Back to Journals » Pragmatic and Observational Research » Volume 14
Real-World Use of Immunotherapy for Hepatocellular Carcinoma
Authors Sara A, Ruff SM, Noonan AM, Pawlik TM
Received 14 June 2023
Accepted for publication 11 August 2023
Published 21 August 2023 Volume 2023:14 Pages 63—74
DOI https://doi.org/10.2147/POR.S397972
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor David Price
Amir Sara,1 Samantha M Ruff,2 Anne M Noonan,1 Timothy M Pawlik2
1Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, USA; 2Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Correspondence: Timothy M Pawlik, Department of Surgery, The Urban Meyer III and Shelley Meyer Chair for Cancer Research, Professor of Surgery, Oncology, Health Services Management and Policy, The Ohio State University, Wexner Medical Center, 395 W. 12th Ave., Suite 670, Columbus, OH, 43210, USA, Tel +1 614 293-8701, Fax +1 614 293-4063, Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related mortality worldwide and accounts for 90% of all primary liver cancers. Chronic inflammation is the hallmark across most prevalent etiologies among which HBV is the leading cause worldwide (33%), followed by alcohol (30%), HCV (21%), other factors like non-alcoholic steatohepatitis linked to insulin resistance/metabolic syndrome, and obesity associated inflammation (16%). Deregulation of the tightly controlled immunological network leads to liver disease, including chronic infection, autoimmunity, and tumor development. While inflammation drives oncogenesis in the liver, HCC also recruits ICOS+ FOXP3+ Tregs and MDSCs and upregulates immune checkpoints to induce a state of immunosuppression in the tumor microenvironment. As such, research is focused on targeting and modulating the immune system to treat HCC. The Checkmate 040 and Keynote 224 studies established the role of immunotherapy in the treatment of patients with HCC. In Phase I and II trials, nivolumab and pembrolizumab demonstrated durable response rates of 15– 20% and were subsequently approved as second-line agents after sorafenib. Due to the success of the IMbrave 150 and HIMALAYA trials, which examined the combination of atezolizumab/bevacizumab and tremelimumab/durvalumab, respectively, the FDA approved these regimens as first-time treatment options for patients with advanced HCC. The encouraging results of immunotherapy in the management of HCC has led researchers to evaluate if combination with locoregional therapies may result in a synergistic effect. Real-world studies represent an invaluable tool to assess and verify the applicability of clinical trials in the bedside setting with a more varied patient population. We herein review current real-life use of ICIs in the management of HCC and highlight some of the ongoing clinical trials that are expected to change current recommended first-line treatment in the near future.
Keywords: hepatocellular carcinoma, immunotherapy, real-world data, immunotherapy biomarkers, liver microenvironment
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related mortality worldwide and accounts for 90% of all primary liver cancers1 HCC is a serious healthcare challenge as it accounted for 830,180 deaths in 2020 and has a projected increase in incidence from 13.5 in 2013 to 17.0 in 2020 and 21.2 in 2030 per 100,000 person-years.2,3 HCC oncogenesis is driven by inflammation.4 Chronic inflammation secondary to Hepatitis B infection (HBV), alcohol, hepatitis C infection (HCV), and non-alcoholic steatohepatitis linked to insulin resistance/metabolic syndrome or obesity associated inflammation is the underlying driver of HCC development.5,6 These etiologies lead to a unique microenvironment through alterations to immune cells, the cytokine milieu, and tumor cell signaling pathways.4
While inflammation drives oncogenesis in the liver, HCC tumor cells also adjust the surrounding microenvironment by recruiting ICOS+ FOXP3+ Tregs and MDSCs and upregulating immune checkpoints (eg PD-1 and CTLA-4) in order to induce a state of immunosuppression.7,8 As such, research is focused on targeting and modulating the immune system to treat HCC. For over a decade, several HHC systemic therapeutic drugs have been developed and have demonstrated modest survival benefits as single agents. However, recent landmark trials with immune-checkpoint inhibitors (ICIs) have revolutionized the management of HCC and changed the standard of care. To this point, HCC clinical research across all stages has increasingly focused on immunotherapy with more than 30 Phase III trials currently ongoing.4,9–11
In addition, the updated Barcelona Clinic Liver Cancer (BCLC) guidelines now incorporate immune checkpoint inhibitor-based combination therapies as first-line treatment for advanced HCC. The spectrum of immunotherapy success in the management of advanced HCC has resulted in a new goal of treating with immunotherapy ± locoregional therapies to downstage patients to enhance resectability. Additionally, immunotherapy is now being leveraged in clinical trials to prevent recurrence after curative-intent resection.12–15 Despite progress in the application of immunotherapy for cancer treatment, reliable response biomarkers to define patients who might benefit the most from immunotherapy are still lacking. We herein review current real-life use of ICIs in the management of HCC and highlight some of the ongoing clinical trials that are expected to change current recommended first-line treatment.
