Back to Journals » Medical Devices: Evidence and Research » Volume 16
Reduction of Eyedrop Volume for Topical Ophthalmic Medications with the Nanodropper Bottle Adaptor
Authors St. Peter DM , Steger JS, Patnaik JL, Davis N, Kahook MY, Seibold LK
Received 15 November 2022
Accepted for publication 28 February 2023
Published 6 April 2023 Volume 2023:16 Pages 71—79
DOI https://doi.org/10.2147/MDER.S397654
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Deidre M St. Peter,1 Jennifer S Steger,2 Jennifer L Patnaik,1 Nicole Davis,1 Malik Y Kahook,1 Leonard K Seibold1
1Department of Ophthalmology, University of Colorado School of Medicine, Aurora, CO, USA; 2Nanodropper, Inc, Rochester, MN, USA
Correspondence: Leonard K Seibold, Department of Ophthalmology, University of Colorado School of Medicine, 1675 Aurora Court, Aurora, CO, 80045, USA, Tel +1 720-848-2020, Fax +1 720-848-5079, Email [email protected]
Purpose: To determine the drop volume and total number of dispensed drops using the Nanodropper eyedrop bottle adaptor (Nanodropper, Inc.) compared to drops dispensed from stock bottles to potentially limit ocular toxicity of these eyedrops and prolong bottle use.
Patients and Methods: Six topical ocular hypotensive medications (5 solutions, 1 suspension), one steroid (suspension) and two artificial tears emulsions were selected for this study. An analytical balance was used to determine the mass per 10 drops with and without the volume-reducing adaptor and repeated until the bottles were completely emptied. The density of each product was determined using the calculated density. The average drop volume and number of drops per bottle for the nine medications were compared with and without the adaptor with paired t-testing.
Results: When all medications were assessed, the drops delivered with the adaptor were 62.1% smaller than eyedrops administered from standard bottles. Compared to stock bottle eyedrops, which had a mean volume of 39.8 ± 2.1 μL, the adaptor resulted in drops with a mean volume of 15.1 ± 1.0 μL, p< 0.0001. The adaptor delivered 2.6x the number of drops dispensed from a standard 2.5 mL bottle (184.1 ± 15.1 drops with adaptor and 69.8 ± 4.9 drops from stock bottle, p< 0.0001).
Conclusion: The Nanodropper eyedrop bottle adaptor can significantly reduce drop volume and increase the overall number of drops dispensed compared with stock eyedrop bottles. Further studies are needed to elucidate the clinical impact of utilizing decreased drop volume with direct comparison to current standards of care.
Keywords: ophthalmic medication, medication delivery, dry eye syndrome, glaucoma medications, medication adherence, drop assist devices
Introduction
Initial treatment of most eye diseases centers on use of topical medications, but significant barriers to adherence can greatly limit efficacy.1 These barriers include the inability to properly self-administer drops, early depletion of drops from stock bottles, forgetfulness, and difficulty with drop administration and medication schedule, among others.1 A variable that can influence treatment adherence is early depletion of drops. There are a few drop volume-related factors that, when considered together, may contribute to premature bottle depletion: 1) Eyedrop bottles dispense drops that exceed the tear volume of the human eye.2 2) Patients regularly miss their eye when administering eyedrops. One review found that for every drop that makes it into a glaucoma patient’s eye, seven drops are wasted in the instillation attempt.3 A previous investigation demonstrated that the different stock bottles deliver a wide range of eyedrop volumes, from 20 to 70 µL per drop.4 There are no current regulations on drop size from stock bottle droppers, so patients may be exhausting their medications prematurely depending on the stock bottle’s drops size. Studies in healthy subjects have also shown that increasing drop volume ≥20 µL does not increase the drug concentration in the tear film, indicating that larger drop volumes provide superfluous medication per dose.2,5,6 Since the commercial eyedrop volume is larger than what the cul-de-sac can hold, most of the medication is washed out following instillation and only 5% of the drug reaches the ocular tissues.7 Excess ophthalmic medications are often systemically absorbed potentially leading to unwanted side effects and potentially worsening medication adherence.8 A review of the determinants of eyedrop volume concluded that the ideal dropper tip consists of a “small diameter outer orifice with a design clearly defining the surface area from which the drop will fall” to limit the drop volume and make administration of medications more streamlined for the patient.4
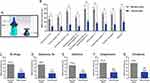
The Nanodropper (Nanodropper, Inc.) is an FDA-listed, volume-reducing adaptor that can be placed onto most eyedrop bottles. It is specifically designed for ease of use and eyedrop volume reduction and has a blue silicone tip for easy visualization (Figure 1A). This design, therefore, should help with the problem of eyedrop aim by making the tip more visible to patients. The adaptor may also help with drop depletion by potentially extending the use of ocular medication bottles. The device creates a small surface area onto which drops will form, reducing the drop volume. By reducing the size of drops, the adaptor may also assist in limiting side effects, which may improve patient adherence. The aim of this study was to examine the adaptor’s efficacy in reducing eyedrop volume and prolonging the life cycle of individual bottles when used with different medications and formulations.
