Back to Journals » Infection and Drug Resistance » Volume 17
Reasons, Efficacy and Safety of Switching to Dolutegravir-Based Regimens Among Virologically Suppressed PLWH: A Retrospective Cohort Study of 96 Weeks
Authors Deng M, Chen N, Lao X, Wang X, Fu J, Xing L, Zhao H
Received 5 December 2023
Accepted for publication 12 April 2024
Published 23 April 2024 Volume 2024:17 Pages 1571—1582
DOI https://doi.org/10.2147/IDR.S451346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Meiju Deng, Na Chen, Xiaojie Lao, Xiaolei Wang, Jiantao Fu, Lulu Xing, Hongxin Zhao
Clinical Center for HIV/AIDS, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
Correspondence: Hongxin Zhao, Clinical Center for HIV/AIDS, Beijing Ditan Hospital, Capital Medical University, No. 8, Jingshun Dongjie, Chaoyang District, Beijing, 100015, People’s Republic of China, Email [email protected]
Purpose: The study aimed to explore the reasons, efficacy, and safety of switching to dolutegravir (DTG) based regimens in virologically suppressed people living with HIV (PLWH) in tertiary hospitals in China. Therefore, the study could provide a valuable reference for the rational clinical use of DTG.
Methods: PLWH’s basic information, treatment details, and reasons for switching were collected, through the electrical clinical medical record system and telephone follow-up. Data included the proportion of PLWH with HIV RNA < 50 copies/mL, changes in immunological indicators, and metabolic metrics at week 48 and week 96.
Results: 319 PLWH were included in the analysis. The three major reasons for switching were neurological toxicity (16.30%), simplification (13.79%), and renal toxicity (11.29%). Our study showed high rates of virologic suppression in the per-protocol analysis (week 48: 99.69%; week 96: 99.29%) after switching to DTG-based regimens. The median CD4+ T cell count increased from 579 cells/μL (IQR 420.5– 758) to 642 cells/μL (IQR 466.5– 854) at week 96 (p< 0.0001). An improvement was observed in liver function (ALT: p< 0.0001; AST: p< 0.0001) and fasting glucose (p< 0.0001). However, there was an elevation in creatinine (Cr) (p< 0.0001) and a slight decrease in the estimated glomerular filtration rate (eGFR) (p< 0.0001). Regarding lipid profile, triglyceride (TG) levels declined, while total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels increased. Further analysis revealed that the increase in TC and LDL-C was associated with the withdrawal of tenofovir disoproxil fumarate (TDF). This observed increase in lipid parameters only concerned the PLWH who switched from a TDF-containing regimen to a non-TDF regimen.
Conclusion: This study confirmed the virologic efficacy of switching to DTG-based regimens in virologically suppressed PLWH over a 96-week period. The findings also expanded the evidence of immune reconstitution and metabolic safety associated with this switch.
Keywords: HIV, dolutegravir, switching, virologically suppressed, lipids profile
Introduction
Treatment optimization has always been the primary focus of managing HIV-1. To achieve better virus suppression and reduce long-term toxicity, researchers are constantly exploring new or substitution drugs. One such pharmaceutical option is dolutegravir (DTG), which is used to combat Human Immunodeficiency Virus type-1 (HIV-1) infection. As a second-generation integrase strand transfer inhibitor (INSTI) of HIV-1, DTG fits loosely into the intasome binding pocket and can retain its binding ability even with conformational changes in the pocket structure.1 This ability to subtly readjust its binding position enhances the genetic barrier to resistance, resulting in high virologic efficacy.2 Additionally, DTG is generally well tolerated, with rare severe side effects and a low potential for drug-drug interactions.3,4
The World Health Organization (WHO) and other guidelines have recommended DTG as a core drug for both naïve and treatment-experienced PLWH.5–7 In China, DTG was approved by the National Medical Products Administration in 2015 for the treatment of PLWH. However, DTG was not included in the Chinese medical insurance reimbursement catalog until 2021. Despite partial costs covered by medical insurance, PLWH still has to bear a portion of the financial burden, which severely hinders the widespread application of DTG. Limited data exists regarding its use in China. Therefore, it’s necessary for us to retrospectively analyze the results of switching to DTG-based regimens.
