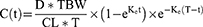Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 15
Recommendations of Gentamicin Dose Based on Different Pharmacokinetic/Pharmacodynamic Targets for Intensive Care Adult Patients: A Redefining Approach
Authors Abbasi MY , Chaijamorn W, Wiwattanawongsa K, Charoensareerat T , Doungngern T
Received 14 April 2023
Accepted for publication 27 June 2023
Published 4 July 2023 Volume 2023:15 Pages 67—76
DOI https://doi.org/10.2147/CPAA.S417298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Mohammad Yaseen Abbasi,1 Weerachai Chaijamorn,2 Kamonthip Wiwattanawongsa,1 Taniya Charoensareerat,3 Thitima Doungngern1
1Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Prince Songkla University, Hat Yai, Songkhla, 90110 Thailand; 2Department of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Chulalongkorn University, Pathum Wan, Bangkok, 10330, Thailand; 3Faculty of Pharmacy, Siam University, Bangkok, 10160, Thailand
Correspondence: Thitima Doungngern, Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90110, Thailand, Tel +66-74288877, Fax +66-74428222, Email [email protected]
Background: In addition to the maximum plasma concentration (Cmax) to the minimum inhibitory concentration (MIC) ratio, the 24-hour area under the concentration-time curve (AUC24h) to MIC has recently been suggested as pharmacokinetic/pharmacodynamic (PK/PD) targets for efficacy and safety in once-daily dosing of gentamicin (ODDG) in critically ill patients.
Purpose: This study aimed to predict the optimal effective dose and risk of nephrotoxicity for gentamicin in critically ill patients for two different PK/PD targets within the first 3 days of infection.
Methods: The gathered pharmacokinetic and demographic data in critically ill patients from 21 previously published studies were used to build a one-compartment pharmacokinetic model. The Monte Carlo Simulation (MCS) method was conducted with the use of gentamicin once-daily dosing ranging from 5– 10 mg/kg. The percentage target attainment (PTA) for efficacy, Cmax/MIC ~8– 10 and AUC24h/MIC ≥ 110 targets, were studied. The AUC24h > 700 mg⋅h/L and Cmin > 2 mg/L were used to predict the risk of nephrotoxicity.
Results: Gentamicin 7 mg/kg/day could achieve both efficacy targets for more than 90% when the MIC was < 0.5 mg/L. When the MIC increased to 1 mg/L, gentamicin 8 mg/kg/day could reach the PK/PD and safety targets. However, for pathogens with MIC ≥ 2 mg/L, no studied gentamicin doses were sufficient to reach the efficacy target. The risk of nephrotoxicity using AUC24h > 700 mg⋅h/L was small, but the risk was greater when applying a Cmin target > 2 mg/L.
Conclusion: Considering both targets of Cmax/MIC ~8– 10 and AUC24h/MIC ≥ 110, an initial gentamicin dose of 8 mg/kg/day should be recommended in critically ill patients for pathogens with MIC of ≤ 1 mg/L. Clinical validation of our results is essential.
Keywords: gentamicin, Cmax/MIC, AUC24h/MIC, nephrotoxicity, critically ill
Introduction
Gentamicin is an aminoglycoside antibiotic which effectively treats serious Gram-negative infections.1 In addition, global Gram-negative resistance rates are increasing and there is limited availability of antibiotics to treat the emergence of resistance.2 An appropriate empirical dose of gentamicin can be suggested based on local epidemiological data and susceptibility patterns. Critical illness and severe infection lead to altered pathophysiology and gentamicin pharmacokinetics. Increased volume of distribution (Vd) is frequently reported in critically ill patients.2,3 Furthermore, augmented renal clearance of gentamicin in patients with sepsis, severe trauma, undergone major surgeries, or use of inotropic agents also plays a role in an alteration of gentamicin pharmacokinetics.4 Therefore, appropriate dosing regimens of gentamicin in a timely manner, particularly during the acute phase of illness, are essential for successful therapeutic outcomes.
Despite the “concentration-dependent” bactericidal activity, the pharmacokinetic and pharmacodynamic (PK/PD) target of gentamicin therapy remains inconclusive in critically ill patients. The maximum gentamicin plasma concentration (Cmax) to the minimum inhibitory concentration (MIC) ratio from 8–10 is advocated to justify the prediction of efficacy in critically ill patients for treatment of infections.4,5 However, in vivo study reported that the 24-hour area under the concentration-time curve to MIC (AUC24h/MIC) ratio around 30–50 was associated with bacteriostasis, and the ratios around 80–100 were associated with 1 and 2 log10 bacterial reduction, respectively.3,6 In addition, the AUC24h/MIC ratio ≥110 significantly improved the clinical cure in patients with serious Gram-negative bacterial infections.7,8 As a result, the AUC24h/MIC ratio ≥110 has recently been proposed as an appropriate PK/PD target for severe infection.6–9 From a safety perspective, AUC24h >700 mg⋅h/L and the minimum gentamicin plasma concentration (Cmin) >2 mg/L are also suggested as the indicator for nephrotoxicity.9,10 To date, no studies have evaluated the achievement of gentamicin both for PK/PD targets for efficacy (Cmax/MIC and AUC24h/MIC ratios) and risk of nephrotoxicity (AUC24h and Cmin) in critically ill patients. The purpose of this is to define optimal once-daily gentamicin dosing regimens for efficacy and safety in critically ill patients during an acute illness (first 72 hours of infection).