Materials and Methods
This experimental study was designed to determine the average drop volume and the total number of dispensed drops per milliliter and bottle when using the adaptor compared with the standard dropper for various topical ophthalmic medications. Nanodropper adaptors (Nanodropper, Inc., Rochester, MN) were provided by the manufacturer. This research did not receive any specific grant funding from agencies in the public, commercial, or not-for-profit sectors.
Nine different topical eye medications were evaluated, including bimatoprost 0.01% (Lumigan®, Allergan USA, Inc., Madison, NJ), timolol maleate 0.5% (Sandoz Inc., Princeton, NJ), brimonidine tartrate 0.15% (Alphagan P®, Allergan, Inc., Irvine, CA), brimonidine tartrate 0.2%/timolol maleate 0.5% (Combigan®, Allergan, Inc. Irvine, CA), dorzolamide hydrochloride 2%/timolol maleate 0.5% (Cosopt®, Akorn, Inc. Lake Forest, IL), brinzolamide 1% (Azopt®, Alcon Laboratories, Inc., Fort Worth, TX), prednisolone acetate 1% (Pred Forte®, Allergan, Inc., Irvine, CA), Systane Complete® (Alcon Laboratories, Inc., Fort Worth, Tx) lubricant drop, and Systane® (Alcon Laboratories, Inc., Fort Worth, Tx) lubricant drop. Drop volume and number of drops were estimated using the densitometric method for volume determination.9 Room temperature (22°C) drops were dispensed from previously unopened bottles held at 90° and measured in increments of ten with a 0.0001 g analytical balance (Sigma-Aldrich, Darmstadt, Germany) through bottle depletion. Drop expression was performed manually and all measurements were performed by the same investigator. For each medication, these measurements were performed with two standard bottles and two bottles using the adaptor. A total of 36 bottles of medication were therefore used in this study.
To determine the volume of each drop, a 200 µL pipette (Sigma-Aldrich, Darmstadt, Germany) was used to draw a 100 µL sample of each medication and the mass was measured. This was repeated four times per medication and the average mass was divided by 0.1 mL to determine the mean density of each medication. This density was then used to determine the volume per drop and per bottle, as the density is equal to the solution’s mass divided by the volume.
