Back to Journals » Clinical Interventions in Aging » Volume 19
Risk Factor Analysis and Nomogram for Early Progression of COVID-19 Pneumonia in Older Adult Patients in the Omicron Era
Authors Qi D, Chen Y, Peng C, Wang Y, Liang Z, Guo J, Gu Y
Received 3 December 2023
Accepted for publication 5 March 2024
Published 11 March 2024 Volume 2024:19 Pages 439—449
DOI https://doi.org/10.2147/CIA.S453057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Maddalena Illario
Daoda Qi,1,* Yang Chen,1,* Chengyi Peng,1 Yuan Wang,2 Zihao Liang,2 Jingjing Guo,1 Yan Gu1
1Department of Geriatrics, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 2Clinical Research Center, The Second Hospital of Nanjing, Affiliated to Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yan Gu; Jingjing Guo, Department of Geriatrics, The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China, Tel +86 25 83626297, Email [email protected]; [email protected]
Background and Objective: Timely recognition of risk factors for early progression in older adult patients with COVID-19 is of great significance to the following clinical management. This study aims to analyze the risk factors and create a nomogram for early progression in older adult patients with COVID-19 in the Omicron era.
Methods: A total of 272 older adults infected with COVID-19 admitted from December 2022 to February 2023 were retrospectively recruited. Risk factor selection was determined using the logistic and the least absolute shrinkage and selection operator (LASSO) regression. A nomogram was then created to predict early progression, followed by the internal validation and assessment of its performance through plotting the receiver operating characteristic (ROC), calibration, and decision curves.
Results: A total of 83 (30.5%) older adult patients presented an early progression on chest CT after 3– 5 days of admission under standard initiate therapy. Six independent predictive factors were incorporated into the nomogram to predict the early progression, including CRP > 10 mg/L, IL-6 > 6.6 pg/mL, LDH > 245 U/L, CD4+ T-lymphocyte count < 400/μL, the Activities of Daily Living (ADL) score ≤ 40 points, and the Mini Nutritional Assessment Scale-Short Form (MNA-SF) score ≤ 7 points. The area under the curve (AUC) of the nomogram in discriminating older adult patients who had risk factors in the training and validation cohort was 0.857 (95% CI 0.798, 0.916) and 0.774 (95% CI 0.667, 0.881), respectively. The calibration and decision curves demonstrated a high agreement in the predicted and observed risks, and the acceptable net benefit in predicting the early progression, respectively.
Conclusion: We created a nomogram incorporating highly available laboratory data and the Comprehensive Geriatric Assessment (CGA) findings that effectively predict early-stage progression in older adult patients with COVID-19 in the Omicron era.
Keywords: early progression, CGA, nomogram, COVID-19, omicron era
Introduction
Adults infected with COVID-19 typically exhibit clinical symptoms persisting for days or weeks, with an average duration of 6.87 days in vaccinated individuals.1 However, older individuals often endure a considerably extended COVID-19 course due to their compromised physical condition and underlying health conditions.2 During the pandemic caused by Omicron variant, even though the mortality rate of the whole population is low, the mortality in the elderly is still high,2 especially in the early days of the epidemic prevention policies changed.
The Comprehensive Geriatric Assessment (CGA) represents a multidimensional tool employed for evaluating overall health status, physical functionality, activities of daily living, nutritional well-being, frailty, pain assessment, prevalent geriatric conditions, cognitive function assessment, mental health evaluation, and analyzing the social environment. Following the assessment, tailored intervention strategies are developed and implemented to safeguard the health and functional well-being of older adults, ultimately enhancing their overall functional capacity and quality of life.3
To date, various predictive models have been developed to anticipate COVID-19 risk factors, with increasing interest in the utility of nomograms in clinical practice. Nomograms are notable for their visual approach in computing predictive model probabilities, highlighting the significance of selected variables, and their straightforward application. Previous studies have created nomograms to predict in-hospital mortality,4 failure of non-invasive respiratory strategies,5 risk of the Intensive Care Unit (ICU) admission in COVID-19 patients,6 etc. However, there are currently little data addressing the risk factors of early-stage progression. Moreover, there was no study combining the risk factors with CGA status in older adult patients. Hence, the objective of this study is to develop a nomogram approach that integrates CGA and validation clinical indicators for predicting risk factors associated with early imaging progression among older adult individuals infected with the Omicron variant of COVID-19.