Liver Microenvironment
The liver is the largest reticuloendothelial system in the body. The liver is continuously challenged by a large burden of antigens and pathogens from both the gastrointestinal tract via the portal vein and the circulatory system via the hepatic artery. As such, the liver needs to maintain a physiologically tolerogenic immunosuppressed niche to downregulate any immunogenic responses and avoid autoimmunity.16 Deregulation of this tightly controlled immunological network can lead to chronic infection, autoimmunity and tumor development.17 Chronic inflammation leads to a persistent pro-inflammatory state, which stimulates liver cell death and regeneration leading to fibrosis and/or subsequent cirrhosis. In turn, fibrosis and cirrhosis often eventually induce the development of dysplastic nodules and tumorigenesis.16
Overall, 80% of the liver parenchyma is constituted of hepatocytes, followed by cholangiocytes, liver sinusoidal endothelial cells (LSECs), hepatic stellate cells (HSCs), and liver-resident immune cells.18 Major immunosuppressive cells implicated in HCC immune evasion include tissue-resident macrophages (mainly Kupffer cells), monocyte-derived macrophages, regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs).16,17,19,20 Dysfunctional dendritic cells (DCs), CD8+PD1+ T cells (in the context of NASH), neutrophils, and regulatory B (Breg) cells produce metalloproteinases and limit the effectiveness of innate and adaptive immunity. Subsequently, this allows for uncontrolled cell growth.21 In turn, this process can produce mitotic mediators (eg EGFR ligands, IL-6, TNFα, FGFs, TGFβ and HGF),22 angiogenesis mediators (eg VEGF, bFGF, TNFα, TGFβ, platelet-derived growth factor and placental growth factor),23 and mediators that support cancer stem cells (eg IL-6 to interact with STAT3).24
Development of Hepatocellular Carcinoma Systemic Treatment
HCC treatment is driven by disease stage and liver function. Early-stage HCC can often undergo curative-intent treatment with surgical resection, liver transplantation, and/or ablation, while intermediate stage disease may require additional treatments modalities like embolization and/or radiation therapy. Systemic therapy (chemotherapy, molecular targeted therapy, immunotherapy, and gene therapy) has been the mainstay treatment for intermediate or advanced-stage-HCC patients.25
Published in 2008, the Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) was the first Phase III, randomized, placebo-controlled trial to demonstrate an overall survival (OS) benefit for patients with advanced HCC and well-preserved liver function (>95% Child-Pugh A) who were treated with a targeted agent. Compared to a placebo, patients treated with sorafenib had improved median OS (sorafenib: 10.7 months versus placebo: 7.9 months, HR in sorafenib cohort: 0.69; p < 0.001).26 Raoul et al published a SHARP trial sub-analysis in 2012 that demonstrated the efficacy of sorafenib irrespective of disease etiology, liver enzyme levels, α-fetoprotein (AFP) levels, bilirubin levels, tumor size or stage.27 Sorafenib was, however, associated with primary and acquired resistance that limited its use and contributed to poor outcomes.28 Subsequently, based on data from the RESORCE and CELESTIAL studies, Regorafenib and Cabozantinib, respectively, received approval for use in patients who were resistant to or intolerant to sorafenib. Cabozantinib has also received approval as a third-line therapeutic option.29,30 Specifically, these agents demonstrated improved OS (26 months) as a second-line therapy to sorafenib compared to placebo as a second-line therapy (OS: 19 months).29,30
The phase III REFLECT randomized trial demonstrated that OS associated with the TKI lenvatinib was noninferior to sorafenib. Lenvatinib also demonstrated a higher objective response rate (ORR: 18% vs 6%) and fewer cases of palmar-plantar erythrodysesthesia. Based on this trial, Lenvatinib is an acceptable alternative to sorafenib as first-line therapy.31 Interestingly, OS related to TKI use in the post-approval phase has been reported to be slightly longer in both clinical trials and in real-life.32–35 Antiangiogenic monoclonal antibodies, such as ramucirumab and bevacizumab, which target VEGF to inhibit the angiogenesis, have also been approved for patients who progressed on sorafenib with AFP levels of ≥400 ng/mL based on data demonstrating improved overall survival versus placebo.36–38
Immunotherapy in Hepatocellular Carcinoma
Immunotherapy has redefined and revolutionized the field of oncology with unprecedented potential to benefit patients with advanced disease. It has proven to be more effective and with a better safety profile than TKIs among patients with advanced HCC (Table 1).
 |
Table 1 Reported Median Overall Survival and Median Progression Free Survival in HCC Immunotherapy Trials |
Single Agent Immunotherapy
The Checkmate 040 and Keynote 224 studies established the role of immunotherapy in the treatment of patients with HCC. In phase I and II trials, nivolumab and pembrolizumab demonstrated response rates of 15–20% and were subsequently approved as second-line agents43,44 However, the phase III CheckMate 459 study failed to demonstrate an improved OS for nivolumab versus sorafenib.45 In the phase III Keynote 240 trial, pembrolizumab was investigated as a second-line therapy, but failed to demonstrate a survival benefit compared to placebo.46 The phase III Keynote 394 trial compared best supportive care with pembrolizumab versus placebo as second-line therapy among patients in Asia with advanced HCC. Compared to the placebo, pembrolizumab significantly improved OS, PFS, and ORR.47 In a Phase II trial, another anti-PD-1 inhibitor camrelizumab demonstrated promise to be utilized as a new treatment in pretreated patients with advanced HCC.48 Atezolizumab, an inti-PDL1 inhibitor, has also been investigated among patients with unresectable HCC, yet the results are pending.49
Combined Agent Immunotherapy
Because single-agent anti-PD1/anti-PDL1 inhibitors have demonstrated limited activity in an immunosuppressive microenvironment like HCC, new immunotherapy combinations have been tested. Combination therapy provides simultaneous blockade of multiple immune checkpoints.
To this point, the IMbrave 150 trial examined the combination of atezolizumab and bevacizumab.39 Compared to sorafenib, this combination treatment regimen demonstrated improved OS (atezo/bev median OS not reached vs 13.2 months with sorafenib) and improved PFS (atezo/bev median PFS: 6.8 months vs 4.3 months with sorafenib). In turn, the FDA approval of this regimen as first-line therapy for advanced HCC.39 An updated analysis with a median follow-up of 15.6 months demonstrated a median overall survival of 19.2 months with atezolizumab and bevacizumab versus 13.4 months with sorafenib alone.40 Of note, the IMbrave 150 study cohort included a patient population with varying etiologies (50% HBV, 20% HCV, and 30% non-viral etiology). Importantly, a recent report noted patients with HCC due to a viral etiology derived a therapeutic benefit from ICI use [HR 0.64], whereas patients with a nonviral HCC etiology (NAFLD/NASH) did not [HR 0.92] (P = 0.03).50 In turn, results of IMbrave 150 need to be interpreted in light of this information as the trial results may be more applicable to viral-induced HCC tumors that are more sensitive to immune microenvironment alterations.39 Two Phase Ib first-line trials examined pembrolizumab plus regorafenib (REG-PEMBRO-HCC) and pembrolizumab plus Lenvatinib (Keynote 524/Study 116), respectively, for advanced HCC.42,51 Both regimens demonstrated promising anti-tumor activity with reported disease control rates of ~90% with no major safety issues.42,51 As such, these combination therapies may be attractive options for NASH-associated HCC. In a phase III trial, Camrelizumab demonstrated improved survival outcomes when received in combination with Rivoceranib (anti-antiangiogenic TKI) as first-line treatment in a cohort of patients with unresectable HCC.41
Anti-CTLA-4 monoclonal antibodies such as tremelimumab and ipilimumab have also emerged as potential effective ICIs. To this end, based on the CheckMate 040 trial, ipilimumab with nivolumab received accelerated approval by the FDA in 2020 for patients with HCC already treated with sorafenib.52 The phase II CheckMate 040 trial demonstrated that nivolumab-ipilimumab combination had a manageable safety profile and promising ORR.52 Nivolumab/ipilimumab versus sorafenib/lenvatinib is currently being studied in a phase III trial (CheckMate 9DW, NCT04039607).