In this study, we presented data on virologically suppressed PLWH and explored the factors that led to switching to DTG-based regimens. A retrospective study over a period of 96 weeks was conducted to assess the virologic efficacy, immunologic profile, and metabolic profile of using DTG as a switch strategy.
Methods
Study Design
We conducted a retrospective analysis of treatment-experienced PLWH who switched to DTG-based regimens and assessed their outcomes over a 96-week period. We enrolled PLWH in Beijing Ditan Hospital from 2018 to 2023 who underwent a therapeutic switch to DTG-based regimens with a follow-up period of 96 weeks. PLWH’s basic information, treatment details, reasons for switching, and laboratory indicators were collected through the electrical clinical medical record system and telephone follow-up. All PLWH met the following inclusion criteria: (a) being at least 18 years old; (b) being on stable (at least 6 months) antiretroviral therapy (ART) with viral suppression (HIV-RNA<50 copies/mL) at the time of switching to DTG-based regimens (week 0); (c) having a follow-up period of at least 48 weeks; (d) having follow-up data.
The primary endpoint was the rate of virologic control (defined as HIV-RNA < 50 copies/mL) at week 48 and week 96. Secondary outcomes of the study were: 1. The reasons for switching to DTG. 2. Immune changes (CD4+ T cell count, CD8+ T cell count and CD4/CD8 ratio). 3. Metabolic changes (liver function: alanine aminotransferase (ALT), aspartate aminotransferase (AST)); fasting glucose (GLU); renal function: urea, creatinine (Cr), uric acid (UA), estimated glomerular filtration rate (eGFR); blood lipids: total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and total cholesterol /high-density lipoprotein cholesterol (TC/HDL-C).
Definitions
The HIV diagnosis and AIDS definitions followed the definition of WHO. Viral suppression was defined as having an undetectable viral load, of less than 50 copies/mL.
Virological failure was defined as a viral load > 50 copies/mL confirmed on two consecutive occasions or a single viral load determination > 400 copies/mL. The definitions for abnormal cholesterol levels were based on the Chinese Guidelines for Lipid Management (2023). A normal level of TC was defined as less than 5.2 mmol/L. Borderline high TC was defined as equal to or greater than 5.2 mmol/L but less than 6.2 mmol/L. A high level of TC was defined as equal to or greater than 6.2 mmol/L. A normal level of LDL-C was defined as less than 3.4 mmol/L. Borderline high LDL-C was defined as equal to or greater than 3.4 mmol/L but less than 4.1 mmol/L. A high level of LDL-C was defined as equal to or greater than 4.1 mmol/L.
Statistical Analysis
Data were displayed as median values and interquartile range (IQR), or simple percentages (%), according to the variable type, continuous or categorical, respectively. The change from week 0 was assessed by raw absolute difference (eg, week 0–96= week 96 value-week 0 value) or percentage of median relative difference (eg, MRD% (week 0–96) =100 × (week 96 value-week 0 value)/week 0 value). The normality of variable distribution was assessed by the Kolmogorov–Smirnov test. Longitudinal analysis was assessed by the paired Wilcoxon test for continuous variables. For all analyses, P < 0.05 was considered statistically significant. All data were analyzed with GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA).
Results
Population Characteristics at the Time of the Switch
Between 2018 and 2023, 579 individuals were screened for eligibility, of which 319 met the inclusion criteria (Figure 1). The characteristics of 319 PLWH at the time of switching were summarized in Table 1. The majority of PLWH were male (94.67%), and the median age was 35 years (IQR 30–41). Prior to switching to DTG-based regimens, the median duration of ART was 3.83 years (IQR 2.17–6.67). A total of 14 individuals among 319 PLWH had experienced virological failure. Out of the 319 PLWH, 193 individuals (60.5%) were on a regimen of two nucleoside reverse transcriptase inhibitors (2NRTIs) plus a non-nucleoside reverse transcriptase inhibitor (NNRTI), with the majority of these individuals (182) receiving efavirenz (EFV). Additionally, 76 individuals (23.82%) were on a regimen of 2NRTIs plus INSTI. The remaining 50 individuals (15.67%) received a regimen of 2NRTIs plus a protease inhibitor (PI). After switching, PLWH received the following combinations: DTG+3TC (49.53%), DTG+3TC+TDF (19.75%), DTG+FTC+TAF (16.61%), DTG+3TC+ABC (10.34%), and DTG+3TC+AZT (3.76%). In other words, 158 individuals (49.53%) switched to a DTG-based two-drug regimen (DTG-2DR), while 161 individuals (50.47%) switched to a DTG-based three-drug regimen (DTG-3DR).