Statistical analyses were performed, and graphs were generated using Prism 9 (GraphPad Software Inc., San Diego, CA, USA). The mean volumes were calculated for standard drops and drops dispensed with the adaptor for each bottle of medication. Two-way ANOVA was used to compare the drop volume with and without the adaptor across all medications, and within-medication comparisons of drop volume were conducted using Sidak’s multiple comparisons. Welch’s t-test was used to compare mean drop volume of standard drops versus drops dispensed with the adaptor when medications were grouped together by indication (glaucoma, steroid, artificial tears), and by formulation (solutions, suspensions, and emulsions). The number of drops per bottle was scaled to a 2.5 mL bottle for each medication to compare the effects of the adaptor on drop number per bottle. Two-way ANOVA was used to compare the mean number of drops dispensed per 2.5 mL bottle with and without the adaptor across all medications, and within-medication comparisons of the number of drops per 2.5 mL were conducted using Sidak’s multiple comparisons. To determine the percent reduction in drop volume of specific medications and formulations that can be solely attributed to use of the adaptor, we normalized the mean volume of drops dispensed with the adaptor to the appropriate mean standard drops volume. For each medication, the mean volume of drops dispensed with the adaptor for each tested bottle (drop volume measurements were performed with two bottles using the adaptor for each medication) was divided by collective mean standard drops volume for this medication. These normalized values were then compared using a one-way ANOVA with Tukey’s multiple comparisons. To evaluate drop volume reduction with the adaptor per formulation, the formulation type of each tested medication was initially noted. The mean volume of drops dispensed with the adaptor for each tested bottle (drop volume measurements were performed with two bottles using the adaptor for each medication) was then divided by the collective mean standard drops volume of all the bottles that are the same formulation type as the medication being evaluated. For example, two suspensions were evaluated in this study, brinzolamide 1% and prednisolone acetate 1%. Using brinzolamide 1% as an example, the volume of drops dispensed with the adaptor for each bottle of brinzolamide 1% was divided by the mean volume of standard drops of the two tested bottles of brinzolamide 1% and the two tested bottles of prednisolone acetate 1% to determine the formulation-normalized drop volume reduction with the adaptor for each medication. These values were then grouped together by formulation and medications were then grouped together by formulation and compared using a one-way ANOVA with Tukey’s multiple comparisons. A p-value of <0.05 was considered statistically significant.
Results
Six topical glaucoma medications, one steroid medication, and two types of artificial tears were selected for study. Of these, five medications were solutions, two were suspensions, and two were emulsions (Table 1). The adaptor (Figure 1A) significantly decreased drop volume compared to the standard dropper for each medication studied (Figure 1B, p<0.0001 for each drug-specific post-hoc comparison). On average, when all medications were combined, compared to the standard dropper, the adaptor reduced the drop volume by 24.7 ± 2.3 μL (mean ± SEM), from 39.8 ± 2.1 μL to 15.1 ± 1.0 μL, corresponding to a 62.1% decrease in drop volume (Figure 1C, p<0.0001). For glaucoma medications, the average drop volume with the standard dropper and the adaptor was 38.9 ± 2.9 μL and 14.2 ± 1.4 μL, respectively, representing a 24.7 ± 3.2 μL reduction in drop volume with the adaptor (Figure 1D, p<0.0001). For solutions, compared to the standard dropper, the adaptor decreased drop volume by 23.4 ± 3.4 μL, from 36.8 ± 3.1 μL to 13.4 ± 1.5 μL (Figure 1E, p<0.0001). For suspensions, compared to the standard dropper, the adaptor decreased drop volume by 27.0 ± 3.3 μL, from 43.8 ± 3.2 μL to 16.8 ± 0.7 μL (Figure 1F, p<0.01). And for emulsions, the average volume was 43.1 ± 3.3 μL with the standard dropper and 17.8 ± 1.7 μL with the adaptor, representing a 25.3 ± 3.7 μL decrease with the adaptor (Figure 1G, p<0.01). Suspensions and emulsions had slightly larger drop volumes both with and without the adaptor, but for all medications, the adaptor drop volume was significantly smaller than the standard drop volume (Figure 1B, p<0.0001 for each drug-specific post-hoc comparison).
 |
Table 1 Classification of Nine Ophthalmic Medications by Indication and Formulation |
The adaptor also increased the total number of drops per bottle for all medications compared with standard bottles. The average number of drops increased from 69.8 ± 4.9 drops per bottle to 184.1 ± 15.1 drops per bottle with the adaptor (scaled to a 2.5mL bottle for all medications, Figure 2, p<0.0001). The adaptor dispensed 2.6x the number of drops delivered from a standard 2.5 mL bottle of medication.