Materials and Methods
Search Strategy, Study Selection and Data Extraction
The following Medical Subject Heading (MeSH) terms: “gentamicin”, “pharmacokinetics”, “critically ill”, “intensive care” and synonymous words were used to search in PubMed, EMBASE, SCOPUS, CINAHL and EBSCO. All original publications of gentamicin traditional pharmacokinetic studies in humans and in English that entered the databases by September 2022 were included. The searched studies were focused to extract individual or study-based pharmacokinetics data with mean and standard deviation. In addition, we also conducted a review on online available secondary references and included these if eligible. Two investigators (AMY and TD) independently found and evaluated studies for potential inclusion and exclusion.
Pharmacokinetic Model Development/Mathematical Pharmacokinetic Models
The traditional gentamicin pharmacokinetic studies in critically ill adult patients were included for evaluation. The studies were excluded if they included pregnant women, patients receiving extracorporeal membrane oxygenation, or renal replacement therapy. All selected published articles based on inclusion and exclusion criteria have undergone extensive scrutiny to gather the required pharmacokinetic parameters of gentamicin to generate the time versus concentration profiles (Figure S1). Data extracted from the selected publications of gentamicin pharmacokinetic parameters (Vd, CL, Ke) and total body weight were combined to calculate mean and standard deviation. In addition, the upper and lower range of each parameter were extracted and used in the MCS.
Furthermore, in a clinical setting gentamicin concentration best fit with the one-compartment pharmacokinetic model.5 Hence, in this study, a one-compartment model with first-order elimination was developed to predict gentamicin concentration for 72 hours of the initial antibiotic therapy. The gentamicin dosage was calculated based on total body weight. The equation used in the model to calculate gentamicin plasma concentrations in individual virtual patients is shown in equation 1. The AUC24h for each day was calculated by using the trapezoidal formula for each virtual individual.
Where D = gentamicin dose (mg/kg), TBW = total body weight (kg), Ke = elimination rate constant, t = infusion time (0.5 hour); T = dosing interval (24 hours), and CL= Clearance.
Monte Carlo Simulations
The mean, standard deviation (SD), upper and lower PK parameters for gentamicin obtained from the selected studies were used to build 10,000 virtual patients using the Crystal Ball, Oracle software.11 Since the targeted population in the simulation was adults, total body weight (kg) was set at the range 40 to infinity in MCS analysis. Gentamicin plasma concentration versus time profiles from 0–72 hours were generated which were divided into first (0–24 h), second (24–48 h), and third day (48–72 h). The processes mentioned in the above section were repeated to assess the various doses regimen ranging from 5–10 mg/kg once-daily gentamicin.
Probability of Target Attainment Prediction
The probability of target attainment (PTA) was estimated employing PK/PD targets for efficacy, i.e., AUC24h/MIC, and Cmax/MIC with a set of distinctive MIC values. The gentamicin dosing efficacy and risk of nephrotoxicity were assessed for the first and third day by considering Cmin >2 mg/L and AUC24h >700 mg⋅h/L.10,12 The optimal gentamicin dose recommended for critically ill patients was defined as the dose that achieves at least 90% of the efficacy target with the minimum risk of nephrotoxicity. MICs were set at 0.5, 1, 2, and 4 mg/L, representing a variety of susceptible pathogen inhibitions as determined by the United States Committee on Antimicrobial Susceptibility Testing (USCAST).13
Results
Gentamicin pharmacokinetic parameters selected from 21 studies were gathered for the simulation (Table S1).14–34 This included critically ill patients admitted to medical, surgical, and traumatic care units. Among a total of 1215 patients, 90.5% of were confirmed for severe infections. Seven studies reported gentamicin PK parameters from the first dose, for which plasma levels during the acute phase (within 48–72 hours) were retrieved. Steady state PK parameters were obtained from another 2 studies. The pooled values (mean ± SD) of Vd, CL, and Ke of gentamicin during the acute phase were 0.33± 0.20 L/kg, 4.70 ± 2.89 L/h, and 0.18 ± 0.10 h−1, respectively. Total body weight (70.8 ± 19.9 kg) was used as dosing weight in these critically ill patients. Additional parameters used in the model are presented in Table S2.
