Back to Journals » Drug Design, Development and Therapy » Volume 16
Research Progress in the Pharmacological Activities, Toxicities, and Pharmacokinetics of Sophoridine and Its Derivatives
Authors Tang Q, Liu Y, Peng X, Wang B, Luan F , Zeng N
Received 15 September 2021
Accepted for publication 14 December 2021
Published 18 January 2022 Volume 2022:16 Pages 191—212
DOI https://doi.org/10.2147/DDDT.S339555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Qiong Tang,1,* Yao Liu,1,2,* Xi Peng,1 Baojun Wang,1 Fei Luan,1 Nan Zeng1
1State Key Laboratory of Southwestern Chinese Medicine Resources, School of Pharmacy, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, 611137, People’s Republic of China; 2School of Laboratory Medicine, Chengdu Medical College, Chengdu, Sichuan, 610083, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Nan Zeng; Fei Luan
School of Pharmacy, Chengdu University of Traditional Chinese Medicine, No. 1166, Liutai Avenue, Wenjiang Distract, Chengdu, Sichuan Province, 611137, People’s Republic of China
Tel/Fax +86-28-6180-0231
Email [email protected]; [email protected]
Abstract: Sophoridine is a natural quinolizidine alkaloid and a bioactive ingredient that can be isolated and identified from certain herbs, including Sophora flavescens Alt, Sophora alopecuroides L, and Sophora viciifolia Hance. In recent years, this quinolizidine alkaloid has gained widespread attention because of its unique structure and minimal side effects. Modern pharmacological investigations have uncovered sophoridine’s multiple wide range biological activities, such as anti-cancer, anti-inflammatory, anti-viral, anti-arrhythmia, and analgesic functions, among others. These pharmacological activities and beneficial effects point to sophoridine as a strong potential therapeutic candidate for the treatment of various diseases, including several cancer types, hepatitis B virus, enterovirus 71, coxsackievirus B3, cerebral edema, cancer pain, heart failure, acute myocardial ischemia, arrhythmia, inflammation, acute lung injury, and osteoporosis. The data showed that sophoridine had adverse reactions, including hepatotoxicity and neurotoxicity. Additionally, analyses of sophoridine’s safety, bioavailability, and pharmacokinetic parameters in animal models of research have been limited, especially in the clinic, as have been investigations on its structure-activity relationship. In this article, we comprehensively summarize the biological activities, toxicity, and pharmacokinetic characteristics of sophoridine and its derivatives, as currently reported in publications, as we attempt to provide an overall perspective on sophoridine analogs and the prospects of its application clinically.
Keywords: sophoridine, pharmacology, pharmacokinetics, toxicity
Introduction
Currently, various plant-derived metabolites are increasingly being used in drug synthesis or semi-synthesis, with a variety of plant extracts seeing extensive use in the treatment of specific diseases. Nitrogen compounds play specific roles in different plant secondary metabolites, including sophoridine, a widely distributed natural quinolizidine alkaloid (Figure 1A) that can be isolated and identified in the Leguminosae of the leguminous family, like Sophora flavescens Alt,1 Sophora alopecuroides L2 and Sophora viciifolia Hance.3 The pharmacological activities of sophoridine, including anti-cancer, anti-inflammatory, anti-viral, anti-arrhythmia, and analgesic properties, have been reported broadly in recent years. The main biological activities and some underlying molecular mechanisms are summarized in Table 1 and suggest that sophoridine is a potential drug candidate for the treatment of a variety of diseases, such as cancers, viruses, and cardiovascular diseases.
 |
Table 1 Pharmacological Activities of Sophoridine Both in vitro and in vivo Studies |
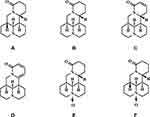 |
Figure 1 The chemical structural formula of sophoridine (A), matrine (B), sophocarpine (C), sophoramine (D), oxymatrine (E) and oxysophoridine (F). |
Over the past decades, the pharmacological properties of sophoridine have seen increased testing, as have its pharmacokinetics and toxicity in recent years. However, most previous reports on the alkaloid are scattered and lack systematic summaries and generalizations. Therefore, we have systematically reviewed and critically discussed the pharmacological activity, pharmacokinetics, and toxicity of sophoridine in this paper, as currently available in publications, in the hope of providing some perspective to the development of sophoridine and the prospects of its application clinically (Figure 2).
 |
Figure 2 The schematic representation of biological activities, toxicity, and pharmacokinetic characteristics of sophoridine and its derivatives. |
Chemical Properties and Plant Sources of Sophoridine
Sophoridine, also known as allomatrine or (5-β)-matridin-15-one, is a common quateracyclic quinolizidine alkaloid. Its basic structure consists of two dipiperidine rings with two trivalent nitrogen atoms, its molecular formula is C15H24N2O (Figure 1A), and its relative molecular weight is 248.36 g/mol, CAS No. 6882-68-4. Sophoridine has several structurally similar derivatives, including sophocarpine, sophoramine, matrine, and oxymatrine (Figure 1B–E), can be chemically converted to oxysophoridine (Figure 1F) by oxidizing its 1-nitrogen with oxygen in the presence of hydrogen peroxide, and is a white or light-yellow crystalline alkaloid monomer soluble in solvents, like water, methanol, ethanol, or carbon tetrachloride.
Sophoridine is a metabolite in plant metabolism and is reportedly one of the major alkaloids in several representative plants, such as Sophora flavescens Alt,1 Sophora alopecuroides L,2 Sophora viciifolia Hance3 and Oxytropis ochrocephala Bunge.4 It is found primarily in the roots, flowers, and seeds of the aforementioned plants. The general steps for its extraction from plants are shown in Figure 3, with the plants yielding varying contents of isolated sophoridine: higher amounts of Sophora alopecuroides L and Sophora flavescens Alt than the others. Differences in the growth period and propagation mode also affect the content of sophoridine in Sophora flavescens Alt roots. According to Hu and his colleagues (2004), sophoridine contents increase with longer growing seasons and are highest during asexual reproduction.5 These contents differ in Sophora alopecuroides L seeds and cotyledon pseudoactinine; cotyledon contains the most significant amount (5.16 mg/g) and the seed coat (2.25 mg/g)6 the least significant amount. However, the alkaloid contents in Sophora flavescens Alt and Sophora alopecuroides L seeds are highly similar.2
 |
Figure 3 Extraction process for sophoridine from Sophora alopecuroides L. |
Pharmacological Activities of Sophoridine
Anti-Cancer Activity
Sophoridine, a natural agent extracted from medicinal plants, exhibits a wide spectrum of pharmacological properties, including anti-cancer effects, and selectively induces apoptotic cell death in a variety of human cancer cells in vitro and in vivo; however, its mechanism of action remains to be elaborated further. The main active ingredient of Kushen extract, the traditional Chinese medicine commonly known as Compound Kushen Injection (CKI) and approved as an anti-cancer agent in 1995, is sophoridine, which exhibits an excellent anti-cancer effect.7 In 2005, the State Food and Drug Administration of China (SFDA) approved the clinical application of sophoridine hydrochloride injection as a treatment for cancer patients;8 it is used to treat patients with malignant trophoblastic tumors who cannot tolerate standard chemotherapy but is contraindicated in patients with a history of hypersensitivity to quinolizidine alkaloids. The recommended dose of this injection is 125 mg/m2 diluted to 500 mL of 5% glucose solution and continuously administered intravenously for no less than 6 hours. A multicenter clinical study on sophoridine conducted in ten hospitals across the country showed that sophoridine had an acceptable therapeutic effect on malignant trophoblastic tumors.8
Broad-spectrum anti-cancer activities of sophoridine have also been reported in cancer cells from different tissues, including lung,9 hepatoma,10 pancreatic,11 gastric,12 and colon13 tissues. Several investigations have demonstrated that sophoridine and most of its derivatives inhibit cancer cell proliferation, as well as induce apoptosis, cell cycle arrest, and reverse multidrug resistance in cancer cells.
Anti-Liver Cancer Cells
Many studies have demonstrated sophoridine’s potency against cancer, with multiple underlying mechanisms. Wang et al (2017) revealed that sophoridine (20, 40 μM) suppresses liver cancer cell survival in vitro by regulating the PTEN/PI3K/Akt, caspase-3/-9, and MMP-2/-9 signaling pathways.10 Bcl-2 and Bax are two significant regulators of mitochondria-dependent apoptosis, with a decrease in Bcl-2 and an increase in Bax resulting in apoptosis.14,15 Additionally, sophoridine has been shown to induce apoptosis in rat liver BRL-3A cells through the excessive ROS accumulation-induced redox imbalance pathway.16 Its effect is also markedly associated with enhancing the expressions of cleaved PARP and Bax in BRL-3A cells and lessening Bcl-2 protein expression. In in vivo experiments, sophoridine considerably reduces tumor volume and tumor weight in nude mice by down-regulating PTEN protein expression.10
Furthermore, one recent study demonstrated sophoridine’s ability to enhance lenvatinib-resistant hepatocellular carcinoma (LR HCC) sensitivity to lenvatinib through a molecular mechanism in which sophoridine drastically suppresses ETS-1 expression to reduce VEGFR2 levels and the downstream RAS/MEK/ERK axis in LR HCC cells. The same investigation established and analyzed an in vivo HepG2-LR cell subcutaneous tumor model in BALB/c nude mice, finding that sophoridine also sensitized LR HCC to lenvatinib via a mechanism of action possibly closely associated with the inhibition of tumor cell proliferation and angiogenesis. Per these reports, combining sophoridine with lenvatinib could be a novel therapeutic strategy for HCC treatment.17
Anti-Stomach Cancer Cells
Stomach cancer is the third leading cause of cancer deaths and the most diagnosed cancer, with an incidence twice as high in men as in women.18 While surgical resection is currently the most effective treatment, most patients tend to be diagnosed with advanced-stage gastric cancer because early-stage gastric cancer is asymptomatic.19 Therefore, the development of new gastric cancer treatments is of great significance to patients with advanced-stage disease. Sophoridine has an anti-gastric cancer effect. In vitro, it has been shown to inhibit the proliferation of MKN45 cells and promote their apoptosis, possibly via a mechanism associated with the down-regulation of HMGB3 protein expression.20 Sophoridine concentration- and time-dependently inhibited the growth of gastric cancer cell lines (SGC7901 and AGS), including suppressing proliferation, migration, invasion, and colony formulation, as well as inducing apoptosis, with IC50 values of 3.52 and 3.91 μM, respectively.