Drop volume data were normalized to the mean volume of the respective drug’s standard drops allowing for comparison of the standard drop volume reduction with the adaptor between medications. There was a difference in drop volume reduction with the adaptor across all medications (Figure 3A, p=0.0035). The drop volume with the adaptor ranged from 31.6 ± 0.5% (timolol maleate 0.5%) to 43.8% ± 3.9% (dorzolamide hydrochloride 2%/timolol maleate 0.5%) of the respective drug’s standard drop volume. Drop volume data were also normalized to the mean volume of the respective formulation’s standard drops. We did not observe a significant between-formulation difference in drop volume reduction with the adaptor. For solutions, suspensions, and emulsions, the adaptor produced drops that were 36.5% ± 4.2%, 38.2% ± 1.7%, and 41.2% ± 3.9%, respectively, of the formulation’s standard drop volumes (Figure 3B, p=0.7707).
Discussion
In this study, we characterized the utility of a novel eyedrop bottle adaptor in reducing the volume of eyedrops administered from a given bottle. When all medications were assessed together, compared to eyedrops administered from standard bottles, the drops delivered with the adaptor were 62.1% smaller, resulting in a 2.6-fold increase in the number of drops dispensed per bottle. Furthermore, the adaptor-mediated reduction in eyedrop volume was significant for solutions, suspensions, and emulsions alike.
Successful glaucoma treatment with topical medications heavily relies on a patient’s ability to administer drops according to a set dosing schedule. Likewise, poor adherence with therapy may result in poorer vision outcomes. A 2020 study showed that the 20-year incidence of blindness for non-adherent glaucoma patients was 13.5% in at least one eye whereas in adherent patients, the rate was 5.4%, indicating worse outcomes for patients with barriers to adherence.10 Unfortunately, studies show alarmingly high rates of non-adherence to topical treatments in glaucoma patients between 30% and 80%.11,12
Several patient survey studies have sought to uncover common barriers to glaucoma medication adherence, specifically drop administration. In a cross-sectional survey of patients with glaucoma treated with topical medications, Newman-Casey et al found that 26.5% of patients self-reported non-adherence and 59% of these non-adherent patients reported a lack of self-efficacy in instilling drops.1 This study also found that one in five patients surveyed were interested in utilizing a drop assist device for controlling the number of drops or assisting in aim.1 Currently available dosing aids help guide instillation, assist in squeezing the bottles, and audibly alert patients to use drops.13
It was found that 18% of non-adherent patients surveyed by Newman-Casey at al. expressed difficulty with controlling the number of drops administered per dose.1 A 2014 survey found that 25.4% of 236 glaucoma patients who self-administered topical ophthalmic medications reported early exhaustion of eyedrops in a one-year period, and 5.1% of patients reported that they ran out of drops early at least five times per year.14 This problem is so prevalent that the American Academy of Ophthalmology has supported legislation to require insurance companies to refill drops early, though many states do not support this measure yet.15 An adaptor such as the Nanodropper that reduces drop volume from stock bottles and extends bottle lifecycle would therefore provide a novel tool for improving adherence as there is currently no other device available on the market to reduce drop size.