The PTAs for the gentamicin efficacy in severe infection (AUC24h/MIC ≥110) are shown in Table 1. Based on AUC24h/MIC ≥110, no gentamicin regimens were sufficient to produce the optimal efficacy in critically ill patients for MIC ≥1 mg/L. Gentamicin 7 mg/kg/day was adequate for MIC ≤0.5 mg/L. Figure 1 presents the PTAs for various AUC24h/MIC targets. Gentamicin 5 and 7 mg/kg/day were the optimal doses for achieving the bacteriostatic (target AUC24h/MIC > 50) for MIC 0.5 and 1 mg/L, respectively.
 |
Table 1 Probability of Target Attainment (% PTA) for Gentamicin Regimens Achieving AUC24h/MIC ≥110 for MIC of 0.5, 1, 2, and 4 mg/L on Day 1 and Day 3 of Therapy in Critically Ill Patients |
For the pathogens with MIC of 0.5 mg/L, the optimal gentamicin dose using Cmax/MIC ≥8 and ≥10 was 5 mg/kg/day. However, when MIC increased to 1 mg/L, the optimal gentamicin doses were 8 and 10 mg/kg/day for the target Cmax/MIC ≥ 8 and ≥ 10, respectively (see Table 2). No gentamicin regimens were sufficient to produce the optimal target of Cmax/MIC in critically ill patients when MIC ≥2 mg/L.
 |
Table 2 Probability of Target Attainment (% PTA) for Gentamicin Regimens Achieving Cmax/MIC Ratio of ≥ 8, and ≥ 10 for MIC of 0.5, 1, 2, and 4 Mg/L on Day 1 of Therapy in Critically Ill Patients |
Table 3 presents the risk of nephrotoxicity, focusing on either AUC24h >700 mg⋅h/L, or Cmin >2 mg/L. In this study, the probability of developing nephrotoxicity depended on gentamicin dose and duration of therapy. Higher incidence of nephrotoxicity was recorded with criteria of minimum plasma gentamicin level (Cmin >2 mg/L), compared with the AUC24h>700 mg⋅h/L.
 |
Table 3 The Probability of Developing Nephrotoxicity Predicted by AUC24h >700 mg*h/L and Cmin > 2 mg/L on Day 1 and Day 3 of Gentamicin Therapy in Critically Ill Patients |
Discussion
Gentamicin is known for its concentration-dependent activity and can be predicted by Cmax and Cmin for the efficacy and risk of nephrotoxicity or ototoxicity. Hence once-daily dosing is more favorable for clinical practice. During therapeutic drug monitoring, individualized therapy using a Bayesian prediction method avoids the problem of inter- or intra-patient variability (IIV) in patients. IIV leads to low or high gentamicin exposure in clinical settings. Therefore, physicians and clinical pharmacists are always working together to improve the effectiveness and reduce the risk of once-daily gentamicin dosing in hospitals throughout the world.9,10,12 Our study employs the MCS method to create virtual populations of critically ill patients. This method was examined for various “what if” scenarios for optimal dosing and risk of nephrotoxicity. This technique generates PK/PD data to assess antibacterial dosing regimens and determine PTA. Previously, several studies have used the MCS to establish optimal antibiotic doses for vulnerable patients, proving its efficacy.35–37 Gathered published pharmacokinetic parameters showed enhanced Vd and fluctuation of clearance due to aggressive fluid resuscitation, vasodilation and capillary leakage, systematic inflammatory response syndrome in acute sepsis. Changes in the Vd (range from 0.27–0.83 L/kg) parameter have been reported in critically ill patients admitted in intensive care units.38 To date, PK/PD targets for gentamicin mostly depend on the plasma concentration and severity of the infection.5 In vitro studies data suggest that Cmax/MIC ~8–10 is sufficient for all pathogens, even Enterobacteriaceae.39 In clinical settings, Cmax/MIC ratio ~8–10 predicts a cure rate of 90% in Gram-negative infected patients.40,41 A more aggressive PK/PD target of Cmax/MIC ≥10 should be considered in infections caused by the high burden resistant pathogens.42,43 The AUC24h/MIC was also reported to be a good predictor of the efficacy in ODDG.6,9
In vivo study proved that there is 1–2 log10 of killing for K. pneumonia when AUC24h/MIC ~80 and 100, respectively.3,6,39 Smith et al8 reported that the AUC24h/MIC ratio ≥110 significantly improved the clinical cure in patients with serious Gram-negative bacterial infections. Higher AUC24h/MIC of ≥150 and ≥175 of gentamicin monotherapy was also associated with fever and leukocytosis resolution in sepsis patients, respectively.40 Therefore, in this study, we utilized both Cmax/MIC and AUC24h/MIC for predicting the efficacy of gentamicin in critically ill patients.