According to Peng and his colleagues (2020), sophoridine induces G2/M cell cycle arrest by inhibiting double-stranded DNA break repairs in gastric cancer cells, which is inseparable from the targeted ESRRG/β-catenin pathway. Furthermore, combining sophoridine and cisplatin enhances the efficacy of cisplatin in gastric cancer cells.21 In their study assessing how CD8+ T cells modulate the tumor microenvironment, Zhuang et al (2020) found sophoridine to exert its anti-gastric cancer effect by inhibiting macrophage-mediated immunosuppression through the TLR4/IRF3 signaling pathway, subsequently up-regulating CD8+ T cytotoxic function against gastric cancer.12
Anti-Colorectal Cancer Cells
Sophoridine has been reported to have an anti-colorectal cancer effect that is possibly linked to the regulation of MAPKAPK2 targeting and could serve as a prognosis marker in colorectal cancer patients.22 Liang et al (2012) showed that sophoridine inhibited SW480 cell (IC50 = 3.14 mM) growth time- and dose-dependently by activating the mitochondrial apoptosis pathway. The studies have reported sophoridine’s exertion of its anti-colorectal carcinoma effect through the activation of apoptosis-related protein caspases (caspase-3, caspase-7, and caspase-9) and PARP regulation, thereby inducing apoptosis. In in vivo xenograft tumor model established via the subcutaneous injection of SW480 cells into the armpits of nude mice, sophoridine (15 and 25 mg/kg) inhibited tumor growth (weight and volume of tumors). Moreover, the pathological and ultrastructure of xenograft tumor cells in the drug treatment group showed apoptotic changes, suggesting that sophoridine-induced apoptosis of tumor cells.13
This demonstrably potent quateracyclic quinolizidine alkaloid has also demonstrated potential apoptosis effects on two pancreatic cancer cell lines, Miapaca-2 and PANC-1. Specifically, it effectively inhibited the proliferation of pancreatic cancer cells by blocking cells in the S phase and decreasing mitochondria-related apoptosis, as well as provoking the initiation of ERK and JNK phosphorylation and inducing ROS generation in pancreatic cancer cells. In vivo, 20 and 40 mg/kg of sophoridine drastically suppressed tumor growth in mouse xenograft models.11 Ren et al (2016) reported that sophoridine can effectively inhibit human pancreatic cancer capan-1 cell proliferation and reduce cell invasion ability by down-regulating the expression levels of MMP-2 and MMP-9.23
Anti-Lung Cancer Cells
Li et al (2015) showed that the intracellular ROS level and apoptosis rate of A549 cells were up-regulated after sophalidine (20 and 40 μM) treatment, and the cell cycle was arrested in G2/M phase. In addition, the expression of pro-apoptotic protein caspase-3/8 was up-regulated, the expression of apoptotic protein Survivin and bcl-2 was down-regulated, and the expression of cycle-related protein CDK-2, adhesion molecule CD44 and matrix metalloproteinase MMP-2/9 were down-regulated. These results suggest that sophoridine inhibits the proliferation and invasion of lung cancer by increasing ROS levels, promoting apoptosis and blocking cell cycle.9 Zhu et al (2020) revealed that sophoridine inhibits the proliferation and colony formation of lung cancer and enhances the effects of cisplatin against lung cancer cells, possibly via the activation of the Hippo and p53 signaling pathways.24 In Lewis mouse model, Zhao et al (2014) found that sophoridine (15 and 25 mg/kg) effectively inhibits tumor growth of non-small lung cancer by activating MAPKs signaling pathway and increasing expression of pro-inflammatory cytokines and M1 surface marker CD86.25
Dong et al (2014) assessment of these properties revealed that mesoporous silica nanospheres effectively regulated the uptake and release of sophoridine; however, how these nanospheres perform in vivo remains unknown.26 Inspired by the above ideas, Wang et al (2016) prepared sophoridine-loaded PLGA microspheres using the O/O emulsion-solvent evaporation method, which exhibited combined lung-targeting and sustained drug release properties.27 Thus, the sustained release of loaded sophoridine by PLGA microspheres suggests that sophoridine has a remarkable potential value for lung cancer treatment.
Others
Forkhead box M1 (FOXM1) is a key regulator of the cell cycle and is expressed during the G1, S, and mitotic phases,28 and the activator protein 1 (AP-1) compound is vital in the event of cancer metastasis.29 Yue et al (2017) demonstrated that sophoridine markedly attenuated cancer cell growth, induced apoptosis by suppressing the FOXM1, NF-κB, and AP-1 signaling pathways, and significantly up-regulated cytotoxicity and caspase-3/8 activity in the human medulloblastoma cell line D283-Med.30 In addition, sophoridine significantly up-regulated cytotoxicity and caspase-3/8 activity in human medulloblastoma. In human glioma U87MG cell, Wang and his colleagues (2015) found that sophoridine shows obvious anti-cancer activity on glioma cells by inducing cell apoptosis, inducing ROS accumulation, and activating mitochondrial signal pathways.31
Anti-Cancer of Sophoridine Derivatives
While sophoridine has been approved as an anti-cancer agent, its anti-cancer property has only been observed in malignant trophoblastic tumors.8 To improve this property, a series of structural modifications have been conducted on sophoridine, and the resulting derivatives have been examined for anti-cancer effects. This endeavor has yielded a well-developed structure–activity relationship (SAR) for sophoridine. Chemically, sophoridine belongs to one kind of matrine-type alkaloid formed from two piperidine rings by the thickening of trivalent nitrogen atoms. Structural modifications on the lactam ring of matrine-type alkaloids are currently a popular subject of research among investigators. Sophoridine derivatives can be classified based on structural modifications: i) at the N-16 position; ii) at the C-14 position of the lactam ring; and iii) on sophoridine by opening the lactam ring (Figure 4).
 |
Figure 4 Synthetic routes of sophoridine derivatives 1, 2 and 3 that have anti-cancer effects. |
The SAR indicates that the introduction of an aliphatic acyl on the 16-nitrogen atom of sophoridine could considerably enhance its anti-cancer effect. For instance, the compound 1-bearing bromoacetyl side-chain is active against four human tumor cell line types: liver, colon, breast, and lung cell lines, with an IC50 value of 0.062 mM and a mechanism of action aimed at inhibiting the activity of DNA topoisomerase I (Figure 4).32 According to findings from SAR analyses, two suitable substitutions on the 16-nitrogen atom and carboxyl region can help maintain a satisfactory anti-tumor activity: two moieties could be introduced as decorators of various substitutions to regulate the drug-like physical-chemical properties of the compounds.33
Among the newly synthesized derivatives, compound 2 displays a potential anti-proliferative activity in both wild MCF-7 and adriamycin (AMD)-resistant MCF-7 (MCF-7/AMD) breast carcinoma cell lines, with an IC50 value of 3.1 μM. Its mechanism of action is associated with the arrest of the cell cycle at the G0/G1 phase, consistent with that of sophoridine. Compound 2 also has a reasonable clogp value and a favorable pharmacokinetic property with rapid absorption at a Tmax of 4.6 h, a favorable half-life of 12 h, and a mean residence time (MRT) of 5.4 h if administered orally at a dose of 25 mg/kg (Figure 4).34 Therefore, the SAR results suggest that a sophoridinic acid with a 3-ring structure scaffold is more promising than sophoridine with a 4-ring scaffold.
Per further SAR analyses, the R-configuration at the 5-position is crucial and the introduction of a chlorobenzyl to the 12-nitrogen atom of sophoridinol significantly enhances sophoridine’s activity.35 Compound 3 is synthesized by introducing a benzyl chloride group to nitrogen atoms, which can result in a more potent anti-proliferative property against various cancer cell line types (HepG2, HCT116, H1299, U87, MCF-7, and KB), with IC50 values ranging from 0.011 to 0.041 mM and much better than that of the parent sophoridine (>0.08 mM) and a mode of action that constitutes inhibiting the activity of DNA topo I, followed by the G0/G1 phase arrest. Compound 3 also has high solubility and decent safety profiles (Figure 4).36
A previous SAR analysis revealed that the 16-N-p-cholobenzyl substituent boosts sophoridine’s anti-tumor activity. Taking 16-N-p-chlorobenzyl Compound 3 as a lead compound, numerous novel sophoridinic derivatives with various 3′-substituents at the 11-attachment have been synthesized and evaluated for their anti-cancer properties. Among them, sophoridinic ketones 4a-b, alkenes 4c-d, and sophoridinic amines 4e-f have displayed adequate anti-proliferative activity, with IC50 values ranging from 3.8 to 5.4 μM. Particularly, compounds 4a and 4d were shown to exhibit an equipotency in both AMD-susceptible and AMD-resistant MCF-7 breast carcinoma cells, indicating that they possess a different mechanism from AMD (Figure 5).37
 |
Figure 5 Synthetic routes of sophoridine derivatives 4a-f and 5 that have anti-cancer effects. |
Recently, Zhao et al (2020) found that compound 5 (Figure 5) induced DNA breakage and cell apoptosis by blocking STAT5a nuclear translocation and inhibiting STAT5a phosphorylation at 694 and 780.38 Compared to other derivatives, compound 5 also displayed outstanding water solubility and lower toxicity properties, and its synthesis is straightforward, suggesting that it could be applied as an anti-cancer compound and, therefore, should be explored further.