Recently, cohort 6 of the Checkmate 040 trial reported that nivolumab/cabozantinib with or without ipilimumab had encouraging preliminary antitumor activity.53 Nivolumab plus cabozantinib had a consistent safety profile compared with other established individual drugs used to treat patients with advanced HCC.53 The phase III LEAP-002 trial demonstrated no difference in OS or PFS when comparing Lenvatinib plus Pembrolizumab versus Lenvatinib alone as first-line treatment.32
The study 22 (a phase II) and HIMALAYA (a phase III) second-line trials demonstrated that a single dose of tremelimumab (anti-CTLA-4) plus durvalumab (anti PD-L1) administered at regular intervals (STRIDE regimen) yielded a median OS of 16.4 versus 13.8 months for patients treated with sorafenib.54,55 HIMALAYA was conducted in 190 centers across 16 countries. Notably, the study population was externally validated and 31% of the patients had HBV, 27% had HCV, and 42% had a non-viral etiology of their chronic liver inflammation.56
A synergistic effect may be the reason for increased efficacy related to combining ICIs and anti-VEGF therapy versus TKIs alone. ICIs block checkpoint inhibition of the immune system, thereby increasing the activation and recruitment of killer lymphocytes. VEGF inhibitors transiently normalize tumor vasculature to convert the immunosuppressive tumor microenvironment to a more immune-supportive one via upregulation of adhesion molecules (ICAM, VCAM), selectins and integrins that facilitate migration of effective killer lymphocytes into the tumor microenvironment.57 Additionally, VEGF inhibitors also decrease the expression of PD-L1 on dendritic cells; in mouse models, VEGF inhibitors have been demonstrated to decrease the expression of inhibitory molecules like PD-1, Tim-3, CTLA-4, and Lag-3, thus leading to increased activation of CD-8+ T cells and CD-8+/T-Reg ratio.57 CTLA-4 is an immune checkpoint that inhibits T-cell activation at secondary lymphoid organs and at the time point of T-cell development. A complementary or synergistic effect may be related to dual ICI therapy based on observation of a distinct immune environment that involves increased CD8+ T-cell recruitment and activation, increased IL-8 and HLA-DR, soluble IL-2R and anti-CTLA-4 upregulated gene expression, and B cell phenotype modulation in the periphery and the cancer TME leading to enhanced tumor rejection.58
Immunotherapy Combined with Locoregional Therapies
The combination of immunotherapy and regional therapies (eg, trans-arterial embolization (TACE), ablation, Yttrium-90 radioembolization (Y90), and/or radiation therapy (RT)) may have a potentially synergistic effect. Regional therapies can alter the microenvironment by inducing necrosis of tumor cells and the release of tumor neo-antigens. In turn, the microenvironment and tumor may be more primed for immune checkpoint inhibitors.59 Currently, this effect of combination therapy has been demonstrated in pre-clinical studies with mouse HCC models and is also being investigated in early phase trials.60,61 Zhu et al treated twenty HCC patients with neoadjuvant TACE and a PD-1 inhibitor as a bridge to surgery.15 Fourteen of patients were successfully downstaged, suggesting that there may be a benefit to this combination therapy. In a separate study, Mizukoshi et al analyzed immune response before and after radiofrequency ablation in 69 patients with HCC.14 Radiofrequency ablation enhanced T cell response to tumor-specific antigens. At 24 weeks post-radiofrequency ablation, there was, however, a decrease in these T cells. These data suggest an association between ablation and change in T cell response, which may be transient in nature. Duffy et al treated 32 HCC patients with tremelimumab and either radiofrequency ablation or chemoablation13 The combination of immunotherapy and ablation was safe and led to the accumulation of intra-tumoral CD8+ T cells. One case report demonstrated the potential of combination of immune checkpoint inhibitors and radiation therapy.62 There are several ongoing trials examining the combination of regional therapy and immune checkpoint inhibitors (NCT 04246177, NCT03482102, NCT03203304, NCT03316872, NCT03817736, NCT04611165, NCT05225116, NCT05185531).
Real World Data of Immunotherapy Efficacy and Safety in Advanced HCC
Real-world studies are crucial to demonstrating that novel therapies can be employed in clinical practice outside strict trial criteria. Liver function is typically assessed with Child‐Pugh classification system. Unfortunately, preserved liver function is often a criterion for these clinical trials and patients with a Child Pugh class B and C are commonly excluded or enrolled in limited numbers.26,31,39,45 Thus, there is limited data on the safety and efficacy of many first-line therapies in patients with Child-Pugh B and C. For this reason, many real-world studies across the globe have been conducted trying to address these questions (Table 2).