For the highly susceptible pathogens with MIC ≤0.5 mg/L, our study revealed that gentamicin 5 mg/kg/day was the optimal dose with Cmax/MIC ≥8–10. When the MIC increased to 1 mg/L, a recommended dose should be 8 mg/kg/day to attain PTA. However, for pathogens with MIC of 2 mg/L, no studied dose achieved the optimal target (>90% PTA). Even with the maximum studied dose of 10 mg/kg/day, the target attainment for Cmax/MIC of 8 or 10 remained lower than 80% (76.2% and 66.9%, respectively). In similar fashion Gonçalves-Pereira et al44 reported that 65.5% of critically ill patients receiving gentamicin 7.4 mg/kg/day were able to reach the target Cmax/MIC of 8 for the treatment of bacterial infections with MIC of 2 mg/L. Rea et al45 also reported that a gentamicin dosing regimen of 7 mg/kg in critically ill patients is inadequate and only fulfills 20% of the Cmax/MIC >10 for MIC of 2 mg/L.
Our simulation showed that when the MIC is 1 mg/L, the optimal gentamicin dose is 8 mg/kg/day to achieve the Cmax/MIC target >8. However, gentamicin 10 mg/kg/day should be considered if the target Cmax/MIC is >10. The French guideline also recommended a maximum gentamicin dose of 8 mg/kg/day for general critically ill and surgical patients with trauma, if the MIC is not more than 0.5 mg/L.46 However, Allou et al47 reported only 30% of ICU patients reached a Cmax of ≥16 mg/L with gentamicin 8 mg/kg/day. The study in critically ill patients with severe sepsis also achieved a Cmax target of ≥30 mg/L in 59% of patients on the first day of therapy.48
While the Cmax/MIC target is commonly used in clinical practice due to its simplicity, Roger et al49 provided strong evidence that the Cmax/MIC >8 is not a reliable indicator of PK/PD target attainment and clinical outcomes based on efficacy and multivariable analysis. AUC24h/MIC has the advantage of representing cumulative exposure throughout the dosing period and being less affected by variations in sampling times for drug concentration.49 The optimal AUC24h/MIC target may vary depending on the severity of the infection and the patient population, ranging from 30–50 for non-critically ill patients with lower and uncomplicated urinary tract infections (UTIs) or those receiving combination therapy to 80–100 for critically ill patients with non-UTI infections or those receiving gentamicin monotherapy.3,6 He et al50 proposed using an AUC24h/MIC ≥100 to guide gentamicin dosing in critically ill patients, particularly for infections with MIC ≤1 mg/L. They suggested a starting gentamicin dose of 7 mg/kg/day.50
In vivo study revealed that the AUC24h/MIC ~30–50 was associated with bacteriostatis; therefore, the AUC24h/MIC ratio >50 should be adequate for prevention of infections, especially in surgical or trauma injury patients.6 These patients commonly received gentamicin in combination with cephalosporins and adequate wound irrigation and debridement for prevention of skin and soft tissue infection.51 In our study, when the MIC was <1 mg/L, the lowest gentamicin dose that attained the AUC24h/MIC >50 for more than 90% was 7 mg/kg/day. Similarly, the USCAST13 recommended a dose of 7 mg/kg/day, which achieved a high PTA (99.8% and 89.5%) with an AUC24h/MIC ≥30.7 based on MIC values of 1 and 2 mg/L, respectively. However, higher AUC24h/MIC targets of ≥80.3 resulted in lower PTA, 58.8% and 2.1% for MIC values of 1 and 2 mg/L, respectively.13
The AUC24h/MIC ratio ≥110 is currently accepted to be beneficial for treating severe or serious infections.8 Our study revealed that the optimal gentamicin dose for the AUC24h/MIC ≥110 target was 7 mg/kg/day for pathogens with MIC ≤0.5 mg/L. However, when the MIC increased to 1 mg/L, approximately 56% of patients reached this target with the dose of 7 mg/kg/day. After increasing the gentamicin dose to 10 mg/kg/day, the AUC24h/MIC target stayed around 80%. The gentamicin dose of 10 mg/kg/day was the only regimen that achieved >90% target of AUC24h/MIC ≥150 for pathogens with MIC ≤0.5 mg/L, but it simultaneously increased the risk of nephrotoxicity. Therefore, gentamicin should not be recommended as monotherapy for pathogens with MIC >2 mg/L when considering AUC24h/MIC >110 in critically ill patients.