The synthesis of novel sophoridine derivatives using acyclic aryloxy phosphoramidate mustard can greatly improve sophoridine’s anti-cancer activity. For instance, compound 6a-6e, containing a phosphoramidate mustard motif substituted at the lactam ring-opened sophoridine skeleton was shown to markedly suppress the viability of S180 and H22 cells (IC50 = 1.01–3.56 μM). In addition, compounds 6a, 6c, and 6e (Figure 6) hindered cancer growth with apparent organ toxicity in mice bearing liver tumors, and molecular docking showed compounds 6a-6e binding well with DNA topoisomerase.39
 |
Figure 6 Synthetic routes of sophoridine derivatives 6a-e and 7 that have anti-cancer effects. |
SAR analyses in a series of sophoridine derivatives show that the introduction of the N-benzyl indole group to the 14-carbon atom of sophoridine can significantly enhance sophoridine’s anti-proliferative activity. Of 58 compounds, 33 displayed improved anti-proliferative properties, with IC50 values less than 10 µM; compound 7 exhibited the most potent anti-proliferative activity in all tested cell lines (HepG2, SMMC-7721, Hela, CNE1, CNE2, and MCF7), with IC50 values ranging from 0.93 to 1.89 μM and much lower than that of the parent sophoridine. Anti-cancer mechanisms are strongly associated with the suppression of DNA topo I and the activation of caspase-3 via molecular docking and enzyme assays. In vivo, anti-tumor assays demonstrated that compound 7 suppresses the growth of HepG2 xenografts in nude mice without obvious side effects.40
Per SAR analyses (Figure 7), i) methoxy and N-(4-(phenylmethoxy) benzyl) groups at the indole ring boost anti-cancer activity; ii) the connecting site of indole (attached to sophoridine) at position 3 is preferable to positions 4 or 5; iii) sophoridine derivatives bearing an α, β-unsaturated ketone scaffold are essential to their anti-cancer inhibitory activity; iv) the (R)-configuration of the 6-chiral center is considerably more adequate for anti-cancer activity enhancement than the (S)-configuration. Therefore, the structural modification of sophoridine is a key strategy to enhance its anti-cancer properties.
 |
Figure 7 Structure activity relationship analysis of sophoridine derivatives. |
Anti-Virus Activity
Hepatitis B virus (HBV) infection is a severe global health problem caused by the hepatotropic double-stranded DNA virus, with long-term infection capable of causing a series of pathological changes in the liver, such as fatty liver, chronic hepatitis, and advanced liver diseases, including cirrhosis and liver cancer.41 Chen et al (2016) reported that sophoridine inhibits HBV in HepG 2.2.15 cells by reducing HBsAg and intracellular HBV DNA levels. Additionally, sophoridine (at doses of 0.4, 0.8, and 1.6 mM) showed a more potent anti-HBV activity than other matrine-type alkaloids (matrine, oxymatrine, and sophocarpine) by effectively down-regulating the mRNA expressions of p38 MAPK, TRAF6, ERK1, NLRP10, and caspase-1; its anti-HBV effect might be partly attributable to the rise in the methylation of HBV DNA.42 Matrine-type alkaloids from Sophora flavescens Alt have also been shown to exhibit potent antiviral activity against HBV.43 Sophoridine was revealed to significantly inhibit HBsAg in the HepG2.2.15 cell line by 40.2% at non-cytotoxic concentrations of 0.4 mM, with a potency degree comparable to matrine’s (34.7% at a concentration of 0.4 mM) but superior to the positive control, lamivudine’s (31.5% at a concentration of 1.0 mM).44 These findings provide a new perspective for the application of sophoridine as an anti-HBV agent.
Enterovirus 71 (EV71) is the main pathogen that causes hand, foot, and mouth disease (HFMD) epidemics.45 It is characterized by single-stranded and positive polarity RNA viruses and belongs to group A of enteroviruses in the family of small RNA viruses.46 Ou et al (2016) found that sophoridine inhibited the EV71 virus adsorption and its RNA replication in vitro (SI = 3.99).47 Using the attachment and penetration assays, Ren et al (2019) established that sophoridine (IC50 = 0.25 mM) displayed significant antiviral activity against EV71 when Vero cells were pretreated with the alkaloid.48
Coxsackievirus B3 (CVB3) also belongs to the enterovirus family of small RNA viruses, which can cause viral myocarditis, hepatitis, and meningoencephalitis in adults.49 Although the pathogenesis of CVB3 has been studied extensively in mouse models and a lot of work has been done on the development of vaccines and therapeutic agents against CVB3, no effective clinical therapeutic methods have been established so far.50,51 Per Zhang et al (2006) sophoridine exhibited antiviral properties against CVB3 both in vitro (primary myocardial cells) and in vivo (BALB/c mice) by speeding up IL-10 and IFN-γ production and shrinking TNF-α levels, possibly through locatine itself rather than via a metabolite.52
Neuroprotection and Analgesic Activities
Sophoridine is a quinolizine alkaloid and a structural isomer of matrine whose biological activities have been scrutinized extensively and shown to be similar to its pharmacological activities. Studies have revealed that matrine is an effective neuroprotective substance against cerebral ischemia.53–55 Researchers examining the effect of sophoridine (10, 20, and 40 mM) on nerves in a model of permanent middle cerebral artery occlusion (PMCO) found that it had a potential neuroprotective activity, including diminishing cerebral edema and infarct volume, possibly via the down-regulation of TRAF6 expression and the up-regulation of erk1/2 phosphorylation.56 In Miao et al’s research (2013), sophoridine significantly improved neural dysfunction in ischemia-reperfusion injury by inhibiting the TLR4/NF-κB signal pathway.57 These findings indicate that sophoridine possesses a decent neuroprotective activity.
Compound matrine injection is clinically used primarily against cancer pain and bleeding, with sophoridine one of its main components.58 Yan et al (2013) found that sophoridine significantly alleviated pain and inhibited tumor progression in rats with W256 tumor cell transfection-induced bone cancer pain by enhancing the mechanical withdrawal threshold and thermal withdrawal latency via a mechanism associated considerably with the inactivation of the expression of cyclooxygenase-2 (COX-2) and vascular endothelial growth factor (VEGF) or linked to nitric oxide (NO),59 a neurotransmitter with a key role in the intermediation stage of pain signaling.60,61 Further research has shown that the mechanism of sophoridine’s analgesic property against bone cancer pain may be associated with its down-regulation of the “NMDAR-nNOS/NO-cGMP” signal transduction pathway that is closely linked to hyperalgesia.62 Zhang et al (2012) also demonstrated sophoridine’s outstanding analgesic activity in traditional pain model-induced hot-plate, writhing, and tenderness tests, which was more potent than those of other matrine-type alkaloids (oxymatrine, sophocarpine, and oxysophocarpine) in acetic acid writhing pain.63 These reports provide experimental bases for the further development of alkaloids; however, their specific mechanisms must be investigated further.
Effect on Cardiovascular System
Heart failure (HF) is characterized by contractile dysfunction and the activation of neurohumoral factors and is escalating major health problems worldwide.64 Studies have demonstrated matrine-type alkaloids’ positive inotropic activity on the myocardium, which could be associated with the activation of the calcium channel. For example, matrine, oxymatrine, sophoridine, and sophocarpine, with common molecular structures of O = C = N-CC-CC-N, possess positive ion effects and hERG blocking activities.65,66 Some investigations have suggested that this structure may be responsible for the positive inotropic effect of matrine-type alkaloids, possibly in association with the activation of calcium channels. The abnormal regulation of Ca2+ by the L-type Ca2+ channel (LTCC)/dihydropyridine receptor (DHPR) and Ca2+-ATPase (SERCA2a) is the underlying mechanism for muscle dysfunctions in HF.67 Our assessment of the effect of sophoridine on heart failure in a rat model of chronic HF revealed that sophoridine (5 and 10mg/kg) improved HF by ameliorating cardiac Ca2+-induced Ca2+ transients through the up-regulation of the dihydropyridine receptor (DHPR).68 The administration of 5 mg/kg of sophoridine provided sufficient significant cardioprotection against myocardial infarction-induced heart injury. Similarly, Hu et al (2014) found that sophoridine protects myocardial in rats with chronic HF by recovering the sarcoplasmic reticulum calcium capacity via a mechanism that is possibly associated with the up-regulation of SERCA2a expression in cardiomyocytes.69 Therefore, sophoridine can become a novel, effective therapeutic drug for the treatment of HF.
Acute myocardial ischemia (AMI) is a pathological condition marked by failure of the heart to function properly in which blood perfusion in the heart rapidly decreases, leading to an abnormal heart function.70,71 Ding et al (2010) established an AMI model in vivo by ligating the left anterior descending coronary artery (LAD) to evaluate the effects of sophoridine on cardiac function in rats with AMI. By recording the cardiac function indexes of pre-LAD occlusion using the BL-420 biological function experimental system, the authors noted that sophoridine improved the cardiac function of rats with LAD acduion-induced acute myocardial ischemia injury, boosting the left ventricular systolic pressure (LVSP) and ± dp/dtmax changes and decreasing the left ventricular end-diastolic pressure (LVEDP) of AMI.72 They also observed that acute myocardial infarction caused irreversible myocardial necrosis due to sudden and persistent AMI, with the changes and recovery of various cardiac function indexes associated with the scope of myocardial infarction. Ding et al (2009) noted that sophoridine ameliorated cardiac damage in a rat model of AMI by shrinking the myocardial ischemic infarct size dose-dependently.73
Sophoridine is protective against arrhythmia too. The human ether-à-go-go-related gene (hERG), which encodes the rapid component of the cardiac delayed rectifier K+ current (IKr), has a significant role in the repolarization of the cardiac action potential.74–76 One study assessed the effects of sophoridine on hERG-encoded K+ channels in HEK293 cells stably transfected with hERG cDNA using the whole-cell patch-clamp technique and found that sophoridine acted as a low potency blocker of hERG K+ channels by altering the channels’ kinetics. Additionally, sophoridine had a higher binding affinity for the open state; however, it had no impact on the generation and transport of the hERG protein.77 According to Hu and his colleagues (2016), the anti-arrhythmia effect of sophoridine is closely associated with the inhibition of cardiomyocytes’ scavenging of hydroxy radicals and effect on the ion channels.68 In other studies, sophoridine significantly inhibited aconitine, BaCl2, and coronary ligation-induced ventricular arrhythmias in rats.78 Therefore, sophoridine harbors significant anti-arrhythmia properties whose specific mechanism must be researched further.