 |
Table 1 Characteristics of the Study Population at the Start of Switching (Week 0) (N=319) |
 |
Figure 1 Study profile. Abbreviation: DTG, dolutegravir. |
The Reasons for Switching
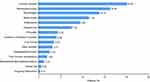
The most common reason for switching, among known causes in our study, was neurological toxicity (16.30%). Other frequent causes included simplification (13.79%), renal toxicity (11.29%), dyslipidemia (9.40%), osteoporosis (7.52%), and pill burden (4.39%) (Figure 2). Totally, most PLWH switched to DTG-based regimens due to adverse reactions, while a minority were seeking an optimal treatment proposal.
Efficacy
No missing data were recorded at week 48, therefore the intention-to-treat analysis (ITT, where missing equal failure) and the per-protocol analysis (PP, where missing equal excluded) yielded the same results. The virologic suppression rate at week 48 was 99.69% (318/319). However, at week 96, there were missing virologic data for a total of 39 PLWH. Among them, the follow-up time for 24 PLWH did not reach 96 weeks, so their viral load at week 96 could not be assessed. Additionally, 15 PLWH were lost to follow-up at week 96, as transferred elsewhere for care. In the ITT analysis at week 96, the rate of virologic suppression was 87.15% (278/319). In the PP analysis, the rate of virologic suppression was 99.29% (278/280). One PLWH had an HIV-RNA of 59 copies/mL at week 48 but achieved virologic suppression again at week 96. Furthermore, two PLWH experienced low-level viremia at week 96 (HIV-RNA 55 copies/mL and 90 copies/mL) (Figure 3A).
Meanwhile, the CD4+ T cell count showed a gradual improvement over the follow-up weeks. The CD4+ T cell absolute count and proportion increased by 31 cells/μL, +6.4% (p<0.0001), and 65 cells/μL, +12.49% (p<0.0001) at week 48 and week 96 compared to week 0, respectively. However, the CD8+ cell count did not change over time (week 0–48: p=0.1619; week 0–96: p=0.22135). A modest increase in the CD4/CD8 ratio was observed (week 0–48: +5.882%, p < 0.001; week 0–96: +13.68%, p < 0.001) (Table 2, Figure 3B).
 |
Table 2 Immunological and Metabolic Changes at Pre-Switching (Week 0) and Post-Switching (Week 48 and Week 96) |
Safety
Liver Function and Fasting Glucose
A significant reduction in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was observed from week 0 to week 96 (ALT: −10.32%, p<0.0001; AST: −12.69%, p<0.0001) (Table 2, Figure 4A). Additionally, there was a parallel decline in fasting glucose (GLU) (week 0–96: −4.519%, p<0.0001) (Table 2, Figure 4A).
Renal Function
No pronounced changes in urea were found over time (week 0–96: p=0.2011). However, creatinine (Cr) elevated from 80.9 mg/dL (IQR 70.53–91.85) at week 0 to 85.2 mg/dL (IQR 78.65–94.45) at week 96 (p<0.0001), with an MRD% (percentual median relative difference) of +4.887%. A similar trend was observed in uric acid (UA) which changed from 361 μmol/L (IQR 307–421) at week 0 to 377 μmol/L (IQR 329–435.5) at week 96 (p<0.0001), with an MRD% of +4.884%. The median estimated glomerular filtration rate (eGFR) slightly decreased from 106.2 mL/min/1.73m2 (IQR 92.58–117.7) at week 0 to 100.6 mL/min/1.73m2 (IQR 89.1–110.3) at week 96 (p<0.0001), with an MRD% of −5.445% (Table 2, Figure 4B). Notably, no significant changes were detected between week 48 and week 96 in terms of the indicators of renal function (UREA: p=0.3867, Cr: p=0.6374, UA: p=0.3630, eGFR: p=0.6716) (Table 2, Figure 4B), suggesting that the renal function of PLWH reduced in the first year after switching and then remained relatively stable in the second year.