Previous studies reported that the risk of gentamicin-induced nephrotoxicity depended on dose, long term use, and concurrent administration of other nephrotoxic agents. It was also provoked by alteration in pathophysiological conditions of individual patients.52,53 Nicolau et al54 found that out of 2184 patients, only 1.2% of patients experienced nephrotoxicity (rise in serum creatinine of >0.5 mg/L) after a median duration of 7 days of therapy. The risk of nephrotoxicity is significantly lower when Cmin <2 mg/L.10 However, Cmin <1 mg/L was recommended in clinical practice to minimize nephrotoxicity.9 The AUC24h >700 mg⋅h/L was also a good predictor of nephrotoxicity in patients receiving gentamicin once-daily dosing for at least 72 hours.12 However, in this study, we selected two parameters i.e., AUC24h >700 mg⋅h/L or Cmin >2 mg/L to predict gentamicin-induced nephrotoxicity. Using the target of AUC24h >700 mg⋅h/L, we found a minimal risk of nephrotoxicity. Our simulation revealed the Cmin >1 mg/L ranged from 19–37.9% after three days of gentamicin 5–10 mg/kg/day therapy in critically ill patients. For Cmin >2 mg/L, the risk of nephrotoxicity ranged from 5.5–18.4% for 5–10 mg/kg/day of gentamicin dosing regimen on day 3. Therefore, gentamicin once-daily dosing for short duration (for 3 days) showed a minimal risk of nephrotoxicity. Routine monitoring of plasma gentamicin concentrations for nephrotoxicity may not be necessary within the first 3 days after gentamicin initiation.
Gentamicin dosing should be based on epidemiological or geographic antimicrobial susceptibility testing and clinical effectiveness on various MICs.55–57 Considering both efficacy targets of Cmax/MIC ~8–10 and AUC24h/MIC ≥110 and the risk of nephrotoxicity, the optimal gentamicin dose of 8 mg/kg/day would be sufficient to initiate therapy in critically ill patients. More than 90% of critically ill patients receiving this dose could achieve both targets for treating pathogens with MIC <0.5 mg/L. For pathogens with MIC of 1 mg/L, the %PTA after 3 days of therapy ranged between ~70–90%. Our gentamicin dosing recommendation of 8 mg/kg/day is consistent with the recommendation by the French guideline.47 However, gentamicin monotherapy should not be recommended for the treatment of pathogens with MIC ≥2 mg/L.
To our knowledge, this is the first study employing both AUC24h/MIC and Cmax/MIC ratios, as the targets for efficacy of gentamicin in critically ill patients. In this study, we focused on the acute phase of illness, i.e., within 3 days of gentamicin initiation, where the culture and sensitivity results would be available thereafter. If the culture results reveal pathogens with the MIC of gentamicin >2 mg/L, then alternative antibiotics or gentamicin use in combination with other antibiotics should be initiated to manage the infection. There is a lack of consensus in the scientific community regarding a single PK/PD target for both efficacy and nephrotoxicity. Bland et al6 have already emphasized the significance of utilizing accurate and effective PK/PD targets. Gentamicin continues to be widely used as an empirical antibiotic and resistance patterns are also changing drastically in significance. Hence, particularly in tropical countries, there is a need for optimal dosing information. This study serves as a valuable resource and guidance for clinicians.
There are a few limitations to this study. First, we gathered the pharmacokinetic data from 21 earlier published studies representing critically ill patients; therefore, inter- or intra-patient variability in pathophysiological conditions could not be excluded in a heterogeneous population. Second, total body weight was used for the prediction; therefore, the recommended optimal gentamicin doses cannot be applicable to the ideal body weight or adjusted body weight for dosing. Third, our result may not apply if the patient has significant changes in pharmacokinetic parameters within the first 72 hours. Our study predicted gentamicin concentrations based on the pharmacokinetic parameters during the acute phase of illness. If clinicians plan to continue treatment for more than 3 days, efficacy and toxicity should be closely monitored.
Conclusion
This is the first study targeting both AUC24h/MIC and Cmax/MIC ratios as targets for efficacy of gentamicin in critically ill patients. The initial gentamicin dose of 8 mg/kg/day was adequate for empirical or documented therapy against a susceptible pathogen, MIC <1 mg/L since it showed both efficacy targets with minimum risk of nephrotoxicity. However, for a documented therapy against a pathogen with MIC ≥2 mg/L, gentamicin monotherapy should not be recommended. While using gentamicin, clinicians should be cautious and maintain close monitoring for therapeutic outcomes and risk of nephrotoxicity. Furthermore, clinical studies are essential to confirm our recommendation.
Abbreviations
AUC24h, 24-hour area under the concentration-time curve; Cmax, maximum gentamicin plasma concentration; Cmin, minimum gentamicin plasma concentration; CL, clearance; Ke, elimination rate constant; IIV, inter- or intra-patient variability; ODDG, once-daily dosing of gentamicin; MCS, Monte Carlo simulation; MIC, minimum inhibitory concentration; PD, pharmacodynamic; PK, pharmacokinetic; PTA, probability of target attainment; USCAST, The United States Committee on Antimicrobial Susceptibility Testing; Vd, volume of distribution.
Data Sharing Statement
The datasets utilized and analyzed in this research are accessible from the corresponding authors upon request in the future without any specific rationale.