Anti-Inflammatory Activity
Inflammation is associated with the development and progression of many diseases, including pneumonia and colitis. Therefore, the search for effective and low-toxicity anti-inflammatory drugs remains a research hotspot. Endotoxin or lipopolysaccharide (LPS) is crucial to the infection-triggered systemic inflammatory response syndrome.79 In recent years, many investigations have focused on the antagonistic effect of sophoridine on LPS. Huang et al (2014) demonstrated sophoridine’s excellent anti-inflammatory activity in which the alkaloid protected mouse macrophages from LPS-induced apoptosis and inhibited the production of inflammatory cytokines (TNF-α and IL-8) and inflammatory media prostaglandin E2 (PGE2) in vitro and in vivo.80 The intercellular adhesion molecule-1 (ICAM-1) gene is a member of the immunoglobulin superfamily (IGSF) of adhesion molecules and plays a pivotal role in the body’s immune response.81,82 In a dextran sulfate sodium (DSS)-induced colitis C57BL/6 mouse model, Zhao et al (2010) revealed for the first time that sophoridine markedly attenuated colonic inflammatory response by negatively mediating the expression of the ICAM-1 gene.83 Another in vivo experiment detailed sophoridine’s significant protective effect against acute LPS-induced liver injury via a mechanism possibly associated with the inhibition of p-ERK expression in liver tissues and TNF-α expression in serum.84 Similarly, Zhang et al (2015) reported that sophoridine suppressed RAW264.7 cell inflammatory response by mitigating the expressions of CD14, P38, and iNOS.85 The TLR4/JNK axis also allegedly participates in the execution of sophoridine’s (125.8 μM) protective effect on LPS-induced RAW264.7 cells.86
Acute lung injury (ALI) is a primary cause of morbidity and mortality worldwide and is characterized by extensive inflammation of the lungs.87 In an LPS-induced lung injury mouse model, researchers found that sophoridine alleviated inflammatory response by suppressing the NF-κB (phosphorylation of p65 and IκB) and MAPK (phosphorylation of p38 and JNK) pathways.88,89 On their part, Yang et al (2012) demonstrated that the anti-inflammatory activity of sophoridine on lungs relies on its inhibition of IL-6, IL-10, NO, and malondialdehyde (MDA) and elevation of superoxide dismutase (SOD).90 These results indicate that sophoridine could treat inflammation-related diseases.
Others
The pharmacological properties of sophoridine against other conditions have also been examined. The formation of osteoclasts (OCs) is one of the decisive pathological processes in the occurrence and development of osteoporosis.91,92 In Zhao et al (2011), ovariectomized mice (OVX) treated with sophoridine (15mg/kg) dose-dependently and time-dependently blocked RANKL-induced OC formation and activation of ERK and c-fos, as well as NFATc1 induction and nuclear translocation. Therefore, sophoridine protected against ovariectomy-induced osteoporosis, possibly by inhibiting OC formation through the RANKL-ERK-NFAT pathway.93
Sophoridine, it is alleged, also inhibits sperm motility in vitro dose-dependently. One study noted that a low dose of sophoridine (44.3 μM) inhibited sperm motor function, while a considerably higher dose (120.8 μM) concentration altered the morphological structure of sperm, including sperm crack. Per a drug sensitivity test, sophoridine did not interfere with the growth and reproduction of normal vaginal flora, especially lactobacillus. Therefore, sophoridine as a vaginal spermatostatic agent cannot destroy the normal physiological environment of the vagina.94
Sophoridine also inhibits a variety of bacteria. Zhou et al (2001) observed that sophoridine significantly inhibited five environmental bacterial strains (Escherichia coli, Enterobacter aerogenes, Proteus vulgaris, Bacillus subtilis, and Staphylococcus epidermidis) at a minimum inhibitory concentration (MIC) of 20–40 mM.95 Another study elaborated on sophoridine’s bacteriostatic effect on bacteria that cause common urogenital tract infection, like Escherichia coli (MIC = 32.2 mM), Pseudomonas aeruginosa (MIC = 32.2 mM), and Staphylococcus epidermidis (MIC = 16.1 mM): sophoridine’s bacteriostatic effect was stronger than that of oxysophoridine.96 Sophoridine undoubtedly possesses a broad spectrum of pharmacological activities and can be used to treat specific diseases (Table 1), which points to its far-reaching application prospects.
Pharmacokinetics of Sophoridine
The development of pharmacochemistry has seen the pharmacokinetic characteristics of drugs become more and more complex and establishing them become progressively more challenging. Presently, the acceptability of a drug requires assessing its characteristics and application prospects based not only on high efficiency and low toxicity but also on excellent pharmacokinetic properties.97 A study found that intravenously administering 20 mg/kg of sophoridine to rats resulted in the following body tissue distribution order: kidney > spleen > uterus > gastrointestinal tract > liver > lung > heart > muscle > brain > blood.98
Liu et al (2014) reported a low absolute bioavailability (Fabs) of sophoridine via gavage (Fabs = 41.8%), as well as an increased area under the plasma concentration–time curve and improved sophoridine bioavailability post-intravenous administration (AUC0-18h = 3005.3 ng.h.mL−1) compared to intragastric administration (AUC0-18h = 1256.2 ng.h.mL−1). The low absolute bioavailability via gavage was probably due to the first-pass effect of oral administration during which part of sophoridine is destroyed in the liver so that the effect of what is left of the drug absorbed into blood circulation is reduced.99 In Wu and colleagues’ (2005) high-performance liquid chromatography-mass spectrometry (HPLC-MS) investigation to examine the pharmacokinetic features of sophoridine in rabbit plasma, they established a decent linear relationship between the peak area and concentration at sophoridine doses ranging from 0.05 to 4 μM.100 In another trial, 16 tumor patients were given sophoridine (75, 125, and 144 mg.m−2) by intravenous infusion at a constant rate for 3 h, and subsequent analyses revealed that the drug was rapidly distributed and eliminated (t1/2β = 5.22, 7.42, and 7.41 h) in the body, eventually excreting mainly in its original form through the kidney.8
A liposome is a type of microcapsule with a bilayer structure similar to a biofilm; it has low toxicity, low irritation, and excellent biocompatibility.101 Nano-liposomes were prepared from sophoridine and used to alter the distribution of drugs in the body, prolong the action time of drugs in target tissues, improve drug efficacy, and reduce the side effects of drugs.102 In an HPLC analysis of the pharmacokinetics and distribution of sophoridine nano-liposome injections in rats after administration through the tail vein using sophoridine injection as control, results showed that the AUC curve of sophoridine nano-liposomes was 2.42 times as much as that of sophoridine injection, with prolonged retention time in vivo.103
Bovine serum albumin (BSA) is the most abundant carrier protein in serum, which can combine with many compounds and is an important carrier and target of drugs. To explore the interaction between drugs and BSA is of great significance for understanding the distribution, transport, efficacy and metabolism of drugs in vivo, revealing pharmacokinetics, guiding clinical rational drug use and new drug design and development.104,105 There is a strong binding effect between sophoridine and BSA, and the main driving force behind this binding effect is electrostatic attraction. In a study on the binding sites of sophoridine on BSA using sites I and II probes, sophoridine liaised with the site I loci on BSA hydrophobic cavities. The results provided important information for studying the storage, transportation and pharmacodynamics of sophoridine in vivo.106
Traditional Chinese medicine often contains multiple ingredients, with other ingredients also capable of impacting the elimination of the drug. Wu et al (2005) found that the elimination half-life of sophoridine in Kuhuang injection (T1/2β = 67.2 min) was shorter than that of standard sophoridine (T1/2β = 84.5 min), suggesting that other components in Kuhuang injection promote the elimination of sophoridine.107 In their investigation, Hu and his colleagues (1995) demonstrated that sophoridine was eliminated primarily through excretion via the kidney and urine after it was injected into the tail vein of rats.98 In Li et al’s research (2006), sophoridine was excreted as a prototype through the kidney in the human body.8 Further relevant pharmacokinetic parameters of sophoridine in vivo are summarized in Table 2; these also provide meaningful references for the pharmacokinetic study of sophoridine.