While ideal drop volume for topical medications is still unclear, standard bottle tips likely deliver a drop larger than necessary. In fact, a drop volume greater than 20 μL exceeds the tear film capacity, and increasing drop volume over this threshold does not increase therapeutic efficacy.5 One study found that a volume in excess of 10 μL is not absorbed from the tear film, suggesting the ideal drop volume is likely between 10 and 20 μL.2 A recent study found that, in a pediatric population, microdrops delivered with a Nanodropper provided non-inferior mydriasis relative to the standard drop size from the bottle dropper.16 Previous studies have detailed average drop volumes much larger than 10–20 μL for various glaucoma medications using the stock bottle dropper. A 2020 study of several combination glaucoma drops common in China found that the average drop volume was between 24.4 and 32.6 μL using the stock bottle droppers, whereas a 2015 study of latanoprost brands showed an average drop volume between 30 and 33.1 μL.17,18 Moore et al characterized the variability in drop volumes for a wide range of glaucoma medications and concluded that the mean volume per drop of glaucoma medication was 79 μL ± 34 μL, with a range of 20.9 to 40.8 drops/mL of medication using the stock bottle dropper.9
Because of the discrepancy between stock bottle drop volumes and the potential ideal drop volume, previous efforts have been made to find an alternative means of reducing drop volumes. Kumar et al attempted to reduce the drop volume from stock bottle droppers by inserting a glass capillary tube with a 0.5 mm inner diameter into the dropper lumen.19 They were able to reduce the average drop volume from 32.7 μL to 18.8–22.9 μL, but this technology is not commercially available.19 Our data shows that among nine different eyedrop medications, the average drop volume from stock bottles was 39.8 μL, which is similar to previously reported volumes, especially when considering the 90° angle we employed, as other studies varied the bottle tilt. We found that the adaptor significantly reduced the drop volume for solutions, emulsions, and suspensions to a potentially more ideal drop volume between 10 and 20 μL.2
Along with reducing drop volume, an effective bottle tip adaptor should increase the number of drops per bottle. A 2016 study of various medications, including five brands common in the United States, showed that for 2.5 mL and 5 mL bottles of latanoprost, the average number of drops per bottle using the stock bottle dropper was between 83.3–85.3 and 127.7–163.3, respectively.20 Similarly, our data show that a 2.5 mL bottle contains between 54 and 97 drops per bottle. The large variation in number is likely because emulsions and suspensions tend to create larger drop volumes and were included along with solutions in our study. We found that the adaptor dispensed 2.6 times the number of drops compared to standard 2.5 mL bottles of medication.
In addition to reducing drop volume and resultant early bottle exhaustion, a smaller drop volume may also impact medication adverse effects. A 2016 review paper stated that the extent of a drug’s systemic absorption depends partly on the volume of the eyedrops, as ocular absorption accounts for less than 10% of the dose administered, and up to 80% of the dose is thought to be systemically absorbed through the conjunctiva and mucous membranes of the nasal passages.8
This suggests that limiting the volume of eyedrops administered should help to limit systemic side effects.14 In fact, studies have found that microdrops reduce the systemic absorption of topical phenylephrine in human subjects without reduced efficacy of pupillary dilation.21,22
Even more common than systemic effects, topical side effects from glaucoma medications are well known. A review of topical glaucoma medications and their side effects showed that topical medications decrease the stability of tear film leading to dry eye and a reduction of quality-of-life measures for patients using topical drops.23 A meta-analysis published in 2020 found multiple large studies demonstrating toxicity to the ocular surface secondary to benzalkonium chloride (BAK), a common additive in glaucoma medications. Side effects from this preservative include decreased tear breakup time, abnormal fluorescein staining, superficial punctate keratopathy, altered tear film osmolarity, worse ocular surface disease index (OSDI) scores, and more pain and discomfort with instillation.24 Animal studies also suggest that exposure to BAK-preserved glaucoma medications may lead to corneal epithelial damage, increased conjunctival inflammation, and decreased conjunctival goblet cell viability.25,26 This preservative is also potentially toxic to the trabecular meshwork, with cultured trabecular meshwork cells showing significantly higher rates of cell death when exposed to BAK as opposed to alternative preservatives or BSS.23,27,28 Additionally, patients who have chronically used preserved glaucoma medications may have poorer outcomes after filtering surgery, likely from chronic inflammatory changes.23 Previous studies have shown that microdrops of pilocarpine, phenylephrine, and tropicamide produced fewer ocular side effects than standard drop sizes.6,29–31 Limiting drop volume with the adaptor can, therefore, reduce the topical exposure to both glaucoma medications and their preservatives, which may prove beneficial to patients on long-term therapy. There is no known threshold for preservative toxicity, so further studies are warranted to determine the true impact of a smaller drop volume on ocular surface disease.