Acknowledgments
We would like to thank Assistant Professor Dr. Sutthiporn Pattharachayakul, and Associate Professor Dr. Wibul Wongpoowarak and for their valuable notes for this manuscript.
Funding
This research work was carried as a part of a PhD study which was funded by the scholarship under Thailand’s Education Hub for ASEAN Countries (TEH-AC), Graduate School and partially by Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Thailand-90110.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Gentamicin Injection USP. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/062366s033lbl.pdf.
2. Dhingra S, Rahman NAA, Peile E, et al. Microbial resistance movements: an overview of global public health threats posed by antimicrobial resistance, and how best to counter. Front Public Health. 2020;8:535668. doi:10.3389/fpubh.2020.535668
3. Craig WA. Optimizing aminoglycoside use. Crit Care Clin. 2011;27(1):107–121. doi:10.1016/j.ccc.2010.11.006
4. Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient - concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11. doi:10.1016/j.addr.2014.07.006
5. Duong A, Simard C, Wang YL, Williamson D, Marsot A. Aminoglycosides in the Intensive Care Unit: what is new in population PK modeling? Antibiotics. 2021;10(5):507. doi:10.3390/antibiotics10050507
6. Bland CM, Pai MP, Lodise TP. Reappraisal of contemporary pharmacokinetic and pharmacodynamic principles for informing aminoglycoside dosing. Pharmacother J Hum Pharmacol Drug Ther. 2018;38(12):1229–1238. doi:10.1002/phar.2193
7. Mouton JW, Jacobs N, Tiddens H, Horrevorts AM. Pharmacodynamics of tobramycin in patients with cystic fibrosis. Diagn Microbiol Infect Dis. 2005;2:123–127. doi:10.1093/jac/dki079
8. Smith PF, Ballow CH, Booker BM, Forrest A, Schentag JJ. Pharmacokinetics and pharmacodynamics of aztreonam and tobramycin in hospitalized patients. Clin Ther. 2001;23(8):1231–1244. doi:10.1016/s0149-2918(01)80103-x
9. Abdul-Aziz MH, Alffenaar JC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med. 2020;46(6):1127–1153. doi:10.1007/s00134-020-06050-1
10. Yamada T, Fujii S, Shigemi A, Takesue Y. A meta-analysis of the target trough concentration of gentamicin and amikacin for reducing the risk of nephrotoxicity. J Infect Chemother. 2021;27(2):256–261. doi:10.1016/j.jiac.2020.09.033
11. Oracle Corporation. Oracle Crystal Ball Classroom Edition [Computer Software]. Version 11.1.2.4. Redwood Shores, CA: Oracle Corporation; 2021.
12. Rybak MJ, Abate BJ, Kang SL, Ruffing MJ, Lerner SA, Drusano GL. Prospective evaluation of the effect of an aminoglycoside dosing regimen on rates of observed nephrotoxicity and ototoxicity. Antimicrob Agents Chemother. 1999;43(7):1549–1555. doi:10.1128/AAC.43.7.1549
13. United States Committee on Antimicrobial Susceptibility Testing (USCAST). Aminoglycoside in vitro Susceptibility Test Interpretive Criteria Evaluations, Version 1.3, 2019. USCAST; 2019.
14. Townsend PL, Fink MP, Stein KL, Murphy SG. Aminoglycoside pharmacokinetics: dosage requirements and nephrotoxicity in trauma patients. Crit Care Med. 1989;17(2):154–157.
15. Sangha KS, Miyagawa CI, Healy DP, Bjornson HS. Pharmacokinetics of once-daily dosing of gentamicin in surgical intensive care unit patients with open fractures. Ann Pharmacother. 1995;29(2):117–119. doi:10.1177/106002809502900201
16. Finnell DL, Davis GA, Cropp CD, Ensom MH. Validation of the Hartford nomogram in trauma surgery patients. Ann Pharmacother. 1998;32(4):417–421. doi:10.1345/aph.17243
17. Niemiec PW Jr, Allo MD, Miller CF. Effect of altered volume of distribution on aminoglycoside levels in patients in surgical intensive care. Arch Surg. 1987;122(2):207–212. doi:10.1001/archsurg.1987.01400140089012
18. Deeter RG, Krauss EA, Penn F, Nahaczewski AE. Comparison of aminoglycoside clearance and calculated serum creatinine clearances. Ther Drug Monit. 1989;11(2):155–161. doi:10.1097/00007691-198903000-00006
19. Triginer C, Fernández R, Izquierdo I, Rello J, Benito S. Gentamicin pharmacokinetic changes related to mechanical ventilation. DICP. 1989;23(11):923–924. doi:10.1177/106002808902301117
20. Triginer C, Izquierdo I, Fernández R, et al. Changes in gentamicin pharmacokinetic profiles induced by mechanical ventilation. Eur J Clin Pharmacol. 1991;40(3):297–302. doi:10.1007/BF00315213
21. Oparaoji EC, Siram S, Shoheiber O, Cornwell EE 3rd, Mezghebe HM. Appropriateness of a 4 mg/kg gentamicin or tobramycin loading dose in post-operative septic shock patients. J Clin Pharm Ther. 1998;23(3):185–190. doi:10.1046/j.1365-2710.1998.00126.x
22. Mann HJ, Wittbrodt ET, Baghaie AA, Cerra FB. Effect of pharmacokinetic sampling methods on aminoglycoside dosing in critically ill surgery patients. Pharmacotherapy. 1998;18(2):371–378. doi:10.1002/j.1875-9114.1998.tb03864.x
23. Dorman T, Swoboda S, Zarfeshenfard F, Trentler B, Lipsett PA. Impact of altered aminoglycoside volume of distribution on the adequacy of a three milligram per kilogram loading dose. Critical Care Res Group Surgery. 1998;124(1):73–78.