 |
Table 2 Pharmacokinetic Parameters of Sophoridine in vivo |
Toxicology of Sophoridine
In addition to the numerous pharmacological studies, experiments to understand the toxicology of sophoridine against normal cells and mice have been conducted. One reported sophoridine’s cytotoxicity against Vero cells (African green monkey kidney cell), with an IC50 value of 5.69 mM via an MTT assay.48 However, in Wang et al’s investigation (2019), sophoridine displayed no cytotoxicity against two non-tumorigenic cell lines (HKC and LX-2), even at a high concentration of 160 μM.22 Another report found sophoridine to concentration- and time-dependently inhibit the activity of BRL-3A cells (rat normal liver cells) at IC50 values of 2.94 mM (12 h), 1.9 mM (24 h), and 1.29 mM (48 h), respectively. Perhaps, sophoridine reduces ATP levels and the mitochondrial membrane potential, thereby inducing apoptosis through the mitochondria-dependent apoptosis pathway.16 Lower degrees of cytotoxicity to human pancreatic ductal epithelial (HPDE) cells and human bronchial epithelial (BEAS-2B) cells have also been demonstrated, with corresponding IC50 values of 0.402 and 0.458 mM.11
Sophoridine toxicity assessments have also been carried out in vivo in recent years. In Shi et al’s (2020) acute evaluations of sophoridine (ip), the alkaloid had an acute toxic LD50 of 62.6 mg/kg in mice. Its continuous administration for 30 days caused substantial organ damage, including the liver and kidney; however, these recovered after 30 days of discontinuing the treatment.108 Yu et al (2006) derived an LD50 of 47.6 mg/kg for sophoridine injection into the tail vein of mice,109 while Zhang et al (2011) showed that a one-time intraperitoneal injection of sophoridine (32–78.1 mg/kg) reduced the autonomous activity of mice, with an LD50 (65.19 mg/kg) of the drug close to LD1 (39.74 mg/kg).110 According to Zhu et al (1995), intraperitoneally injecting 27 mg/kg (about 27 times the clinical therapeutic dose) of sophoridine resulted in embryotoxicity in Wistar pregnant mice, including decreased weight gain and increased stillbirth rates; however, no teratogenic effect was noted.111 In contrast, Zhang et al (2006) reported no sophoridine-related toxicity in mice, not even at an oral dose of 40 mg/kg, suggesting it can be used in a clinical setting.52
After intraperitoneally injecting rats with sophoridine (32 mg/kg) for 60 days, Li et al (2004) found that the rats developed various neurotoxic reactions, including fluffiness, tail erection, muscle tremor, and convulsion. However, the investigators established no histopathological changes or delayed alterations in the nervous system. Therefore, sophoridine-caused neurological symptoms are functional, stressful, and reversible without late-onset and sequelae.112
Cheng et al’s analyses (2010) yielded sophoridine (47.83 mg/kg)-induced increases in the expressions of ERK1, ERK2, and p-ERK1/2 proteins in hippocampal CA1 neurons and epileptic damage to rat hippocampal tissues through the activation of the ERK signaling pathway.113 Another investigation examined the effects of sophoridine on the morphology and ultrastructure of hippocampal neurons in the CA3 region and revealed that sophoridine induced hippocampal neuronal damage, including the destruction of the nuclear membrane bilayer structure, severe nuclear pyknosis, and mitochondrial swelling, which resulted in epilepsy.114 The authors subsequently demonstrated that sophoridine shortens the incubation period of epilepsy, prolongs the onset of time, and its effect is stronger than that of penicillin,115 with the mechanisms possibly associated with sophoridine’s elevation of TNF-α, IL-2, and IL-6 in serum.116 The contradictory nature of reports on sophoridine toxicity suggests that the toxicology of this alkaloid requires further research and exploration to ascertain its safety and efficacy. The toxicity of sophoridine is summarized in Table 3 for additional reference.
 |
Table 3 Toxicity of Sophoridine Both in vitro and in vivo Studies |
Conclusion and Future Perspectives
Natural products are key sources for the development of new drugs.117–119 In recent years, artemisinin, camptothecin, and others have been examined and have seen increased use thanks to their acceptable efficacy and low toxicity. Sophoridine is another natural compound with a unique chemical structure and pharmacological action. It can be extracted from some commonly used traditional herbal medicines. In this review, we have systematically summarized the chemical properties, plant origin, pharmacological activities, toxicity, and pharmacokinetic characteristics of sophoridine and hope that the material we have put together provides a reference point for future research and the further development of sophoridine.
At present, sophoridine is used mostly to treat malignant trophoblastic tumors that cannot be attended to with standard chemotherapy. However, many investigations have demonstrated excellent inhibitory effects of this substance on other forms of tumors. As mentioned previously, sophoridine has shown good anti-cancer effects by blocking cells in the S phase and decreasing mitochondrial-related apoptosis, up-regulating cytotoxicity in human medulloblastoma, and regulating the TLR4/IRF3, RAS/MEK/ERK, NF-κB, and PTEN/PI3K/Akt signaling pathways. In addition, sophoridine also regulated apoptosis proteins and cell cycle-related genes (cleaved caspase-3, caspase-7, caspase-9, Bcl-2, MMP-2, and MMP-9) in tumor cells. Research can, therefore, utilize nanotechnology and structural modifications (new sophoridine derivatives) to promote its anti-tumor activity for clinical use.
Because the risk of cancer is further amplified by chronic inflammation considered one of the hallmarks of cancer,120,121 we have also presented findings on sophoridine’s significant anti-inflammatory activity mediated mainly by modulating immune cells and related cytokines, including TNF-α, IL-6, IL-8, IL-10, NO and iNOS, etc. Furthermore, we have digested results on sophoridine’s therapeutic potential against viruses and cardiovascular diseases. As mentioned above, experiments with some animal models have demonstrated that sophoridine not only inhibits cardiomyocytes’ scavenging of hydroxyl radicals and effect on cardiac ion channels but also has positive inotropic effects. Sophoridine, as presented above, also inhibits HBV, EV71, and CBV3 viruses. According to literature reports, sophoridine through structural modification (benzyl indole group or chlorobenzyl), can improve its anti-tumor pharmacological activity, but in other pharmacological activities (inflammatory and viruses) are relatively lack, later can increase this aspect of research, screen more activity and lower side effects of sophoridine derivatives. Its therapeutic mechanism must also be established with the help of metabolomics and pharmacology.
In addition to the therapeutic effects of drugs, their pharmacokinetic properties and safety are key factors in determining their potential for clinical application. It has been reported that sophoridine is widely distributed in various tissues, including kidney, liver, gastrointestinal, uterus, lung and other organs. Sophoridine showed hepatotoxicity, reproductive toxicity and neurotoxicity. Therefore, it is necessary to further study the methods to reduce the toxicity of sophoridine in order to improve its medicinal value. The structure can be modified to produce sophoridine derivatives that are specific to specific organs and reduce their widespread distribution and resulting toxicity.
Added to that, a recent study reported that the support vector machine (SVM) classifier and physiologically based pharmacokinetic (PBPK) modeling can be optimized to predict the oral hepatotoxicity dose of natural products derived from traditional Chinese medicines (NP-TCMs).122,123 Therefore, if these methods can be used to predict the hepatotoxicity of sophoridine in future experiments, evaluating its safety and clinical applicability will take one step forward. To better assess its side effects or toxicity and render it safe to use, rigorous in vivo testing must be performed.
Sophoridine and berberine are both bioactive alkaloids derived from many herbs, but they have some differences in pharmacological activity and pharmacokinetics. Berberine is widely used to treat diabetes mellitus, and its mechanism probably consists of inhibiting hepatic gluconeogenesis and inducing hyperglycolysis and insulin sensitivity.124 On the other hand, sophoridine is the focus of anti-tumor research, particularly malignant trophoblastic tumors. Many experimental studies have confirmed that berberine is metabolized rapidly in vivo, primarily by the liver and intestinal flora, and its content and structure change after metabolism.124 In contrast, research shows that sophoridine is metabolized primarily through urine excretion as a prototype of the human kidney.8,98 Still, the difference in LD50 between sophoridine and berberine (65.19 vs 57.6 mg/kg) by intraperitoneal injection is miniscule.110,125
Collectively, sophoridine has multiple biological activities. To promote its development for acceptable clinical application, future research should consider focusing primarily on the following aspects: (i) elucidating its anti-cancer molecular mechanism using scientific techniques and methods; (ii) conducting more in-depth investigations to demonstrate the potential of sophoridine in the treatment of viruses and arrhythmias; (iii) performing more in vivo experiments to clarify its safety; (iv) improving oral availability and drug treatment potential through nanotechnology and chemical structure modification.
Abbreviations
Akt, protein kinase B; ALI, acute lung injury; AMD, adriamycin; AMI, acute myocardial ischemia; AP-1, activator protein 1; ATP, adenosine triphosphate; CC50, cytotoxic concentration 50%; CCK-8, cell counting kit-8; cGMP, cyclic guanosine monophosphate; CL, clearance; Cmax, maximum concentration; COX-2, cyclooxygenase-2; CVB3, coxsackie virus B3; DCM, dichloromethane; DHPR, dihydropyridine receptor; DMSO, dimethyl sulfoxide; ERK, extracellular signal-regulated kinase; EV71, enterovirus 71; FDA, Food and Drug Administration; FOXM1, forkhead box m1; HBV, hepatitis B virus; hERG, human ether-a-go-go related gene; HF, heart failure; HFMD, foot and mouth disease; HPLC-MS, high performance liquid chromatography mass spectrometry; IC50, half maximal inhibitory concentration; ICAM-1, intercellular adhesion molecule-1; IFN-gamma, interferon-gamma; IGSF, immunoglobulin superfamily; IL-10, interleukin 10; IL-8, interleukin 8; IRF3, interferon regulatory factor 3; JNK, c-Jun N-terminal kinase; LAD, left anterior descending coronary artery; LPS, lipopolysaccharide; LTCC, L-type Ca2+ channel; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure; MAPK, mitogen-activated protein kinase; MAPK, mitogen-activated protein kinase; MAPKAPK2, mitogen activated protein kinase activated protein kinase 2; MDA, malondialdehyde; MMPs, matrix metalloproteinase; MRT, mean residence time; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor kappa-B; NMDAR, N-methyl-D-aspartic acid receptor; nNOS, neuronal nitric oxide synthase; NO, nitric oxide; PARP, poly-ADP-ribose polymerase; PGE2, prostaglandin E2; PI3K, phosphatidylinositol 3-kinase; PLGA, plastic land grid array; PMCO, permanent middle cerebral artery occlusion; PTEN, phosphate and tension homology deleted on chromosome ten; RANKL, receptor activator of nuclear factor-κ B ligand; ROS, reactive oxygen species; SAR, structure–activity relationship; SERCA2a, sarcoplasmic reticulum Ca2+-ATPase; SOD, superoxide dismutase; SOD, superoxide dismutase; STAT5a, signal transducer and activator of transcription 5a; STB, Sodium tert-butoxide; T1/2, half-life; TEA, triethylamine; THF, Tetrahydrofuran; TLR4, Toll-like receptor 4; Tmax, mean time to peak concentration; TNF-α, tumor necrosis factor-alpha; TRAF6, tumor necrosis factor-associated factor 6; VEGF, vascular endothelial growth factor.
Acknowledgments
This project was financially supported by National Natural Science Foundation of China (No. 82074094) and the Xinglin Scholar Research Promotion Project of Chengdu University of Traditional Chinese Medicine (No. CXTD2018014).