Methods
Subjects
A total of 272 adults over the age of 65 years, who were hospitalized at our center with confirmed COVID-19 infections during the period from December 2022 to February 2023, were enrolled in this retrospective study (Figure 1).
 |
Figure 1 Flow chart of study design. |
Inclusion Criteria and Exclusion Criteria
Inclusion criteria: (1) adults over the age of 65 years; (2) COVID-19 diagnosis in accordance with the 10th edition of China’s Guidelines for the Diagnosis and Treatment of COVID-19 Infection; (3) underwent chest computed tomography (CT) examinations on admission and 3–5 days after treatment; (4) complete clinical data were available; (5) cooperated with the experiments.
Exclusion criteria: (1) history of pulmonary nodules, lung cancer, pulmonary tuberculosis, or other severe pulmonary diseases; (2) mental illnesses. Informed consent was obtained from all subjects.
Data Collection
Clinical data of eligible subjects were collected as follows: (1) baseline characteristics, including the gender, age, COVID-19 vaccination, underlying diseases and malignancies; (2) complete blood counts, including the white blood cells (WBC), neutrophils (NEU), lymphocytes (LYM), and neutrophil-to-lymphocyte ratio (NLR); (3) inflammatory factors, including the C-reactive protein (CRP), procalcitonin (PCT), and interleukin 6 (IL-6); (4) liver and kidney function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (γ-GT), albumin (ALB), globulin (GLB), and serum creatinine (Scr); (5) disease severity indicators, including lactate dehydrogenase (LDH); (6) immune-related indicators (lymphocyte subset counting), including CD4+ T-lymphocyte count (CD4+ T-LYM), CD8+ T-lymphocyte count (CD8+ T-LYM), CD4+ T-lymphocyte count/CD8+ T-lymphocyte count (CD4+/ CD8+), B-lymphocyte count (B-LYM), and natural killer cell count (NKC); (7) chest CT imaging data.
Comprehensive Geriatric Assessment
CGA was conducted for all enrolled subjects, encompassing the evaluation of nutritional status, the Activities of Daily Living (ADL), and frailty.
Briefly, nutritional status was assessed via grading the Prognostic Nutritional Index (PNI) and the Mini Nutritional Assessment Scale-Short Form (MNA-SF) score. PNI was calculated based on the individualized ALB level and LYM count as previously reported.7 MNA-SF consisted of six sections: appetite or eating problems, recent weight loss, mobility impairment, acute illness/stress, dementia or depression, and body mass index (BMI). An MNA-SF score ranging from 0 to 7 points indicated malnutrition.
ADL assessment comprised the evaluation of ten fundamental functional tasks, including feeding, bathing, grooming, dressing, bowel and bladder control, toilet use, bed-to-chair-and-back transfers, mobility on level surfaces, and navigating stairs. ADL scores were categorized as independent (80–100 points), minimally dependent (60–79 points), partially dependent (40–59 points), very dependent (20–39 points), and totally dependent (<20 points).8
Frailty assessment utilized the FRAIL scale, which considered dimensions such as fatigue, resistance, ambulation, illness, and weight loss. Subjects with scores of 3–5 points were categorized as frail, those with 1–2 points as pre-frail, and those with 0 points as healthy.9
Initiate Therapeutic Regimen
Patients received standard treatments as deemed necessary in accordance with the guidelines outlined in the 10th edition of China’s Guidelines for the Diagnosis and Treatment of COVID-19 Infection. These treatment modalities encompassed a comprehensive approach, including general management, oxygen therapy (respiratory support therapy if SPO2≤93%), antiviral therapy (Paxlovid or Molnupiravir), anti-inflammatory factor therapy (Baricitinib or Tocilizumab), antibiotics, symptomatic measures, and anticoagulation therapy.
