):381–388. doi:10.1111/hae.12878
12. Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323. doi:10.1056/NEJMoa1305074
13. Mancuso ME, Lubetsky A, Pan-Petesch B, et al. Long-term safety and efficacy of rIX-FP prophylaxis with extended dosing intervals up to 21 days in adults/adolescents with hemophilia B. J Thromb Haemost. 2020;18(5):1065–1074. doi:10.1111/jth.14778
14. Japanese Society for Thrombosis and Hemostasis. Hemostasis treatment guidelines for hemophilia patients without inhibitors; 2013; Available from: https://www.jsth.org/wordpress/wp-content/uploads/2015/04/03_inhibitor_H1_B.pdf.
15. Tortella BJ, Alvir J, McDonald M, et al. Real-world analysis of dispensed IUs of coagulation factor IX and resultant expenditures in hemophilia B patients receiving standard half-life versus extended half-life products and those switching from standard half-life to extended half-life products. J Manag Care Spec Pharm. 2018;24(7):643–653. doi:10.18553/jmcp.2018.17212
16. Cortesi PA, D’Angiolella LS, Lafranconi A, et al. Modern treatments of haemophilia: review of cost-effectiveness analyses and future directions. Pharmacoeconomics. 2018;36(3):263–284. doi:10.1007/s40273-017-0588-z
17. Guh S, Grosse SD, McAlister S, Kessler CM, Soucie JM. Healthcare expenditures for males with haemophilia and employer-sponsored insurance in the United States, 2008. Haemophilia. 2012;18(2):268–275. doi:10.1111/j.1365-2516.2011.02692.x
18. Machin N, Ragni MV, Smith KJ. Gene therapy in hemophilia A: a cost-effectiveness analysis. Blood Adv. 2018;2(14):1792–1798. doi:10.1182/bloodadvances.2018021345
19. Tortella BJ, Fogarty PF, Alvir J, et al. Real world data analysis of coagulation factor IX costs in specific patients with hemophilia B switching from standard half-life to extended half-life products [abstract]. Blood. 2016;128:4963.
20. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187–197. doi:10.1016/s0140-6736(15)01123-x
21. Chhabra A, Spurden D, Fogarty PF, et al. Real-world outcomes associated with standard half-life and extended half-life factor replacement products for treatment of haemophilia A and B. Blood Coagul Fibrinolysis. 2020;31(3):186–192. doi:10.1097/mbc.0000000000000885
22. The Japanese society for pediatric endocrinology. Available from: http://jspe.umin.jp/medical/taikaku.html.
23. General Counter of Official Statistics (e-Stat). National health and nutrition survey: average and standard deviation of height and weight. Available from: https://www.e-stat.go.jp/dbview?sid=0003224177.
24. Ministry of Health Labor and Welfare. Various information of medical fee. Available from: https://shinryohoshu.mhlw.go.jp/shinryohoshu/.
25. Bank of Japan. Transactions on the Tokyo foreign exchange market (in 2019); 2020; Available from: https://www.boj.or.jp/statistics/market/forex/fxdaily/ex2019.pdf.
26. Shirahata A. Treatment for hemophilia from the economic points of view. Jpn J Thromb Hemost. 2021;32(1):55–63. doi:10.2491/jjsth.32.55
27. Buckner TW, Bocharova I, Hagan K, et al. Health care resource utilization and cost burden of hemophilia B in the United States. Blood Adv. 2021;5(7):1954–1962. doi:10.1182/bloodadvances.2020003424
28. Nagao A, Bingo M, Yamaguchi T, Fukutake K. Real-world use of albutrepenonacog alfa, A recombinant coagulation factor IX albumin fusion protein, for personalized prophylaxis in Japanese individuals with hemophilia B: a case series. Cureus. 2023;15(1):e33573. doi:10.7759/cureus.33573
29. Japan Foundation for AIDS Prevention. National survey of blood coagulation disorders; 2021; Available from: https://api-net.jfap.or.jp/library/project.html.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