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Zhang H, Zhu Z, Qian D, et al. Analysis and evaluation of alkaloids and flavonoids in flower of Sophora flavescens from Shanxi province. Zhongguo Zhong Yao Za Zhi. 2016;41(24):4621–4627. doi:10.4268/cjcmm20162422
2. Weng Z, Duan J, Guo S, et al. Evaluation analysis of alkaloids in seed of Sophora flavescens from Shanxi province and exploration of its utilization value. China j Chin materia medica. 2016;41(17):3265–3271. doi:10.4268/cjcmm20161724
3. Wen M, Mao X. Research status of chemical constituents and anti-inflammatory and antiallergic effects of Sophora viciifolia Hance. Yunnan J Traditional Chin Med and Materia Medicina. 2006;2(1):63–64.
4. Tan C, Zhao Y, Goto M, et al. Alkaloids from Oxytropis ochrocephala and antiproliferative activity of sophoridine derivatives against cancer cell lines. Bioorg Med Chem Lett. 2016;26(5):1495–1497. doi:10.1016/j.bmcl.2015.09.010
5. Chen X, Yi C, Yang X, Wang X. Liquid chromatography of active principles in Sophora flavescens root. J Chromatography B. 2004;812(1–2):149–163. doi:10.1016/S1570-0232(04)00679-8
6. Wang H, Guo S, Qian D, Qian Y, Duan J. Comparative analysis of quinolizidine alkaloids from different parts of Sophora alopecuroides seeds by UPLC–MS/MS. J Pharm Biomed Anal. 2012;67–68. doi:10.1016/j.jpba.2012.04.024
7. Niu T, Chen S, Wang H, Zhao J, Hai L, Qin W. Simultaneous determination of seven constituents in compound kushen injection with quantitative analysis of multi-components by single-marker. Chin J Modern App Pharm. 2019;036(10):1187–1192.
8. Li X, Wu Y, Pan D, et al. Sophoridine is a new antitumor medicine with new molecular structure. Chine J New Drugs. 2006;15(8):654–657.
9. Li M, Wang Y, Dong L. Effect and mechanism of allomatrine in proliferation and invasion in vitro inhibition of human lung cancer A549 cell line. J Chin Pharm Sci. 2015;50(13):1111–1116.
10. Wang B, Xu J, Wang H, Chang S, Liu N. Effect and Mechanism of Sophoridine to suppress Hepatocellular carcinoma in vitro and vivo. Biomed Pharmacother. 2017;95:324–330. doi:10.1016/j.biopha.2017.08.029
11. Xu Z, Zhang F, Bai C, et al. Sophoridine induces apoptosis and S phase arrest via ROS-dependent JNK and ERK activation in human pancreatic cancer cells. J Exp Clin Cancer Res. 2017;36(1):124. doi:10.1186/s13046-017-0590-5
12. Zhuang H, Dai X, Zhang X, Mao Z, Huang H. Sophoridine suppresses macrophage-mediated immunosuppression through TLR4/IRF3 pathway and subsequently upregulates CD8+ T cytotoxic function against gastric. Biomed Pharmacother. 2020;121. doi:10.1016/j.biopha.2019.109636
13. Liang L, Wang X, Zhang X, et al. Sophoridine exerts an anti-colorectal carcinoma effect through apoptosis induction in vitro and in vivo. Life Sci. 2012;91(25–26):1295–1303. doi:10.1016/j.lfs.2012.09.021
14. Tsujimoto Y. Role of Bcl family proteins in apoptosis: apoptosomes or mitochondria. Genes Cells. 1998;3(11):697–707. doi:10.1046/j.1365-2443.1998.00223.x
15. Zheng JH, Follis AV, Kriwacki RW, Moldoveanu T. Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J. 2016;283(14):2690–2700. doi:10.1111/febs.13527
16. Qiu M, Shi F, Dai F, et al. A reactive oxygen species activation mechanism contributes to Sophoridine-induced apoptosis in rat liver BRL-3A cells. J Ethnopharmacol. 2018;213:376–383. doi:10.1016/j.jep.2017.10.030
17. Zhao Z, Zhang D, Wu F, et al. Sophoridine suppresses lenvatinib-resistant hepatocellular carcinoma growth by inhibiting RAS/MEK/ERK axis via decreasing VEGFR2 expression. J Cell Mol Med. 2021;25(01):549-560. doi:10.1111/jcmm.16108
18. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
19. Salati M, Orsi G, Smyth E, et al. Gastric cancer: translating novels concepts into clinical practice. Cancer Treat Rev. 2019;79:101889. doi:10.1016/j.ctrv.2019.101889
20. Chen X, Hua X, Kong X, Wang XW. Sophoridine inhibits the proliferation of human gastric cancer MKN45 cells and promotes apoptosis. Sheng LI Xue Bao Acta Physiologica Sinica. 2018;70(4):391–396.
21. Peng Z, Guan Q, Luo J, et al. Sophoridine exerts tumor-suppressive activities via promoting ESRRG-mediated β-catenin degradation in gastric cancer. BMC Cancer. 2020;20(1):582. doi:10.1186/s12885-020-07067-x
22. Wang R, Liu H, Shao Y, et al. Sophoridine Inhibits Human Colorectal Cancer Progression via Targeting MAPKAPK2. Mol Cancer Res. 2019;17(12):2469–2479. doi:10.1158/1541-7786.MCR-19-0553
23. Ren L, Liu X, Jin S. Effects of sophoridine on proliferation and invasion of human pancreatic cancer cell line Capan-1. J Henan Univ Sci Tech. 2016;34(4):244–246.
24. Zhu L, Huang S, Li J, et al. Sophoridine inhibits lung cancer cell growth and enhances cisplatin sensitivity through activation of the p53 and Hippo signaling pathways. Gene. 2020;742:144556. doi:10.1016/j.gene.2020.144556
25. Zhao B, Hui X, Zeng H, et al. Sophoridine Inhibits the Tumour Growth of Non-Small Lung Cancer by Inducing Macrophages M1 Polarisation via MAPK-Mediated Inflammatory Pathway. Front Oncol. 2021;11:634851. doi:10.3389/fonc.2021.634851
26. Dong L, Peng H, Wang S, et al. Thermally and Magnetically Dual- Responsive Mesoporous Silica Nanospheres: preparation, Characterization, and Properties for the Controlled Release of Sophoridine. J Appl Polym Sci. 2014;1:131.
27. Wang W, Cai Y, Zhang G, et al. Sophoridine-loaded PLGA microspheres for lung targeting: preparation, in vitro, and in vivo evaluation. Drug Deliv. 2016;23(9):3674–3680. doi:10.1080/10717544.2016.1223210
28. Zhao F, Lam EW. Role of the forkhead transcription factor FOXO-FOXM1 axis in cancer and drug resistance. Front Med. 2012;6(4):376–380. doi:10.1007/s11684-012-0228-0
29. Chambers M, Kirkpatrick G, Evans M, Gorski G, Foster S, Borghaei RC. IL-4 inhibition of IL-1 induced Matrix metalloproteinase-3 (MMP-3) expression in human fibroblasts involves decreased AP-1 activation via negative crosstalk involving of Jun N-terminal kinase (JNK). Exp Cell Res. 2013;319(10):1398–1408. doi:10.1016/j.yexcr.2013.04.010
30. Yue Z, Si T, Pan Z, et al. Sophoridine suppresses cell growth in human medulloblastoma through FoxM1, NF-κB and AP-1. Oncol Lett. 2017;14(6):7941–7946. doi:10.3892/ol.2017.7224
31. Wang W, Sun Z, Chen H, Xu B, Wang F. Role and mechanism of Sophoridine on proliferation inhibition in human glioma U87MG cell line. Int J Clin Exp Med. 2015;8(1):464–471.
32. Li X, Hao W, Jiang J, et al. Synthesis, structure–activity relationship and biological evaluation of anticancer activity for novel N-substituted sophoridinic acid derivatives. Bioorg Med Chem Lett. 2011;21(18):5251–5254. doi:10.1016/j.bmcl.2011.07.038
33. Li D, Dai L, Zhang N, Tao Z. Synthesis, structure-activity relationship and biological evaluation of novel nitrogen mustard sophoridinic acid derivatives as potential anticancer agents. Bioorg Med Chem Lett. 2015;25(19):4092–4096. doi:10.1016/j.bmcl.2015.08.035
34. Bi C, Zhang C, Li Y, et al. Synthesis and biological evaluation of sophoridinol derivatives as a novel family of potential anticancer agents. ACS Med Chem Lett. 2014;5(11):1225–1229. doi:10.1021/ml500289h
35. Zhao W, Zhang C, Bi C, et al. Sophoridinol derivative 05D induces tumor cells apoptosis by topoisomerase1-mediated DNA breakage. Onco Targets Ther. 2016;9:2805–2817. doi:10.2147/OTT.S103671
36. Bi C, Zhang C, Li Y, et al. Novel N-substituted sophoridinol derivatives as anticancer agents. Eur J Med Chem. 2014;81:95–105. doi:10.1016/j.ejmech.2014.04.069
37. Bi C, Ye C, Li Y, Zhao W, Shao R, Song D. Synthesis and biological evaluation of 12-N-p-chlorobenzyl sophoridinol derivatives as a novel family of anticancer agents. Acta Pharm Sin B. 2016;6(3):222–228. doi:10.1016/j.apsb.2016.03.004
38. Zhao W, Xing Y, Ye C, et al. The novel quinolizidine derivate IMB-HDC inhibits STAT5a phosphorylation at 694 and 780 and promotes DNA breakage and cell apoptosis via blocking STAT5a nuclear translocation. Acta Pharmacol Sin. 2020;41(5):686–697. doi:10.1038/s41401-019-0333-6
39. Li D, Dai L, Zhao X, Zhi S, Shen H, Yang Z. Novel Sophoridine Derivatives Bearing Phosphoramide Mustard Moiety Exhibit Potent Antitumor Activities In Vitro and In Vivo. Molecules. 2018;23(8):1960. doi:10.3390/molecules23081960
40. Li Z, Luo M, Cai B, et al. Design, synthesis, biological evaluation and structure-activity relationship of sophoridine derivatives bearing pyrrole or indole scaffold as potential antitumor agents. Eur J Med Chem. 2018;157:665–682. doi:10.1016/j.ejmech.2018.08.021
41. Shu Z, Shahen WH, Guo Z, et al. Clarifying of the potential mechanism of Sinisan formula for treatment of chronic hepatitis by systems pharmacology method. Biomed Pharmacother. 2018;100:532–550. doi:10.1016/j.biopha.2018.02.047
42. Chen J, Shen H, Niu M, et al. Anti-hepatitis B virus effect of matrine-type alkaloid and involvement of p38 mitogen-activated protein kinase and tumor necrosis factor receptor-associated factor 6. Virus Res. 2016;215:104–113. doi:10.1016/j.virusres.2015.12.005
43. Liu X, Shen H, Chen J, Bai Z, Xiao X. Thymopolypeptides combined with matrine type alkaloids suppress HBV replication. China j Chin materia medica. 2016;41(7):1275–1281. doi:10.4268/cjcmm20160719
44. Zhang Y, Luo D, Yang L, et al. Matrine-Type Alkaloids from the Roots of Sophora flavescens and Their Antiviral Activities against the Hepatitis B Virus. J Nat Prod. 2018;81(10):2259–2265. doi:10.1021/acs.jnatprod.8b00576
45. Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9(11):1097–1105. doi:10.1016/S1474-4422(10)70209-X
46. Zhong J, Xia Y, Hua L, et al. Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif Cells Nanomed Biotechnol. 2019;47(1):3485–3491. doi:10.1080/21691401.2019.1640716
47. Yang-liujian Ou CW. Effect of sophoridine against EV71 virus in culture cell system. Gansu Sci Tech. 2016;32(23):130–134.