Blood Lipids
Regarding lipid profile, no significant change was found in total cholesterol (TC) between week 48 and week 0 (p=0.7394). Nonetheless, there was a mild median increase of 0.09 mmol/L in TC (MRD%=+2.062%, p=0.0456) and 0.18 mmol/L in low-density lipoprotein cholesterol (LDL-C) (MRD%=+7.042%, p<0.0001) at week 96 (Table 2, Figure 4C). PLWH experienced a significant 8.824% reduction in triglyceride (TG) at week 96 (p=0.0099). There were no statistical changes in high-density lipoprotein cholesterol (HDL-C) (p=0.6979) and TC/HDL-C ratio (p=0.1051) at week 96. Median values and changes from week 0 were presented in Table 2.
Further analysis was performed to investigate the cause of the increase in TC and LDL-C. It was speculated that the rise in blood lipids could be attributed to the discontinuation of tenofovir disoproxil fumarate (TDF), considering the positive impact of TDF on lipid parameters. Thus, the individuals were divided into different groups based on their pre-switch and post-switch ART regimens. Group 1 consisted of individuals who switched from a TDF-containing regimen to a non-TDF regimen (201/319). Group 2 included those who switched from a TDF-containing regimen to another TDF-containing regimen (58/319). Group 3 comprised individuals who switched from a non-TDF regimen to another non-TDF regimen (55/319). Group 4, which had a small sample size (5/319), was excluded from the analyses.
In Group 1, there was a significant increase in TC and LDL-C at week 96 (TC: p<0.0001; LDL-C: p<0.0001). Conversely, Group 2 showed a decrease in TC and LDL-C at week 48 (TC: p<0.0001; LDL-C: p=0.0434), but no significant change at week 96 (TC: p=0.3169; LDL-C: p=0.5572). Group 3 did not experience any significant changes in TC or LDL-C over time (TC: p=0.3161; LDL-C: p=0.3161) (Figure 5A). Furthermore, the severity grading of dyslipidemia indicated a mild increase in Group 1 and a decrease in both Group 2 and Group 3 (Figure 5B). Notably, Group 2 consistently maintained lower levels of TC and LDL-C compared to Group 1 and Group 3 throughout the observation period (Table 2).
Discussion
The results of this retrospective, observational study supported the use of DTG-based regimens as effective maintenance therapies in virologically suppressed PLWH. DTG-based regimens provided long-lasting virological suppression and promoted immunological recovery. Furthermore, there was some improvement in metabolic changes.
In our study, PLWH had a long treatment history before transitioning to DTG-based regimens, with a median time since ART initiation of 3.83 years (IQR 2.17–6.67). It was worth noting that the majority of PLWH (53.61%) were compelled to switch drugs due to intolerable adverse reactions. Previous studies have reported that PLWH was plagued by severe EFV-related adverse events, particularly neurological toxicity and dyslipidemia.8,9 Moreover, TDF was found to potentially cause renal impairment and reduce bone mineral density.10,11 Gastrointestinal toxicity12 and dyslipidemia, especially an increase in TC and TG,13 were common side effects of Lopinavir/ritonavir (LPV/r). In our study, 182 individuals (57.05%) received EFV, 230 individuals (72.10%) received TDF, and 50 individuals (15.67%) received LPV/r in their pre-switch regimens. Overall, for most PLWH, the decision to switch drugs was made after experiencing serious adverse events, rather than for preventing severe side effects. It is crucial for PLWH, especially those at a high risk of diabetes, cardiovascular diseases, or other underlying diseases, to switch to drugs with effective viral suppression and rare severe side effects in a timely manner.