Definition of Early-Stage Imaging Progression on Chest CT
All recruited patients underwent chest CT examinations on admission and 3–5 days after treatment. Early-stage imaging progression on chest CT was characterized by the presence of ground glass opacity (GGO), an increased number and/or enlarged extent of consolidation shadows, and the absorption of one pulmonary lesion accompanied by the emergence of a new lesion or the progressive merging of existing lesions (Supplemental Figure 1A). Conversely, cases where GGO decreased, the number of consolidation shadows diminished, and the scope of consolidation either reduced or remained similar following treatment were classified as progression-free on chest CT (Supplemental Figure 1B).
Statistical Analysis
Statistical analyses were carried out utilizing SPSS 23.0 and the R package. Measurement data were expressed as mean ± standard deviation (X ± SD) and compared by the Student’s t-test between groups. Enumeration data were expressed as the percentage and compared by the chi-square test. Risk factors for early-stage progression were identified by the univariable and backward stepwise multivariable logistic regression analysis. The odds ratio (OR) and corresponding 95% confidence interval (CI) were calculated. Using the least absolute shrinkage and selection operator (LASSO) regression, selected factors were then incorporated to create a nomogram. The 1000 bootstrap resamples were used to conduct the internal validation of the nomogram. The discriminating ability of the nomogram was assessed by plotting the receiver operating characteristic (ROC) curves and calculating the area under the curve (AUC) in the training and validation cohort (a random allocation in a ratio of 7:3). Calibration and decision curve analysis (DCA) were performed to assess the goodness-of-fit and the net benefit of the nomogram. All reported P-values were two sided, and confidence intervals referred to 95% boundaries, and a P-value ≤0.05 was considered statistically significant.
Results
Clinical Data of Older Adult Patients
A total of 272 eligible older adult patients infected with COVID-19 were recruited in the study and subsequently categorized into two groups: those with early progression (n = 83) or progression-free (n = 189) after initiating therapy. Table 1 presented a comprehensive overview of the clinical characteristics observed. Significantly older age and a higher proportion of subjects with malignancies were detected in the progression group than those in the progression-free group (both P<0.05). In terms of hematological parameters, the progression group displayed significantly lower WBC, NEU, and LYM counts compared to the progression-free group (all P<0.05). Furthermore, inflammatory markers, including CRP, PCT, and IL-6, were notably elevated in the progression group as compared to the progression-free group (all P<0.05). Additionally, patients in the progression group exhibited significantly higher levels of AST, LDH, and Scr compared to those in the progression-free group, suggesting impaired liver and kidney function in the former (all P<0.05). T-cell subset counting data revealed significantly lower CD4+ T-LYM, CD8+ T-LYM, and B-LYM in patients of the progression group than those of the progression-free group, suggesting hypoimmunity within them (all P<0.05).
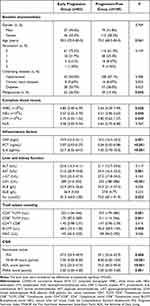 |
Table 1 Clinical Data of 272 Older Adult Patients Infected with Omicron Sub-Variants of COVID-19 with Early Progression or Progression-Free on Chest CT |
CGA Status of Older Adult Patients
CGA showed significantly lower PNI, MNA-SF, and ADL scores and a higher FRAIL score in patients of the progression group than those in the progression-free group, suggesting a less favorable nutritional status, decreased quality of life, and increased frailty among patients in the progression group (all P<0.05).