 |
Table 2 Reported Median Overall Survival and Median Progression Free Survival in HCC Immunotherapy Real-World Studies |
In 2022, a retrospective study by De Castro et al, the efficacy and safety of atezolizumab/bevacizumab was evaluated in 147 HCC patients from Germany and Austria. This study divided the patients into two cohorts: patients who met the inclusion criteria of IMBrave150 (IMBrave-IN) versus patients with at least one major exclusion criteria of the IMBrave150 trial (IMBrave-OUT). The IMBrave-OUT group had 35 Child-Pugh B pts (23.8%), 6 Child-Pugh C pts (4.1%), 7 BCLC D Pts (4.8%), and 13 ALBI III Pts (8.8%). The IMbrave-IN cohort had improved median OS and median PFS compared to the IMbrave-OUT cohort (mOS: 15.0 months versus 6.0 months and mPFS: 8.7 months versus 3.7 months). Additionally, the impact on OS also corresponded with worsening liver function (CPS >7 and ALBI grade >2), which was in line with consistent with other real-world studies.71,74 Finally, the IMBrave-OUT group had increased risk for hepatic encephalopathy and/or ascites.63 Following this study, a recent similar evaluation conducted by Rimini et al noted that adherence to the IMbrave150 trial inclusion criteria was associated with improved prognosis of patients receiving atezolizumab/bevacizumab. Of note, patients with Child–Pugh class A had improved OS and PFS compared to patients with Child–Pugh class B (OS: 16.3 months vs 5.9 months; PFS 7.9 months vs 5.3 months).64 In another retrospective study, Tanaka et al reported on 457 Japanese patients with unresectable HCC, 89 of whom were classified as Child-Pugh B, while the remainder were classified as Child-Pugh.65 PFS and OS among CP-A patients were better than that of patients with CP-B disease, with the therapeutic efficacy correlating with worsening liver function (mALBI grade of 2b or 3).65
Separate reports by Kim et al and D’Alessio et al noted that the Atezolizumab/Bevacizumab regimen was a safe option as a first-line treatment for unresectable HCC.66,67 OS was, however, inferior among patients with Child-Pugh B versus Child-Pugh A disease. The rate of grade 3 or 4 adverse events was higher among patients with Child-Pugh B.66,67 Cheon et al reported similar findings in which patients with Child–Pugh B HCC receiving first-line Atezolizumab/Bevacizumab had an ORR of 11.1%, a median PFS of 3.0 months, and a median OS 7.7 months, while Child–Pugh A patients had an ORR of 34.1%, a median PFS of 9.6 months, and a median OS that was not reached.68 Again, grade 3–4 adverse events were more commonly observed among patients with Child–Pugh B versus patients with Child–Pugh A. In addition, there were differences even within the Child–Pugh B subset; patients with Child–Pugh B7 demonstrated a trend toward better ORR, median PFS, and OS results compared with the Child–Pugh B8–B9 group.68
Jost-Brinkmann et al evaluated the efficacy of Atezolizumab plus bevacizumab as a second-line therapy after either sorafenib or Lenvatinib.75 In this study, the Atezolizumab/Bevacizumab regimen was noted to be safe among patients with liver disease. Of note, patients with Child–Pugh B had similar ORR (objective response rate) yet shorter median OS and PFS versus patients with preserved liver function.75 In another study, Rimini et al reported that patients with unresectable HCC and Child-Pugh B liver disease, had better outcomes following treatment with Lenvatinib versus atezolizumab/bevacizumab.69 The study recruited 217 CP B HCC patients from Italy, Germany, Republic of Korea and Japan. The median OS among patients receiving Lenvatinib was 13.8 months versus 8.2 months among patients receiving atezolizumab/bevacizumab as first-line treatment and there was no difference in median PFS. Additional analysis demonstrated that patients who received first-line Lenvatinib had longer median OS compared to patients receiving atezolizumab/bevacizumab.69 These findings were in line with another recent study by Casadei-Gardini et al, which noted that atezolizumab plus bevacizumab was not associated with a survival advantage over Lenvatinib.70 This study included a total of 214 Child-Pugh B patients, 62 of whom were in atezolizumab plus bevacizumab arm (7.2%), and 152 in the Lenvatinib arm (11.4%). OS was prolonged by atezolizumab plus bevacizumab over lenvatinib among patients with a viral etiology of HCC; in contrast, OS was better among patients treated with lenvatinib in the setting of non-alcoholic steatohepatitis/non-alcoholic fatty liver disease.70 Chen et al noted that among patients with HCC in a real-world clinical practice, Lenvatinib was safe and efficacious as a second-line systemic therapy after progression on atezolizumab/bevacizumab.76 The IMBrave050, a phase III trial by Chow et al published very recently showed that adjuvant treatment ATE/BEV combination achieved a statistically significant recurrence free survival compared to active surveillance alone in patients with high-risk HCC following resection or ablation. 12-month recurrence free survival rate was 78% in ATE/BEV group vs 65% in active surveillance group.12
The efficacy of nivolumab and pembrolizumab for treatment of unresectable HCC has also been evaluated in the real-world clinical setting. Wong et al studied 61 patients with unresectable HCC; 72.1% (n=44) were Child-Pugh B and 27.9% (n = 17) were of Child-Pugh C.71 Patients received either single agent nivolumab or pembrolizumab. The ORR of Child-Pugh B and Child-Pugh C patients were 6.8% and 0%, respectively, and time to progression were 2.1 months and 1.4 months, respectively. Child-Pugh B patients had a better OS than Child-Pugh C patients (3.1 months, vs 1.7 months).71 A different retrospective, international, multicenter observational study evaluated the safety and efficacy of nivolumab in 233 patients (n=75, 32.2% Child-Pugh B) treated outside clinical trials from eight centers in North America, Europe, and Asia.72 In this study, nivolumab was noted to be safe and effective in advanced HCC across various lines of therapy and degrees of liver dysfunction, however, OS was shorter among patients with Child-Pugh B versus A disease (7.3 months vs 16.3 months).72 Choi et al indicated that Child–Pugh class B was an independent negative predictor for objective response to nivolumab among patients with HCC.74 Chapin et al also recently compared nivolumab to sorafenib for first systemic therapy among patients with HCC and Child‐Pugh B cirrhosis.73 Among patients with HCC and Child‐Pugh B cirrhosis, nivolumab was associated with improved OS and tolerability compared to sorafenib. Therefore, nivolumab should be considered first-line in this patient population.73 A recent study assessed the efficacy and safety of ipilimumab plus nivolumab in advanced HCC patients who received pembrolizumab alone or with the addition of TKI. Results showed improved OS and PFS with reasonable safety data suggesting that ipi/nivo combination could represent a reasonable second-line regimen in advanced HCC.77
Current Guidelines for the Treatment of HCC, BCLC 2022 Recommendations