Aside from direct clinical benefits, there would be a cost benefit to increasing the number of drops per bottle for patients, assuming a relatively stable price per bottle of medication. Studies have shown that cost is a significant barrier to adherence in patients with glaucoma.32–34 A 2018 study compared the price and medication waste of single-use blister packs with bottles of medication and determined that bottles were, overall, more cost-effective than single-use medications even after accounting for over- or under-consumption of medication on a monthly basis.35 As the most cost-effective means of obtaining topical medications is a stock bottle, the adaptor could, therefore, extend the use of these medications beyond what is currently available through other products. Moreover, one study determined through cost-adjusted quality of life algorithms that a measure costing $550/year or less to improve medication adherence for glaucoma patients would be considered highly cost effective.10 The adaptor may improve adherence to medication through ease of use and prolonging the lifecycle of eyedrop bottles at a far lower cost.
The potential benefits of the adaptor should be contrasted with the potential drawbacks of the device. The adaptor should extend the lifespan of the stock bottle by reducing the drop size and some may argue that this will increase the risk of medication contamination. Indeed, a 2019 study conducted in Kenya, including both inpatient and outpatient ocular medication bottles, showed that up to 72.8% of eye bottles used for an average of two weeks become contaminated with bacterial microorganisms.36 However, a recent multi-society position paper endorsed by the American Academy of Ophthalmology, the American Glaucoma Society, the Outpatient Ophthalmic Surgery Society and the American Society of Refractive Surgery was published in 2022 regarding reducing topical drug waste. The second of three evidence-based recommendations made in this position paper is that “Topical drugs in multidose containers can be used until the manufacturer’s labeled date of expiration”.37 It is thought that the preservatives in eyedrop bottled should prevent contamination of medications until the stated expiration date, unless otherwise stated on the manufacturer label.38 These guidelines assume proper training for safely administering eyedrops, something which not all patients may have undergone, potentially increasing the risk of contamination. Given the Academy’s current recommendations, it is likely that the adaptor’s ability to prolong the use of medication bottles will not lead to increased risk of contamination allowing that proper eyedrop installation techniques are used. Furthermore, though much was discussed regarding the potential ideal volume of topical ophthalmic medications, the fact remains that the existing data suggest a smaller drop size may be adequate and provide the benefits we detail, but this requires further study to confirm.
We acknowledge several limitations of our study including the lack of variation in medication brand/manufacturer that were tested. Although formulations of medication are strictly regimented, there may be variations on surface tension between brands that might lead to a variation in drop volume, which were not accounted for in our study. An additional study to look at brand differences for these ophthalmic medications is not likely needed given the clear demonstration of a significant reduction in drop size amongst all medications studied. Additionally, there may be slight differences in total bottle volume based on manufacturer regulations. Furthermore, a non-masked single researcher collected data and manually expressed drops so there was no control for the force at which the eyedrop bottles were squeezed during data collection. The densitometric method was used for study and the density-based values used for volume calculations may have been subject to systematic error. Additionally, the bottle volume and number of drops per bottle varied in our study, which is consistent with reported deviations between true and stated bottle volumes.20
Conclusion
Our data demonstrate for the first time how a specifically engineered adaptor can significantly reduce the drop volume of ophthalmic medications compared with the standard dropper to increase the number of drops per bottle of medication. This adaptor could alleviate many barriers to ophthalmic medication adherence and may improve patient outcomes. Future studies should examine patient medication adherence and satisfaction with the adaptor in addition to the adaptor’s effect on bioavailability of medications. IOP-lowering efficacy and cost analyses for medication use with and without the adaptor are also warranted.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Newman-Casey PA, Robin AL, Blachley T, et al. The most common barriers to glaucoma medication adherence: a cross-sectional survey. Ophthalmology. 2015;122(7):1308–1316. doi:10.1016/j.ophtha.2015.03.026
2. Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol Vis Sci. 1966;5(3):264–276.