DTG showed high rates of virologic suppression in PLWH based on the PP analysis at week 48 and week 96, achieving a rate of virological suppression close to 100%. Nonetheless, in the ITT analysis, the rate of virologic suppression at week 96 was 87.15% (278/319), with 39 PLWH missing virologic data. Consequently, the statistical power of the analyses involving week 96 was slightly reduced due to incomplete follow-up of all participants. Increasing the sample size or obtaining as much data as possible could enhance the statistical power of the analyses. Additionally, the CD4+ T cell count and CD4/CD8 ratio showed consistent improvement, as reported in other studies.14–16 Overall, these findings suggested that switching to DTG-based regimens effectively maintains viral suppression and continuously improves the immunity of PLWH.
Consistent with previous studies,17 the liver function of PLWH was beneficial, even though DTG was primarily metabolized by uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) and only a minor substrate for cytochrome P450 (P450) 3A4 (CYP3A4) in the liver.3,4 This was partly explained by that DTG had no inhibition or induction of the UGT1A1 and CYP enzymes at clinically relevant concentrations.3 However, clinical trials excluded PLWH with severe hepatic impairment. Therefore, further research is needed to investigate the potential liver toxicity of DTG in PLWH with severe hepatic impairment.
Despite renal excretion of DTG being negligible,3,18 a slight decrease in renal function at week 48 was observed in our study, with overall stability from week 48–96. In fact, early investigations revealed that DTG mediated a modest, non-progressive increase in Cr by inhibiting organic cation transporter (OCT) 2, resulting in a reduction in tubular secretion of Cr without affecting actual eGFR.3,19 However, in our data, glomerular filtration rate (eGFR) was estimated by serum Cr. Therefore, serum Cr-based estimated GFR (eGFRCr) of PLWH received DTG could be lower than actual eGFR. Serum Cystatin C is another marker for estimation of GFR (eGFRCysC). Produced by nucleated cells at a constant rate, Cystatin C is freely filtered without tubular secretion or reabsorption and catabolized in the proximal tubule. Therefore, it is more reliable to use Cystatin C levels for assessing glomerular filtration rate (eGFRCysC) than merely serum Cr when evaluating renal function (eGFRcr) in DTG-treatment PLWH.20,21 Thus, it is necessary to regularly monitor Cystatin C levels and combinate more renal biomarkers, such as microalbumin and β2-microglobulin in urine to accurately assess renal function.
Data from the EuroSIDA study revealed that cardiovascular events accounted for approximately one-third of non-AIDS-defining events.22 Atherogenic dyslipidemia (elevated TC and decreased HDL-C), recognized as a risk factor for cardiovascular disease, was found to be the most common pattern of lipid abnormalities in PLWH.23,24 This dyslipidemia has become an important factor affecting the prognosis and quality of life of PLWH.25,26 Previous studies demonstrated a favorable lipid profile among treatment-experienced PLWH switching to DTG-2DR and DTG-3DR, or at least no detrimental effects.27–31 The TANGO trial,14 a large clinical study on treatment simplification, showed that switching from triple TAF-based regimens to DTG+3TC brought about declines of 4.5% in TC, 5.5% in LDL-C, and 11.2% in TG. While the SALSA study15 found that the lipid profile remained generally unchanged from baseline. In the case of DTG-3DR, the NEAT022 study showed that TC and other lipid fractions (except HDL-C) prominently improved after switching from a boosted PI-based regimen to a DTG-based regimen.16 Consistent with the results of the NEAT022 study, the CHARACTERISE study also reported a slightly improved lipid profile.31 However, our study observed an increase in the proatherogenic lipid fractions (TC and LDL-C), despite a significant improvement in TG. This variation could be partly explained by the high proportion of individuals (63.01%, 201/319) switching away from TDF-containing regimens, considering the previously described metabolic effects of TDF.32,33 As expected, further analysis confirmed that the increase in TC and LDL-C was related to the withdrawal of TDF. Thus, it appears necessary to closely monitor lipid parameters in PLWH who switch away from TDF-containing regimens.