The BCLC guidelines divide HCC into 5 stages: 0 (Very Early), A (Early), B (Intermediate), C (Advanced), D (Terminal) (Figure 1). These 5 stages are linked to prognosis and treatment recommendations. The BCLC was most recently updated in 2022.78,79 For very early stage (BCLC 0) disease, defined as a solitary HCC < 2 cm without vascular invasion or extrahepatic spread in a patient with preserved liver function and no cancer-related symptoms, liver transplant (LT) is the first choice followed by ablation/resection if LT is not feasible.78 For early stage (BCLC A), disease defined as solitary HCC irrespective of size or as a multifocal HCC up to 3 nodules (none of them >3 cm), without macrovascular invasion, extrahepatic spread or cancer-related symptoms (PS-0), surgical resection and LT are considered depending on number, location, burden of hepatic lesions, and presence of clinically significant portal hypertension (CSPH) defined by a hepatic venous pressure gradient [HVPG] >10 mmHg.78
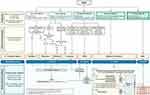 |
Figure 1 The BCLC system establishes a prognosis in accordance with the 5 stages that are linked to first-line treatment recommendation. The expected outcome is expressed as median survival of each tumour stage according to the available scientific evidence. Individualised clinical decision-making, according to the available data on November 15, 2021, is defined by teams responsible for integrating all available data with the individual patient’s medical profile. Note that liver function should be evaluated beyond the conventional Child-Pugh staging. *Except for those with tumor burden acceptable for transplant. ^Resection may be considered for single peripheral HCC with adequate remnant liver volume. Reprinted with copyright permission from Reig M, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol, 2022;76(3):681–693. © 2021 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.78 Abbreviations: AFP, alpha-fetoprotein; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; BSC, best supportive care; ECOG-PS, Eastern Cooperative Oncology Group-performance status; LT, liver transplantation; MELD, model of end-stage liver disease; TACE, transarterial chemoembolisation. |
Intermediate stage (BCLC-B) is defined as multifocal HCC outside of BCLC A criteria with preserved liver function, no cancer-related symptoms, and no vascular invasion or extrahepatic spread. This stage is divided into three groups. The first cohort meets extended liver transplant criteria and LT should be considered for these patients. The second cohort includes patients who have preserved portal flow and defined tumor burden, suggesting the feasibility of selective access to feeding tumor arteries, but are not candidates for LT. These patients are candidates for arterial based therapies like trans-arterial chemoembolization (TACE). If patients meet neither the “extended liver transplant criteria” nor TACE criteria, then systemic therapy should be considered. The third subgroup within BCLC-B includes patients with diffuse, infiltrative, extensive HCC liver involvement. These patients should be referred for systemic therapy.78
Advanced stage (BCLC-C) patients are defined as having vascular invasion or extrahepatic spread who are still relatively fit with preserved liver function. BCLC-C patients should be evaluated for systemic therapy.78 Based on the positive results from IMBrave and HIMALAYA studies, the current recommended first-line option in the updated BCLC algorithm should be targeted therapy. For advanced stage HCC, first-line treatments include atezolizumab plus bevacizumab or durvalumab plus tremelimumab. If these options are not feasible, then sorafenib, lenvatinib, or durvalumab are other options. Second-line therapy post sorafenib typically involves regorafenib, cabozantinib, or ramucirumab.78,79
Immunotherapy Response Biomarkers
Despite the encouraging results of the IMBrave 150 and HIMALAYA trials, identifying biomarkers that predict clinical response to immunotherapy remains crucial to identify which patients may benefit the most. Biomarkers will improve patient selection and treatment outcomes, which is critical as roughly 25% of patients will develop grade 3–4 adverse events from immunotherapy.
In general, there is no standard biomarker to predict ICIs outcomes among patients with HCC. Tumor PD-L1 expression is the most widely studied biomarker in HCC, but the data on its predictive potential is controversial.80,81 Other potential biomarkers related to HCC include infiltrating lymphocytes,13,80,82 tumor mutational burden (TMB),83–85 neutrophil-to-lymphocytes ratio and platelet-to-lymphocytes ratio,80 circulating tumor DNA (ctDNA),86–89 circulating tumor cells (CTCs),90 and gut microbiota.91,92 Unfortunately, the evidence is not yet strong enough to support implementation of these biomarkers in the clinical setting, thus more studies are needed beyond the pre-clinical setting.
Analysis of histopathology, imaging, and immune signatures is likely the most comprehensive way to assess treatment effect.93 A recent study classified HCC into four groups based on microenvironment lymphocytic infiltration and angiogenic factor expression. These studies included immune-high/angiostatic (IH/AS), immune-mid/angio-mid (IM/AM), immune-low/angiogenic (IL/AG), and immune-low/angio-low (IL/AL). A reciprocal interaction between antitumor immunity and tumor angiogenesis, and an association between poor prognosis with decreased lymphocyte infiltration, and increased vessels encapsulating tumor clusters (VETC)/macrotrabecular-massive (MTM) pattern formation was noted. In turn, this classification may potentially be used to better identify patients expected to respond to immunotherapy alone.94 Mutations in the CTNNB1 gene lead to activation of the Wnt/β-catenin pathway and may serve as a predictor of response to ICIs. Mutations in Wnt/B-catenin were associated with increased resistance to immunotherapy and poorer prognosis in both pre-clinical and clinical setting.95,96 These mutations have been observed in “cold” HCC tumors and were associated with IL/AG subtype and VETC positivity, which correlated with tumors that were devoid of CD8+ T-cells and NK cells and rich in T-Regs.94,97
Conclusion/Expert Opinion
HCC is a rare and aggressive tumor with a high incidence of recurrence and poor prognosis. The unique environment of the liver is balanced between immune tolerance and activation. When this balance is disrupted by chronic inflammation, immune cell exhaustion and fibrosis can occur making the liver vulnerable to carcinogenesis. This perturbation may prove, however, an opportunity to leverage immunotherapy to treat HCC. The IMbrave150 and HIMALAYA trials established that atezolizumab/bevacizumab and durvalumab/tremelimumab can effectively treat advanced HCC. Real-world data has further confirmed the survival benefit of immunotherapy in advanced HCC, as well as patients with more advanced underlying liver disease. Unfortunately, only about 30–40% of patients with HCC respond to immunotherapy and a large proportion of individuals will eventually progress even after demonstrating an initial response. Recent research has focused on overcoming primary and secondary resistance, but the mechanisms remain poorly understood and reliable response biomarkers are still needed. Future progress will require close collaboration and translational work between basic science labs and clinical trial investigators.