3. Robin AL, Covert D. Beyond compliance: getting the drops. Rev Ophthalmol. 2005;112(5):863–868. doi:10.1016/j.ophtha.2004.12.026
4. Van Santvliet L, Ludwig A. Determinants of eye drop size. Surv Ophthalmol. 2004;49(2):197–213. doi:10.1016/j.survophthal.2003.12.009
5. Nagataki S, Mishima S. Pharmacokinetics of instilled drugs in the human eye. Int Ophthalmol Clin. 1980;20(3):33–49. doi:10.1097/00004397-198002030-00006
6. File R, Patton T. Topically applied pilocarpine. Human pupillary response as a function of drop size. Arch Ophthalmol. 1980;98(1):112–115. doi:10.1001/archopht.1980.01020030114010
7. Agrahari V, Mandal A, Agrahari V, et al. A comprehensive insight on ocular pharmacokinetics. Drug Deliv Transl Res. 2016;6(6):735–754. doi:10.1007/s13346-016-0339-2
8. Farkouh A, Frigo P, Czejka M. Systemic side effects of eye drops: a pharmacokinetic perspective. Clin Ophthalmol. 2016;10:2433–2441. doi:10.2147/OPTH.S118409
9. Moore DB, Beck J, Kryscio RJ. An objective assessment of the variability in number of drops per bottle of glaucoma medication. BMC Ophthalmol. 2017;17(1). doi:10.1186/s12886-017-0473-8
10. Newman-Casey PA, Salman M, Lee PP, Gatwood JD. Cost-utility analysis of glaucoma medication adherence. In: Ophthalmology. Vol. 127. Elsevier Inc.;2020:589–598. doi:10.1016/j.ophtha.2019.09.041
11. Olthoff C, Schouten J, van de Borne B, Webers C. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112(6):953–961. doi:10.1016/j.ophtha.2004.12.035
12. Schwartz G, Quigley H. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;58:S57–68. doi:10.1016/j.survophthal.2008.08.002
13. Kahook MY. Developments in dosing aids and adherence devices for glaucoma therapy: current and future perspectives. Expert Rev Med Devices. 2007;4(2):261–266. doi:10.1586/17434440.4.2.261
14. Moore DB, Walton C, Moeller KL, Slabaugh MA, Mudumbai RC, Chen PP. Prevalence of self-reported early glaucoma eye drop bottle exhaustion and associated risk factors: a patient survey. BMC Ophthalmol. 2014;14(1). doi:10.1186/1471-2415-14-79
15. Early eyedrop prescription refills. Academy-backed Eyedrop Refill Bill becomes law in Pennsylvania. American Academy of Ophthalmology. Available from: https://www.aao.org/advocacy/eyedrop-refills
16. Hoppe CB, Yonamine S, Kao BW, et al. Randomized Trial to evaluate the efficacy of the nanodropper device for pupillary dilation and cycloplegia in children. Ophthalmology. 2022;130(3):324–330. doi:10.1016/j.ophtha.2022.10.016
17. Xu C, Guo R, Huang D, Ji J, Liu W. Daily costs and cost effectiveness of glaucoma fixed combinations in China. J Ophthalmol. 2020;2020:1–5. doi:10.1155/2020/2406783
18. Queen JH, Feldman RM, Lee DA. Variation in Number of Doses, Bottle Volume, and Calculated Yearly Cost of Generic and Branded Latanoprost for Glaucoma. Am J Ophthalmol. 2016;163:70–74.e1. doi:10.1016/j.ajo.2015.11.021
19. Kumar S, Karki R, Meena M, Prakash T, Rajeswari T, Goli D. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J Adv Pharm Technol Res. 2011;2(3):192–194. doi:10.4103/2231-4040.85540
20. Al-Jumaian N, Malik R, Khandekar R, et al. Bottle characteristics of topical international glaucoma medications versus local brands in Saudi Arabia. Middle East Afr J Ophthalmol. 2016;23(4):296–301. doi:10.4103/0974-9233.194077
21. Whitson JT, Love R, Brown RH, Lynch MG, Schoenwald RD. The effect of reduced eyedrop size and eyelid closure on the therapeutic index of phenylephrine. Am J Ophthalmol. 1993;115(3):357–359. doi:10.1016/S0002-9394(14)73588-3