24. Tang GJ, Tang JJ, Lin BS, Kong CW, Lee TY. Factors affecting gentamicin pharmacokinetics in septic patients. Acta Anaesthesiol Scand. 1999;43(7):726–730. doi:10.1034/j.1399-6576.1999.430707.x
25. Chelluri L, Jastremski MS. Inadequacy of standard aminoglycoside loading doses in acutely ill patients. Crit Care Med. 1987;15(12):1143–1145. doi:10.1097/00003246-198712000-00015
26. Hassan E, Ober JD. Predicted and measured aminoglycoside pharmacokinetic parameters in critically ill patients. Antimicrob Agents Chemother. 1987;31(11):1855–1858. doi:10.1128/AAC.31.11.1855
27. Chelluri L, Warren J, Jastremski MS. Pharmacokinetics of a 3 mg/kg body weight loading dose of gentamicin or tobramycin in critically ill patients. Chest. 1989;95(6):1295–1297. doi:10.1378/chest.95.6.1295
28. Rodvold KA, Pryka RD, Kuehl PG, Blum RA, Donahue P. Bayesian forecasting of serum gentamicin concentrations in intensive care patients. Clin Pharmacokinet. 1990;18(5):409–418. doi:10.2165/00003088-199018050-00005
29. Zarowitz BJ, Robert S, Mlynarek M, Peterson EL, Horst HM. Determination of gentamicin pharmacokinetics by bioelectrical impedance in critically ill adults. J Clin Pharmacol. 1993;33(6):562–567. doi:10.1002/j.1552-4604.1993.tb04704.x
30. Buijk SE, Mouton JW, Gyssens IC, Verbrugh HA, Bruining HA. Experience with a once-daily dosing program of aminoglycosides in critically ill patients. Intensive Care Med. 2002;28(7):936–942. doi:10.1007/s00134-002-1313-7
31. Wallace AW, Jones M, Bertino JS Jr. Evaluation of four once-daily aminoglycoside dosing nomograms. Pharmacotherapy. 2002;22(9):1077–1083. doi:10.1592/phco.22.13.1077.33529
32. van Maarseveen E, Man WH, Proost J, Neef C, Touw D. Chronopharmacokinetics of once daily dosed aminoglycosides in hospitalized infectious patients. Int J Clin Pharm. 2015;37(2):342–347. doi:10.1007/s11096-015-0066-7
33. Triginer C, Izquierdo I, Fernández R, et al. Gentamicin volume of distribution in critically ill septic patients. Intensive Care Med. 1990;16(5):303–306. doi:10.1007/BF01706354
34. Beckhouse MJ, Whyte IM, Byth PL, Napier JC, Smith AJ. Altered aminoglycoside pharmacokinetics in the critically ill. Anaesth Intensive Care. 1988;16(4):418–422. doi:10.1177/0310057X8801600406
35. Ambrose PG. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the society of infectious diseases pharmacists. Pharmacotherapy. 2006;26(1):129–134. doi:10.1592/phco.2006.26.1.129
36. Lodise TP, Lomaestro BM, Drusano GL. Society of Infectious Diseases Pharmacists. Application of antimicrobial pharmacodynamic concepts into clinical practice: focus on beta-lactam antibiotics: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2006;26(9):1320–1332. doi:10.1592/phco.26.9.1320
37. Trang M, Dudley MN, Bhavnani SM. Use of Monte Carlo simulation and considerations for PK-PD targets to support antibacterial dose selection. Curr Opin Pharmacol. 2017;36:107–113. doi:10.1016/j.coph.2017.09.009
38. Fish DN. Extended-interval dosing of aminoglycoside antibiotics in critically ill patients. J Pharm Pract. 2002;15(2):85–95. doi:10.1106/WKHR-J8JL-FHU5
39. Andes D, Craig WA. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents. 2002;19(4):261–268. doi:10.1016/s0924-8579(02)00022–5.