Given the propensity for HCC to arise in a dysfunctional liver, patients are prone to recurrence even after a successful resection or ablation. In particular, the underlying non-tumorous environment can promote ongoing carcinogenesis and give rise to either new or recurrent disease. In turn, trials that involve the neoadjuvant and adjuvant setting have been an area of increased interest to treat micrometastatic disease and/or prevent recurrence. In addition, given the relatively high number of patients who present with advanced HCC, there is also interest in using immunotherapy to downstage patients to a resection. Several, small neoadjuvant trials have demonstrated promising results and are currently ongoing. The IMbrave050 trial, a randomized controlled phase III trial designed to study the use of atezolizumab/bevacizumab in the adjuvant setting after resection or ablation of high-risk HCC, recently demonstrated promising results on the interim analysis. To advance treatment, patients with HCC need to be screened and accrued for appropriate clinical trials to accelerate innovation and discovery related to HCC.
Disclosure
Dr Anne M Noonan is a member of steering committee for clinical trial for Astra Zeneca, and a member of the Advisory Board for Elevar Therapeutics and Eisai, outside the submitted work. The authors report no conflict of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi:10.1002/hep.29913
3. Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787–1794. doi:10.1200/JCO.2015.64.7412
4. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
5. Akinyemiju T, Abera S, Ahmed M, et al.; Global Burden of Disease Liver Cancer, C. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi:10.1001/jamaoncol.2017.3055
6. Ahmad MI, Khan MU, Kodali S, et al. Hepatocellular Carcinoma due to nonalcoholic fatty liver disease: current concepts and future challenges. J Hepatocell Carcinoma. 2022;9:477–496. doi:10.2147/JHC.S344559
7. Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13(3):267–276. doi:10.1038/cmi.2016.3
8. Tu J-F, Ding Y-H, Ying X-H, et al. Regulatory T cells, especially ICOS+ FOXP3+ regulatory T cells, are increased in the hepatocellular carcinoma microenvironment and predict reduced survival. Sci Rep. 2016;6(1):35056. doi:10.1038/srep35056
9. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
10. Stefanini B, Ielasi L, Chen R, et al. TKIs in combination with immunotherapy for hepatocellular carcinoma. Expert Rev Anticancer Ther. 2023;23(3):279–291. doi:10.1080/14737140.2023.2181162
11. Rimassa L, Finn RS, Sangro B. Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 2023;79(2):506–515. doi:10.1016/j.jhep.2023.03.003
12. Chow P, Chen M, Cheng AL, et al. IMbrave050: Phase 3 study of adjuvant atezolizumab+ bevacizumab versus active surveillance in patients with hepatocellular carcinoma (HCC) at high risk of disease recurrence following resection or ablation.
13. Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66(3):545–551. doi:10.1016/j.jhep.2016.10.029
14. Mizukoshi E, Yamashita T, Arai K, et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57(4):1448–1457. doi:10.1002/hep.26153
15. Zhu C, Dai B, Zhan H, et al. Neoadjuvant transarterial chemoembolization (TACE) plus PD-1 inhibitor bridging to tumor resection in intermediate-stage hepatocellular carcinoma patients. Ir J Med Sci. 2023;192(3):1065–1071. doi:10.1007/s11845-022-03131-6
16. Ringelhan M, Pfister D, O’Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–232. doi:10.1038/s41590-018-0044-z
17. Hernandez–Gea V, Toffanin S, Friedman SL, et al. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144(3):512–527. doi:10.1053/j.gastro.2013.01.002
18. Giraud J, Chalopin D, Blanc J-F, et al. Hepatocellular Carcinoma immune landscape and the potential of immunotherapies. Front Immunol. 2021;12:655697. doi:10.3389/fimmu.2021.655697
19. Zheng C, Zheng L, Yoo J-K, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356 e16. doi:10.1016/j.cell.2017.05.035
20. Heymann F, Peusquens J, Ludwig‐Portugall I, et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology. 2015;62(1):279–291. doi:10.1002/hep.27793
21. Powles T, van der Heijden MS, Castellano D, et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (Danube): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574–1588. doi:10.1016/S1470-2045(20)30541-6
22. Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. 2020;31(7):942–950. doi:10.1016/j.annonc.2020.04.001
23. Rizvi NA, Cho BC, Reinmuth N, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer. JAMA Oncol. 2020;6(5):661–674. doi:10.1001/jamaoncol.2020.0237
24. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. doi:10.1016/S1470-2045(20)30539-8
25. Wege H, Li J, Ittrich H. Treatment lines in Hepatocellular Carcinoma. Visc Med. 2019;35(4):266–272. doi:10.1159/000501749
26. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
27. Raoul J-L, Bruix J, Greten TF, et al. Relationship between baseline hepatic status and outcome, and effect of sorafenib on liver function: SHARP trial subanalyses. J Hepatol. 2012;56(5):1080–1088. doi:10.1016/j.jhep.2011.12.009
28. Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5(7):345–352. doi:10.4254/wjh.v5.i7.345
29. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
30. Abou-Alfa GK, Meyer T, Cheng A-L, et al. Cabozantinib in patients with advanced and progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
31. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
32. Finn R, Kudo M, Merle P, et al. LBA34 primary results from the phase III LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2022;33:S1401. doi:10.1016/j.annonc.2022.08.031
33. Kelley RK, Rimassa L, Cheng A-L, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(8):995–1008. doi:10.1016/S1470-2045(22)00326-6
34. Granito A, Forgione A, Marinelli S, et al. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol. 2021;14:17562848211016959. doi:10.1177/17562848211016959