22. Lynch MG, Brown RH, Goode SM, Schoenwald RD, Chien DS. Reduction of phenylephrine drop size in infants achieves equal dilation with decreased systemic absorption. Arch Ophthalmol. 1987;105(10):1364–1365. doi:10.1001/archopht.1987.01060100066027
23. Baudouin C. Detrimental effect of preservatives in eyedrops: implications for the treatment of glaucoma. Acta Ophthalmol. 2008;86(7):716–726. doi:10.1111/j.1755-3768.2008.01250.x
24. Zhang X, Vadoothker S, Munir WM, Saeedi O. Ocular surface disease and glaucoma medications: a clinical approach. Eye Contact Lens. 2019;45(1):11–18. doi:10.1097/ICL.0000000000000544
25. Kahook MY, Noecker RJ. Comparison of corneal and conjunctival changes after dosing of travoprost preserved with SofZia, latanoprost with 0.02% benzalkonium chloride, and preservative-free artificial tears. Cornea. 2008;27(3):339–343. doi:10.1097/ICO.0b013e31815cf651
26. Kahook MY, Noecker R. Quantitative analysis of conjunctival goblet cells after chronic application of topical drops. Adv Ther. 2008;25(8):743–751. doi:10.1007/s12325-008-0078-y
27. Rasmussen CA, Kaufman PL, Kiland JA. Benzalkonium chloride and glaucoma. J Ocular Pharmacol Therap. 2014;30(2–3):163–169. doi:10.1089/jop.2013.0174
28. Ammar DA, Kahook MY. Effects of glaucoma medications and preservatives on cultured human trabecular meshwork and non-pigmented ciliary epithelial cell lines. Br J Ophthalmol. 2011;95(10):1466–1469. doi:10.1136/bjophthalmol-2011-300012
29. Gray RH. The influence of drop size on pupil dilatation. Eye. 1991;5(5):615–619. doi:10.1038/eye.1991.107
30. Lal A, Kataria V, Rajpal A, Khanna N. Pharmacodynamic effects of pilocarpine eye drop enhanced by decreasing its volume of instillation. Indian J Physiol Pharmacol. 1995;39(3):267–270.
31. Craig EW, Griffiths PG. Effect on mydriasis of modifying the volume of phenylephrine drops. Br J Ophthalmol. 1991;75(4):222–223. doi:10.1136/bjo.75.4.222
32. Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surg. 1995;26(3):233–236.
33. Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–436. doi:10.1016/j.ophtha.2005.10.034
34. Schmier JK, Halpern MT, Jones ML. The economic implications of glaucoma: a literature review. Pharmacoeconomics. 2007;25(4):287–308. doi:10.2165/00019053-200725040-00003
35. Na KH, Yoo C, Park JH, Kim YY. Eye drop dispenser type and medication possession ratio in patients with glaucoma: single-use containers versus multiple-use bottles. Am J Ophthalmol. 2018;188:9–18. doi:10.1016/j.ajo.2018.01.011
36. Tamrat L, Gelaw Y, Beyene G, Gize A. Microbial contamination and antimicrobial resistance in use of ophthalmic solutions at the department of ophthalmology, Jimma University Specialized Hospital, Southwest Ethiopia. Can J Infect Dis Med Microbiol. 2019;2019:5372530. doi:10.1155/2019/5372530
37. Palmer DJ, Robin AL, McCabe CM, Chang DF. Reducing topical drug waste in ophthalmic surgery multisociety position paper. J Cataract Refract Surg. 2022;4(8):1073–1077. doi:10.1097/j.jcrs.0000000000000975
38. Chaudhary OR. How long can you use prescription eye drops after opening them? Am Acad Ophthalmol. 2022. Available from: https://www.aao.org/eye-health/ask-ophthalmologist-q/how-long-after-opening-is-it-safe-to-use-eye-drops
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.