Our study had several limitations. Firstly, it was a single-center study and potential bias existed in our population of PLWH. Secondly, this study was a retrospective study with some pertinent data missing. And we did not have detailed ART adherence information. Finally, we only collected routine laboratory data, with bone density measures and urine protein biomarkers not included in our study.
Conclusion
The primary reason for PLWH to switch ART regimens was adverse reactions. After switching to DTG-based regimens, HIV viral suppression was consistently maintained and the parameters of immunity and metabolism were partly improved. These findings reinforce the reliability of DTG-based regimens as an effective switching strategy for PLW. DTG-based treatments prove to be successful options for individuals who are virologically suppressed and may potentially improve patient adherence. There’s a need to enhance doctor-patient communication to help patients in selecting more effective treatments in a timely manner.
Ethical Approval
This study was approved by the Committee of Ethics at Beijing Ditan Hospital, Capital Medical University, Beijing, China (No. 2021-22-01). Written informed consent was obtained from all participants before study initiation. The study complied with the Declaration of Helsinki.
Acknowledgments
We would like to thank the PLWH who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Capital health development scientific research project (2024-2-2175).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Hare S, Smith SJ, Métifiot M, et al. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572). Mol Pharmacol. 2011;80(4):565–572. doi:10.1124/mol.111.073189
2. Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res. 2017;239:1–9. doi:10.1016/j.virusres.2016.07.006
3. Reese MJ, Savina PM, Generaux GT, et al. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41(2):353–361. doi:10.1124/dmd.112.048918
4. Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet. 2013;52(11):981–994. doi:10.1007/s40262-013-0093-2
5. World Health Organization. WHO recommends dolutegravir as preferred HIV treatment option in all populations. Available from: https://www.who.int/news/item/22-07-2019-whorecommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations.
6. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324(16):1651–1669. doi:10.1001/jama.2020.17025
7. Ryom L, Cotter A, De Miguel R, et al.; EACS Governing Board. 2019 update of the European AIDS Clinical Society Guidelines for treatment of people living with HIV version 10.0. HIV Med. 2020;21(10):617–624. doi:10.1111/hiv.12878
8. Ciccarelli N, Fabbiani M, Di Giambenedetto S, et al. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76(16):1403–1409. doi:10.1212/WNL.0b013e31821670fb
9. Prasithsirikul W, Chongthawonsatid S, Ohata PJ, et al.; PROGRESS study team. Depression and anxiety were low amongst virally suppressed, long-term treated HIV-infected individuals enrolled in a public sector antiretroviral program in Thailand. AIDS Care. 2017;29(3):299–305. doi:10.1080/09540121.2016.1201194
10. Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. 2013;24(10):1519–1527. doi:10.1681/ASN.2012080857
11. Del Palacio M, Romero S, Casado JL. Proximal tubular renal dysfunction or damage in HIV-infected patients. AIDS Rev. 2012;14(3):179–187.
12. Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372(9639):646–655. doi:10.1016/S0140-6736(08)61081-8
13. Hill A, Sawyer W, Gazzard B. Effects of first-line use of nucleoside analogues, efavirenz, and ritonavir-boosted protease inhibitors on lipid levels. HIV Clin Trials. 2009;10(1):1–12. doi:10.1310/hct1001-1
14. Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the Phase 3, Noninferiority TANGO randomized trial. Clin Infect Dis. 2022;75(6):975–986. doi:10.1093/cid/ciac036
15. Llibre JM, Brites C, Cheng CY, et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with Human Immunodeficiency Virus 1 (HIV-1): week 48 results from the Phase 3, noninferiority SALSA randomized trial. Clin Infect Dis. 2023;76(4):720–729. doi:10.1093/cid/ciac130
16. Gatell JM, Assoumou L, Moyle G, et al.; European Network for AIDS Treatment 022 (NEAT022) Study Group. Immediate versus deferred switching from a boosted protease inhibitor-based regimen to a dolutegravir-based regimen in virologically suppressed patients with high cardiovascular risk or age ≥50 years: final 96-week results of the NEAT022 Study. Clin Infect Dis. 2019;68(4):597–606. doi:10.1093/cid/ciy505
17. Santos JR, Saumoy M, Curran A, et al.; Tenofovir/emtricitabine inflUence on LIPid metabolism (TULIP) Study Group. The lipid-lowering effect of tenofovir/emtricitabine: a randomized, crossover, double-blind, placebo-controlled trial. Clin Infect Dis. 2015;61(3):403–408. doi:10.1093/cid/civ296
18. Weller S, Borland J, Chen S, et al. Pharmacokinetics of dolutegravir in HIV-seronegative subjects with severe renal impairment. Eur J Clin Pharmacol. 2014;70(1):29–35. doi:10.1007/s00228-013-1590-9
19. van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. doi:10.1016/S1473-3099(11)70290-0
20. Galizzi N, Galli L, Poli A, Spagnuolo V, Castagna A, Gianotti N. Glomerular filtration rate estimated by cystatin C formulas in HIV-1 patients treated with dolutegravir, rilpivirine or cobicistat. New Microbiol. 2018;41(4):256–261.