35. Tovoli F, Ielasi L, Casadei-Gardini A, et al. Management of adverse events with tailored sorafenib dosing prolongs survival of hepatocellular carcinoma patients. J Hepatol. 2019;71(6):1175–1183. doi:10.1016/j.jhep.2019.08.015
36. Zhu AX, Park JO, Ryoo B-Y, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi:10.1016/S1470-2045(15)00050-9
37. Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26(18):2992–2998. doi:10.1200/JCO.2007.15.9947
38. Zhu AX, Kang Y-K, Yen C-J, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
39. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
40. Cheng A-L, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi:10.1016/j.jhep.2021.11.030
41. Qin S, Chan LS, Gu S, et al. LBA35 Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): a randomized, phase III trial. Ann Oncol. 2022;33(7):S1401–S1402. doi:10.1016/j.annonc.2022.08.032
42. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of lenvatinib plus pembrolizumab in patients with unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/JCO.20.00808
43. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
44. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19(7):940–952. doi:10.1016/S1470-2045(18)30351-6
45. Yau T, Park J-W, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
46. Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced Hepatocellular Carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
47. Qin S, Chen Z, Fang W, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40(4_suppl):383. doi:10.1200/JCO.2022.40.4_suppl.383
48. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/S1470-2045(20)30011-5
49. Lee MS, Ryoo B-Y, Hsu C-H, et al. Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study. Lancet Oncol. 2020;21(6):808–820. doi:10.1016/S1470-2045(20)30156-X
50. Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021;592(7854):450–456. doi:10.1038/s41586-021-03362-0
51. El-Khoueiry AB, Kim RD, Harris WP, et al. Updated results of a phase 1b study of regorafenib (REG) 80 mg/day or 120 mg/day plus pembrolizumab (PEMBRO) for first-line treatment of advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2021;39(15_suppl):4078. doi:10.1200/JCO.2021.39.15_suppl.4078
52. Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced Hepatocellular Carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564. doi:10.1001/jamaoncol.2020.4564
53. Yau T, Zagonel V, Santoro A, et al. Nivolumab plus cabozantinib with or without ipilimumab for advanced Hepatocellular Carcinoma: results from cohort 6 of the CheckMate 040 trial. J Clin Oncol. 2023;41(9):1747–1757. doi:10.1200/JCO.22.00972
54. Kemp A. Imfinzi Plus Tremelimumab Significantly Improved Overall Survival in HIMALAYA Phase III Trial in 1st-Line Unresectable Liver Cancer. Cambridge, Inglaterra: AstraZeneca; 2021.
55. Kelley RK, Sangro B, Harris W, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39(27):2991–3001. doi:10.1200/JCO.20.03555
56. Castria TBD, Khalil DN, Harding JJ, et al. Tremelimumab and durvalumab in the treatment of unresectable, advanced hepatocellular carcinoma. Future Oncol. 2022;18(33):3769–3782. doi:10.2217/fon-2022-0652
57. Di Federico A, Rizzo A, Carloni R, et al. Atezolizumab-bevacizumab plus Y-90 TARE for the treatment of hepatocellular carcinoma: preclinical rationale and ongoing clinical trials. Expert Opin Investig Drugs. 2022;31(4):361–369. doi:10.1080/13543784.2022.2009455
58. Willsmore ZN, Coumbe BGT, Crescioli S, et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: treatment of melanoma and immune mechanisms of action. Eur J Immunol. 2021;51(3):544–556. doi:10.1002/eji.202048747
59. Llovet JM, De Baere T, Kulik L, et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi:10.1038/s41575-020-00395-0
60. Friedman D, Baird JR, Young KH, et al. Programmed cell death-1 blockade enhances response to stereotactic radiation in an orthotopic murine model of hepatocellular carcinoma. Hepatol Res. 2017;47(7):702–714. doi:10.1111/hepr.12789
61. Sheng H, Huang Y, Xiao Y, et al. ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer. 2020;8(1):e000340. doi:10.1136/jitc-2019-000340
62. Chen L, Zhang R, Lin Z, et al. Radiation therapy in the era of immune treatment for hepatocellular carcinoma. Front Immunol. 2023;14:1100079. doi:10.3389/fimmu.2023.1100079
63. de Castro T, Jochheim LS, Bathon M, et al. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol. 2022;14:17588359221080298. doi:10.1177/17588359221080298
64. Rimini M, Persano M, Tada T, et al. Real-world data for atezolizumab plus bevacizumab in unresectable Hepatocellular Carcinoma: how does adherence to the IMbrave150 trial inclusion criteria impact prognosis? Target Oncol. 2023;18(2):221–233. doi:10.1007/s11523-023-00953-x
65. Tanaka T, Hiraoka A, Tada T, et al. Therapeutic efficacy of atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma in patients with Child-Pugh class A or B liver function in real-world clinical practice. Hepatol Res. 2022;52(9):773–783. doi:10.1111/hepr.13797
66. Kim H, Cheon J, Ha Y, et al. Atezolizumab plus bevacizumab in Child-Pugh B advanced hepatocellular carcinoma patients. J Clin Oncol. 2022;40(4_suppl):397. doi:10.1200/JCO.2022.40.4_suppl.397
67. D’Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology. 2022;76(4):1000–1012. doi:10.1002/hep.32468
68. Cheon J, Kim H, Kim HS, et al. Atezolizumab plus bevacizumab in patients with child–Pugh B advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2023;15:17588359221148541. doi:10.1177/17588359221148541
69. Rimini M, Persano M, Tada T, et al. Survival outcomes from atezolizumab plus bevacizumab versus Lenvatinib in Child Pugh B unresectable hepatocellular carcinoma patients. J Cancer Res Clin Oncol. 2023;149(10):7565–7577. doi:10.1007/s00432-023-04678-2
70. Casadei-Gardini A, Rimini M, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a large real-life worldwide population. Eur J Cancer. 2023;180:9–20. doi:10.1016/j.ejca.2022.11.017
71. Wong JS, Kwok GW, Tang V, et al. Nivolumab/Pembrolizumab in Child-Pugh Grade B/C Patients with Advanced HCC. Wolters Kluwer Health; 2021.