40. Kashuba AD, Nafziger AN, Drusano GL, Bertino JS Jr. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by gram-negative bacteria. Antimicrob Agents Chemother. 1999;43(3):623–629. doi:10.1128/AAC.43.3.623
41. Zelenitsky SA, Harding GK, Sun S, Ubhi K, Ariano RE. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J Antimicrob Chemother. 2003;52(4):668–674. doi:10.1093/jac/dkg403
42. Zelenitsky SA, Rubinstein E, Ariano RE, Zhanel GG. Canadian Antimicrobial Resistance Alliance. Integrating pharmacokinetics, pharmacodynamics and MIC distributions to assess changing antimicrobial activity against clinical isolates of Pseudomonas aeruginosa causing infections in Canadian hospitals (CANWARD). J Antimicrob Chemother. 2013;68(1):i67–i72. doi:10.1093/jac/dkt028
43. Sumi CD, Heffernan AJ, Lipman J, Roberts JA, Sime FB. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin Pharmacokinet. 2019;58(11):1407–1443. doi:10.1007/s40262-019-00791-z
44. Gonçalves-Pereira J, Martins A, Póvoa P. Pharmacokinetics of gentamicin in critically ill patients: pilot study evaluating the first dose. Clin Microbiol Infect. 2010;16(8):1258–1263. doi:10.1111/j.1469-0691.2009.03074.x
45. Rea RS, Capitano B, Bies R, Bigos KL, Smith R, Lee H. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit. 2008;30(6):674–681. doi:10.1097/FTD.0b013e31818b6b2f
46. Agence française de sécurité sanitaire des produits de santé. Update on good use of injectable aminoglycosides, gentamycin, tobramycin, netilmycin, amikacin. Pharmacological properties, indications, dosage, and mode of administration, treatment monitoring. Med Mal Infect. 2012;42(7):301–308. doi:10.1016/j.medmal.2011.07.007
47. Allou N, Allyn J, Levy Y, et al. Assessment of the National French recommendations regarding the dosing regimen of 8mg/kg of gentamicin in patients hospitalised in intensive care units. Anaesth Crit Care Pain Med. 2016;35(5):331–335. doi:10.1016/j.accpm.2015.12.012
48. Roger C, Nucci B, Louart B, et al. Impact of 30 mg/kg amikacin and 8 mg/kg gentamicin on serum concentrations in critically ill patients with severe sepsis. J Antimicrob Chemother. 2016;71(1):208–212. doi:10.1093/jac/dkv291
49. Roger C, Louart B, Elotmani L, et al. An international survey on aminoglycoside practices in critically ill patients: the AMINO III study. Ann Intensive Care. 2021;11(1):49. doi:10.1186/s13613-021-00834-4
50. He S, Cheng Z, Xie F. Pharmacokinetic/pharmacodynamic-guided gentamicin dosing in critically ill patients: a revisit of the Hartford nomogram. Int J Antimicrob Agents. 2022;59(6):106600. doi:10.1016/j.ijantimicag.2022.106600
51. Hoff WS, Bonadies JA, Cachecho R, Dorlac WC. EAST practice management guidelines work group: update to practice management guidelines for prophylactic antibiotic use in open fractures. J Trauma. 2011;70(3):751–754. doi:10.1097/TA.0b013e31820930e5
52. Llanos-Paez CC, Hennig S, Staatz CE. Population pharmacokinetic modelling, Monte Carlo simulation and semi-mechanistic pharmacodynamic modelling as tools to personalize gentamicin therapy. J Antimicrob Chemother. 2017;72(3):639–667. doi:10.1093/jac/dkw461
53. Drusano GL, Louie A. Optimization of aminoglycoside therapy. Antimicrob Agents Chemother. 2011;55(6):2528–2531. doi:10.1128/AAC.01314-10
54. Nicolau DP, Freeman CD, Belliveau PP, Nightingale CH, Ross JW, Quintiliani R. Experience with a once-daily aminoglycoside program administered to 2184 adult patients. Antimicrob Agents Chemother. 1995;39(3):650–655. doi:10.1128/AAC.39.3.650
55. Avent ML, Rogers BA, Cheng AC, Paterson DL. Current use of aminoglycosides: indications, pharmacokinetics and monitoring for toxicity. Intern Med J. 2011;41(6):441–449. doi:10.1111/j.1445-5994.2011.02452.x
56. Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70(3):195–283. doi:10.2146/ajhp120568
57. Gonzalez-Padilla M, Torre-Cisneros J, Rivera-Espinar F, et al. Gentamicin therapy for sepsis due to carbapenem-resistant and colistin-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2015;70(3):905–913. doi:10.1093/jac/dku432
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.