21. Lu L, Li X, Liu X, et al. Comparison of renal function biomarkers of serum creatinine and cystatin C in HIV-infected people on dolutegravir-containing therapy. Infect Drug Resist. 2022;15:1695–1706. doi:10.2147/IDR.S347054
22. Mocroft A, Reiss P, Gasiorowski J, et al.; EuroSIDA Study Group. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55(2):262–270. doi:10.1097/QAI.0b013e3181e9be6b
23. da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: old and new drugs. World J Virol. 2015;4(2):56–77. doi:10.5501/wjv.v4.i2.56
24. Myerson M. Lipid management in human immunodeficiency virus. Endocrinol Metab Clin North Am. 2016;45(1):141–169. doi:10.1016/j.ecl.2015.09.010
25. Stanley TL, Grinspoon SK. Body composition and metabolic changes in HIV-infected patients. J Infect Dis. 2012;205(Suppl 3):S383–S390. doi:10.1093/infdis/jis205
26. Paula AA, Schechter M, Tuboi SH, et al. Continuous increase of cardiovascular diseases, diabetes, and non-HIV related cancers as causes of death in HIV-infected individuals in Brazil: an analysis of nationwide data. PLoS One. 2014;9(4):e94636. doi:10.1371/journal.pone.0094636
27. Borghetti A, Baldin G, Lombardi F, et al. Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med. 2018;19(7):452–454. doi:10.1111/hiv.12611
28. Baldin G, Ciccullo A, Rusconi S, et al. Long-term data on the efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multi-centre cohort of HIV-1-infected, virologically suppressed patients. Int J Antimicrob Agents. 2019;54(6):728–734. doi:10.1016/j.ijantimicag.2019.09.002
29. Maggiolo F, Gulminetti R, Pagnucco L, et al. Lamivudine/dolutegravir dual therapy in HIV-infected, virologically suppressed patients. BMC Infect Dis. 2017;17(1):215. doi:10.1186/s12879-017-2311-2
30. Bendala-Estrada AD, Diaz-Almiron M, Busca C, et al. Change in metabolic parameters after switching from triple regimens with tenofovir alafenamide to dolutegravir-based dual therapy. Bi-lipid study. HIV Med. 2023;24(5):558–567. doi:10.1111/hiv.13432
31. Bosch B, Akpomiemie G, Chandiwana N, et al. Weight and metabolic changes after switching from Tenofovir Alafenamide/Emtricitabine (FTC)+Dolutegravir (DTG), Tenofovir Disoproxil Fumarate (TDF)/FTC + DTG, and TDF/FTC/Efavirenz to TDF/Lamivudine/DTG. Clin Infect Dis. 2023;76(8):1492–1495. doi:10.1093/cid/ciac949
32. Kauppinen KJ, Aho I, Sutinen J. Switching from tenofovir alafenamide to tenofovir disoproxil fumarate improves lipid profile and protects from weight gain. AIDS. 2022;36(10):1337–1344. doi:10.1097/QAD.0000000000003245
33. Milinkovic A, Berger F, Arenas-Pinto A, Mauss S. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS. 2019;33(15):2387–2391. doi:10.1097/QAD.0000000000002350
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.