48. Ren G, Ding G, Zhang H, et al. Antiviral activity of sophoridine against enterovirus 71 in vitro. J Ethnopharmacol. 2019;236:124–128. doi:10.1016/j.jep.2019.02.045
49. Huang Y, Li Y, Wei B, Wu W, Gao X. CD80 Regulates Th17 Cell Differentiation in Coxsackie Virus B3-Induced Acute Myocarditis. Inflammation. 2018;41(1):232–239. doi:10.1007/s10753-017-0681-7
50. Dai K, Wang Y, Tai S, et al. Fasudil exerts a cardio-protective effect on mice with coxsackievirus B3-induced acute viral myocarditis. Cardiovasc Ther. 2018;36(6):e12477. doi:10.1111/1755-5922.12477
51. Ge M, Wang H, Zhang G, Yu S, Li Y. The antiviral effect of jiadifenoic acids C against coxsackievirus B3. Acta Pharmaceutica Sinica B. 2014;4(4):277–283. doi:10.1016/j.apsb.2014.06.008
52. Zhang Y, Zhu H, Ye G, et al. Antiviral effects of sophoridine against coxsackievirus B3 and its pharmacokinetics in rats. Life Sci. 2006;78(17):1998–2005. doi:10.1016/j.lfs.2005.09.034
53. Chen L, Wang F, Han Z, Hu Y. Effects of matrine on inflammatory cytokines for rats with cerebral ischemia reperfusion injury. J Em Traditional Chin Med. 2010;19(12):2098–2099.
54. Zhao P, Zhou R, Zhu X, et al. Matrine attenuates focal cerebral ischemic injury by improving antioxidant activity and inhibiting apoptosis in mice. Int J Mol Med. 2015;36(3):633–644. doi:10.3892/ijmm.2015.2260
55. Chen J, Huang C, Ye L, Yao B, Yang M, Cai Q. Effect of matrine on JAK2/STAT3 signaling pathway and brain protection in rats with cerebral ischemia-reperfusion. Adv Clin Exp Med. 2020;29(8):959–966. doi:10.17219/acem/123352
56. Liu Z, He D, Zhang X, et al. Neuroprotective effect of early and short-time applying sophoridine in pMCAO rat brain: down-regulated TRAF6 and up-regulated p-ERK1/2 expression, ameliorated brain infaction and edema. Brain Res Bull. 2012;88(4):379–384. doi:10.1016/j.brainresbull.2012.04.003
57. Miao J, Zhu C, Wang L, Zhang X. The underlying regulation mechanisms of TLR4/ NF-κB pathway activity in ischemia-reperfusion injury. J Brain Nervous Dis. 2013;21(02):127–130.
58. Qi L, Zhang J, Zhang Z. Determination of four alkaloids in compound Kushen Injection by high performance liquid chromatography with ionic liquid as mobile phase additive. Chin j Chromatography. 2013;31(03):249–253. doi:10.3724/SP.J.1123.2012.10039
59. Yan J, Yang Y, Wang Y, Jing K. Study on effect of sophoridine against bone cancer pain and its mechanism. China J Chin Materia Medica. 2013;38(23):4134–4137.
60. Gomes FIF, Cunha FQ, Cunha TM. Peripheral nitric oxide signaling directly blocks inflammatory pain. Biochem Pharmacol. 2020;176:113862. doi:10.1016/j.bcp.2020.113862
61. Bahardokht T, Ladan D, Ameneh R. Blockade of NMDA Receptors and Nitric Oxide Synthesis Potentiated Morphine-Induced Anti-Allodynia via Attenuating Pain-Related Amygdala pCREB/CREB Signaling Pathway. J Pain. 2019;20(8):885–897. doi:10.1016/j.jpain.2019.01.329
62. Yan J, Wang Y, Yang Y, Wang Y. Effects of sophoridine on expression of NR2B mRNA and nNOS mRNA in spinal cord of rats with bone cancer pain. Chin Traditional Patent Med. 2014;36(6):1142–1146.
63. Qian L, Dai W, Zho G, Wang L, Wang H. Study on analgesic and anti-inflammatory effects of main alkaloids from Sophora flavescens and Sophora alopecuroides. Chin Traditional Patent Med. 2012;34(8):1593–1596.
64. Ziaeian B, Fonarow G. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13(6):368–378. doi:10.1038/nrcardio.2016.25
65. Moorthy NSHN, Ramos MJ, Fernandes PA. Human ether-a-go-go-related gene channel blockers and its structural analysis for drug design. Curr Drug Targets. 2013;14(1):102–113. doi:10.2174/138945013804806460
66. Chen X, Yi C, Yang X, Wang X. Liquid chromatography of active principles in Sophora flavescens root. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812(1–2):149–163.
67. Middlekauff HR, Vigna C, Verity MA, et al. Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: a shared mechanism. J Card Fail. 2012;18(9):724–733. doi:10.1016/j.cardfail.2012.07.005
68. Hu S, Shen Y, Gong J, Yang Y. Effect of Sophoridine on Ca2+ Induced Ca2+ Release During Heart Failure. Physiol Res. 2016;65(1):43–52. doi:10.33549/physiolres.933052
69. Hu S, Gong J, Zhou Y, Liu H. Myocardial protective effect of sophoridine on chronic heart failure in rats. Ningxia Med J. 2014;36(9):769–771.
70. Neri M, Riezzo I, Pascale N, Pomara C, Turillazzi E. Ischemia/Reperfusion Injury following Acute Myocardial Infarction: a Critical Issue for Clinicians and Forensic Pathologists. Mediators Inflamm. 2017;2017:7018393. doi:10.1155/2017/7018393
71. Choi KH, Lee JM, Kim HK, et al. Fractional Flow Reserve and Instantaneous Wave-Free Ratio for Nonculprit Stenosis in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv. 2018;11(18):1848–1858. doi:10.1016/j.jcin.2018.06.045
72. Ding J, Zhao W, Peng T, Nie L, Zhou Y, Zhou X. Effects of Sophoridine on the Cardiac Function of Rats with AMI. Lishizhen Med Materia Med Res. 2010;21(2):348–350.
73. Ding J, Zhao W, Nie L, Peng T, Zhou Y, Zhou X. Effect of Sophoridine on the MIS in Rats with AMI. J Ningxia Med Univ. 2009;31(01):22–23.
74. Zhang C, Zhou Y, Gu S, et al. In silico prediction of hERG potassium channel blockage by chemical category approaches. Toxicol Res (Camb). 2016;5(2):570–582. doi:10.1039/C5TX00294J
75. Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440(7083):463–469. doi:10.1038/nature04710
76. Wang S, Li Y, Xu L, Li D, Hou T. Recent developments in computational prediction of HERG blockage. Curr Top Med Chem. 2013;13(11):1317–1326. doi:10.2174/15680266113139990036
77. Zhao X, Gu D, Qi Z, et al. Comparative effects of sophocarpine and sophoridine on hERG K+ channel. Eur J Pharmacol. 2009;607(1–3):15–22. doi:10.1016/j.ejphar.2009.02.013
78. Zhang H, Li H. Anti-arrhythmic effects of sophoridine and oxysophoridine. Acta Pharmacol Sin. 1999;20(06):517–520.
79. Miao S, Lv C, Liu Y, et al. Pharmacologic Blockade of 15-PGDH Protects Against Acute Renal Injury Induced by LPS in Mice. Front Physiol. 2020;11:138. doi:10.3389/fphys.2020.00138
80. Huang X, Li B, Shen L. Studies on the anti-inflammatory effect and its mechanisms of sophoridine. J Anal Methods Chem. 2014;2014:502626. doi:10.1155/2014/502626
81. Pola R, Flex A, Gaetani E, et al. Intercellular adhesion molecule-1 K469E gene polymorphism and Alzheimer’s disease. Neurobiol Aging. 2003;24(2):385–387. doi:10.1016/S0197-4580(02)00087-8
82. Gottrand G, Courau T, Thomas-Vaslin V, et al. Regulatory T-cell development and function are impaired in mice lacking membrane expression of full length intercellular adhesion molecule-1. Immunology. 2015;146(4):657–670. doi:10.1111/imm.12533
83. Zhao W, Song L, Deng H. Effect of sophoridine on dextran sulfate sodium-induced colitis in C57BL/6 mice. J Asian Nat Prod Res. 2010;12(11):925–933. doi:10.1080/10286020.2010.505188
84. Gao X, Zhou Y, Huang L, Zhao J, Wang N. Effect of sophoridine on endotoxin-induced acute liver injury in mice and its intervention action of the p-ERK and TNF-α expression. Ningxia Med J. 2010;32(5):
85. Zhang W, Li Y, Zhang G, Zhou Y. Effects of sophoridine on lipopolysaccharide-induced expressions of CD14, iNOS and P38 in RAW264.7 cells. Immunol J. 2015;31(02):100–104.