72. Fessas P, Kaseb A, Wang Y, et al. Post-registration experience of nivolumab in advanced hepatocellular carcinoma: an international study. J Immunother Cancer. 2020;8(2):e001033. doi:10.1136/jitc-2020-001033
73. Chapin WJ, Hwang W-T, Karasic TB, et al. Comparison of nivolumab and sorafenib for first systemic therapy in patients with hepatocellular carcinoma and Child-Pugh B cirrhosis. Cancer Med. 2023;12(1):189–199. doi:10.1002/cam4.4906
74. Choi W-M, Lee D, Shim JH, et al. Effectiveness and safety of nivolumab in Child–Pugh b patients with hepatocellular carcinoma: a real-world cohort study. Cancers. 2020;12(7):1968. doi:10.3390/cancers12071968
75. Jost‐Brinkmann F, Demir M, Wree A, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma: results from a German real-world cohort. Aliment Pharmacol Ther. 2023;57(11):1313–1325. doi:10.1111/apt.17441
76. Chen Y-H, CHEN -Y-Y, WANG J-H, et al. Efficacy and safety of Lenvatinib after progression on first-line Atezolizumab Plus Bevacizumab treatment in advanced Hepatocellular Carcinoma patients. Anticancer Res. 2023;43(3):1377–1384. doi:10.21873/anticanres.16286
77. Alden SL, Lim M, Kao C, et al. Salvage ipilimumab plus nivolumab in advanced hepatocellular carcinoma after prior anti-PD-(L)1 blockade. J Clin Oncol. 2023;41(16_suppl):4091. doi:10.1200/JCO.2023.41.16_suppl.4091
78. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
79. Mauro E, Forner A. Barcelona clinic liver cancer 2022 update: linking prognosis prediction and evidence-based treatment recommendation with multidisciplinary clinical decision-making. Liver Int. 2022;42(3):488–491. doi:10.1111/liv.15180
80. Sangro B, Melero I, Wadhawan S, et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J Hepatol. 2020;73(6):1460–1469. doi:10.1016/j.jhep.2020.07.026
81. Sangro B, Park J, Finn R, et al. LBA-3 CheckMate 459: long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. Ann Oncol. 2020;31:S241–S242. doi:10.1016/j.annonc.2020.04.078
82. Kaseb AO, Vence L, Blando J, et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in Hepatocellular CarcinomaHCC with complete response after immunotherapy. Cancer Immunol Res. 2019;7(9):1390–1395. doi:10.1158/2326-6066.CIR-18-0605
83. Ang C, Klempner SJ, Ali SM, et al. Prevalence of established and emerging biomarkers of immune checkpoint inhibitor response in advanced hepatocellular carcinoma. Oncotarget. 2019;10(40):4018. doi:10.18632/oncotarget.26998
84. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377(25):2500–2501. doi:10.1056/NEJMc1713444
85. Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers TMB predicts response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi:10.1158/1535-7163.MCT-17-0386
86. Hsu C-H, Lu S, Abbas A, et al. Longitudinal and personalized detection of circulating tumor DNA (ctDNA) for monitoring efficacy of atezolizumab plus bevacizumab in patients with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2020;38(15_suppl):3531. doi:10.1200/JCO.2020.38.15_suppl.3531
87. Reinert T, Henriksen TV, Christensen E, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–1131. doi:10.1001/jamaoncol.2019.0528
88. Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nature Cancer. 2020;1(9):873–881. doi:10.1038/s43018-020-0096-5
89. Li J, Jiang W, Wei J, et al. Patient specific circulating tumor DNA fingerprints to monitor treatment response across multiple tumors. J Transl Med. 2020;18(1):1–12. doi:10.1186/s12967-020-02449-y
90. Winograd P, Hou S, Court CM, et al. Hepatocellular Carcinoma–circulating tumor cells expressing PD-L1 are prognostic and potentially associated with response to checkpoint inhibitors. Hepatol Commun. 2020;4(10):1527–1540. doi:10.1002/hep4.1577
91. Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):1–7. doi:10.1186/s40425-019-0650-9
92. Peng Z, Cheng S, Kou Y, et al. The gut microbiome is associated with clinical response to anti–PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res. 2020;8(10):1251–1261. doi:10.1158/2326-6066.CIR-19-1014
93. Gok Yavuz B, Hasanov E, Lee SS, et al. Current landscape and future directions of biomarkers for immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:1195–1207. doi:10.2147/JHC.S322289
94. Kurebayashi Y, Matsuda K, Ueno A, et al. Immunovascular classification of HCC reflects reciprocal interaction between immune and angiogenic tumor microenvironments. Hepatology. 2022;75(5):1139–1153. doi:10.1002/hep.32201
95. Harding JJ, Nandakumar S, Armenia J, et al. Prospective genotyping of Hepatocellular Carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin Cancer Res. 2019;25(7):2116–2126. doi:10.1158/1078-0432.CCR-18-2293
96. Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, et al. β-catenin activation promotes immune escape and resistance to anti–PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9(8):1124–1141. doi:10.1158/2159-8290.CD-19-0074
97. Rizzo A, Ricci AD. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: how can they assist drug clinical trials? Expert Opin Investig Drugs. 2022;31(4):415–423. doi:10.1080/13543784.2021.1972969
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