86. Liu J, Li B, Zhou Y. Effect of two Treatments of Sophoridine on TLR4-JNK Signaling Pathway of RAW264.7 Cells Induced by LPS. J Chin Med Materials. 2016;39(08):1854–1859.
87. Zhou M, Fang H, Du M, et al. The Modulation of Regulatory T Cells via HMGB1/PTEN/β-Catenin Axis in LPS Induced Acute Lung Injury. Front Immunol. 2019;10:1612. doi:10.3389/fimmu.2019.01612
88. Liang J, Han H, Zhou Y. Effects of Sophoridine on p38 MAPK/AP-1 Signaling Pathways in Mouse with Endotoxemia. J Ningxia Med Coll. 2012;34(12):1239–1242.
89. Zhu J, Wan H, Xiong Q, Ye H, Yang C. Antioxidant Effect of Sophoridine on Lung Injury Induced by Lipopolysaccharide in Mice and Its Influence on NF-κB Expression. Chin J Exp Traditional Med Formulae. 2011;17(20):182–186.
90. Yang C, Yang X, Tian Z, Cai C, Liu J, Hong-jiao W. Effect of serum IL-6, IL-10, NO, SOD and MDA of sophoridine on acute lung injury mice induced by lipopolysaccharide. Pharmacol Clin Chin Materia Medica. 2012;28(03):31–33.
91. Li J, Li X, Liu D, et al. eIF2α signaling regulates autophagy of osteoblasts and the development of osteoclasts in OVX mice. Cell Death Dis. 2019;10(12):921. doi:10.1038/s41419-019-2159-z
92. Lorenzo J. The many ways of osteoclast activation. Joseph Lorenzo. 2017;127(7):2530–2532.
93. Zhao X, Mei L, Pei J, et al. Sophoridine from Sophora Flower Attenuates Ovariectomy Induced Osteoporosis through the RANKL-ERK-NFAT Pathway. J Agric Food Chem. 2017;65(44):9647–9654. doi:10.1021/acs.jafc.7b03666
94. Wang Y, Li Y, Cui Y, Yuan H. The effect of sophoridine, a alkaloid of sophoridine, on the spermatogenesis in vitro and Lactobacillus of normal human vagina. J Henan Med Univ. 1995;29–31.
95. Xia L, Zhao B, Ju Y, Dai L. Inhibitory effect of bis (piperidine) alkaloids on five environmental bacterial strains. J Nanjing Forestry Univ. 2001;81:81–84.
96. Quan X, Liang F, Ma R, et al. Antibacterial effect of sophoridine and oxysophoridine on common urogenital tract infection bacteria. J Ningxia Med Univ. 2016;38(04):463–465.
97. Fang Z, Zhang J, Li P, et al. Toward a new age of cellular pharmacokinetics in drug discovery. Drug Metab Rev. 2011;43(3):335–345. doi:10.3109/03602532.2011.560607
98. Hu Y, Huang S. Physiological pharmacokinetic modeling of sophoridine. Chine J Pharmacol Toxicol. 1995;1:133–136.
99. Wang L, Wang S, Zeng J, et al. Pharmacokinetics of sophoridine in mice. Central South Pharm. 2014;12(05):442–444.
100. Wu Y, Chen J, Cheng Y. A sensitive and specific HPLC-MS method for the determination of sophoridine, sophocarpine and matrine in rabbit plasma. Anal Bioanal Chem. 2005;382(7):1595–1600. doi:10.1007/s00216-005-3316-z
101. Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent Progress in Bioconjugation Strategies for Liposome-Mediated Drug Delivery. Molecules. 2020;25:23. doi:10.3390/molecules25235672
102. Zhang M, Shen Y. Research progresses on clinical pharmacological action of anti-virus of matrine-type alkaloids. Anti Infect Pharm. 2018;15(02):185–191.
103. Hu P, Zheng Q, Chen H, Wu Z, Yue P, Yang M. Pharmacokinetics and distribution of sophoridine nanoliposomes in rats. Chine J New Drugs. 2012;21(22):2662–2666.
104. Dang J, Shao F, Ma J, Yang Y, Sun W. Fluorescence spectroscopy for interaction of bovine serum albumin and rutin. J Gansu Normal Coll. 2016;21(09):5–7.
105. Wang F, Pei M, Tang G, Zheng X. Applications of fluorescence technology on the spectroscopy in interactions between drug and serum albumin. J Dalian Univ. 2009;30(3):
106. Zhang G, Zhao N, Pan J, Chen X. Studies on the Interaction of Sophoridine with Bovine Serum Albumin. Nat Product Res Dev. 2010;22(01):16–19.
107. Wu Y, Chen J, Cheng Y. Pharmacokinetics of sophoridine, sophocarpine and matrine in rabbit by high performance liquid chromatography/mass spectrometry. Chin J Analytical Chem. 2005;33(11):1627–1630.
108. Shi F, Feng P, Su Y, et al. Acute and Subacute Toxicity of Sophoridine. Progress Veterinary Med. 2020;41(05):44–50.
109. Yu J, Jiang Y. Effects of sophoridine on central nervous system in mice. Chin Traditional Herbal Drugs. 2006;3(11):1688–1691.
110. Liang L, Zhang X, Wang X, Deng H. Acute toxicity of sophoridine by intraperitoneal injection. Lishizhen Med Materia Med Res. 2011;22(05):1252–1253.
111. Zhu Y, Zhu H, Teng S, Huang X. Study on toxicity and teratogenicity of sophoridine in rat embryos. Carcinogenesis Teratogenesis Mutagenesis. 1995;152–155.
112. Li X, Zhuang W, Jiang L, Pan D, Yu Y, Gong Z. Observation of pathological morphology on nervous system of rat with maximum dose sophoridine in chronic toxic test. Chin J Cancer. 2004;1(S1):1376–1378.
113. Chen X, Bai J, Zhang L. Effects of sophoridine on the ERK signal transduction pathway of hippocampal CA1 in rats. J Sichuan Traditional Chin Med. 2010;28(10):20–23.
114. Chen X, Zhang L, Duan H, Bai J. Observation of ultrastructure and morphological deformation of hippocampus in sophoridine induced epileptic rat. Lishizhen Med Materia Med Res. 2010;21(11):2736–2738.
115. Zhang L, Li L, Bai J. Compare the function epilepsy induced by large dose abdomen administration of sophoridine and penicillin. Ningxia Med J. 2012;34(11):
116. Chen X, Zhang Z, Yang X, et al. Study of the change of serum cytokines and hippocapal electroencephalograph in waking state of rats with epilepsy induced by sophoridine. Shanxi J Traditional Chine Med. 2007;2(3):57–59.
117. Seo M, Shin HK, Myung Y, Hwang S, No KT. Development of Natural Compound Molecular Fingerprint (NC-MFP) with the Dictionary of Natural Products (DNP) for natural product-based drug development. J Cheminform. 2020;12(1):1582–1614. doi:10.1186/s13321-020-0410-3
118. Li R.Natural Product-Based Drug Discovery. Med Res Rev. 2016;36(1):3. doi:10.1002/med.21380
119. Shen B, New Golden A. Age of Natural Products Drug Discovery. Cell. 2015;163(6):1297–1300. doi:10.1016/j.cell.2015.11.031
120. Chen X, Xu C, Hong S, et al. Immune Cell Types and Secreted Factors Contributing to Inflammation-to-Cancer Transition and Immune Therapy Response. Cell Rep. 2019;26(7):1965–1977. doi:10.1016/j.celrep.2019.01.080
121. Piazuelo MB, Riechelmann RP, Wilson KT, Algood HMS. Resolution of Gastric Cancer-Promoting Inflammation: a Novel Strategy for Anti-cancer Therapy. Curr Top Microbiol Immunol. 2019;421:319–359. doi:10.1007/978-3-030-15138-6_13
122. Si-ze Li YY, Xiao-lan Bian LY, Min Li YL, Jing Yuan HL, Lucy Liu BH, Xiang X. Prediction of oral hepatotoxic dose of natural products derived from traditional Chinese medicines based on SVM classifier and PBPK modeling. Arch Toxicol. 2021;95(5):1683–1701. doi:10.1007/s00204-021-03023-1
123. Wiebke Albrecht FK, Tim Brecklinghaus RS, Rosemarie Marchan MZ, et al. Prediction of human drug-induced liver injury (Dili) in relation to oral doses and blood concentrations. Arch Toxicol. 2019;93(6):1609–1637. doi:10.1007/s00204-019-02492-9
124. Han Y, Xiang Y, Shi Y, et al. Pharmacokinetics and Pharmacological Activities of Berberine in Diabetes Mellitus Treatment. Evid Based Complement Alternat Med. 2021;2021:9987097. doi:10.1155/2021/9987097
125. Kheir MM, Wang Y, Hua L, et al. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem Toxicol. 2010;48(4):1105–1110. doi:10.1016/j.fct.2010.01.033
126. Gao H, Ji H, Deng N, et al. Determination of the concentration of sophoridine in rat plasma by HPLC. Northwest Pharmaceutical Journal. 2016;31(02):183-185.
127. Liu X, Huang W, Wang H et al. Study on the concentration and pharmacokinetics of sophoridine from Sophora alopecuroides in rats. J Chin Med Materials. 2011;34(04):592-594.
128. Feng C, She C, Liu D, et al. Pharmacokinetic properties of sophocarpine and sophoridine. Chinese Journal of New Drugs. 2009;18(08):759-762
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