Risk Factors for the Early Progression
To streamline the predictive model and enhance its reproducibility and applicability across diverse healthcare settings, the values of the 18 factors above with statistically significant differences were converted into binary variables according to the clinical reference limits. To create a simple nomogram involving highly available factors, 272 subjects were randomly allocated into the training cohort (n = 191) and validation cohort (n = 81) in a ratio of 7:3. Comparative analysis of clinical data between these two cohorts revealed no significant differences, except for the absolute value of neutrophils (Supplemental Table 1).
Logistic regression analysis of the training cohort was performed to identify potential factors for predicting the progression. Univariable logistic regression highlighted that a history of malignancies, LYM < 0.8×109/L, CRP > 10 mg/L, PCT > 0.052 ng/mL, IL-6 > 6.6 pg/mL, LDH > 245 U/L, CD4+ T-LYM < 400/µL, B-LYM < 50/µL, PNI > 40, MNA-SF score ≤ 7 points, ADL score ≤ 40 points, and FRAIL score ≥3 points were significantly correlated with the early-progression. Subsequently, by employing backward stepwise multivariable logistic regression, CRP > 10 mg/L (OR, 4.037; 95% CI 1.818–9.427; P=0.001), IL-6 > 6.6 pg/mL (OR, 3.661; 95% CI 1.527–9.444; P=0.005), LDH > 245 U/L (OR, 2.811; 95% CI 1.264–6.484; P=0.013), CD4+ T-LYM < 400/µL (OR, 3.002; 95% CI 1.177–8.327; P=0.026), MNA-SF score ≤ 7 points (OR, 3.706; 95% CI 1.487–9.815; P=0.006), and ADL score ≤40 points (OR, 3.374; 95% CI 1.381–8.685; P=0.009) were identified as independent risk factors for the early progression in older adult patients (Table 2).
 |
Table 2 Univariable and Multivariable Logistic Regression to Identify Risk Factors for Older Adult Patients Infected with Omicron Sub-Variants of COVID-19 with Early Progression on Chest CT |
Factor Selection and Nomogram Creation
Factors incorporated into the nomogram were selected by LASSO regression. Through cross-validation, a set of non-zero coefficients was selected from 18 significantly different clinical data identified between the early progression group and the progression-free group. The log lambda (λ) associated with CRP, IL-6, LDH, CD4+ T-LYM, ADL score, and MNA-SF score were determined to be 0.41, 0.20, 0.29, 0.28, 0.60, and 0.45, respectively (Figure 2). Notably, factors selected via LASSO regression were consistent with risk factors identified by multivariable logistic regression, including the high-level inflammatory factors (CRP and IL-6) and the index of disease severity (LDH), the hypoimmunity indicator (CD4+ T-LYM), and CGA data (ADL and MNA-SF scores). We then created a nomogram to directly predict the early progression by calculating the total points of predicted values (Figure 3).
Performance Validation of the Nomogram
ROC curves were constructed to assess the diagnostic utility of the nomogram, with AUC as the performance metric. In the training cohort, the AUC for discriminating early progression was 0.857 (95% CI 0.798–0.916), while in the validation cohort, it stood at 0.774 (95% CI 0.667–0.881) (Figure 4A and B). After 1000 bootstrap resampling, the AUC of the nomogram in the two cohorts was 0.857 (95% CI 0.796–0.911) and 0.775 (95% CI 0.660–0.879), respectively (Figure 4C and D).
To further evaluate the model’s performance, calibration curves were generated to assess goodness of fit. The Brier scores for the training cohort and validation cohort were calculated as 0.128 and 0.207, respectively. Notably, both cohorts exhibited Brier scores below 0.25, indicative of the nomogram’s high predictive accuracy. In addition, the calibration curves of the nomogram and bootstrap resampling closely approximated the ideal curve, affirming a strong agreement between predicted probabilities and actual outcomes in both the training and validation cohorts (Figure 5). Finally, DCA was performed, and an acceptable net benefit was obtained at a threshold probability of 0.04 or above (Figure 6).
 |
Figure 6 Decision curve analysis for the nomogram. |
Discussion
In contrast to prior prediction models, our study introduced a novel nomogram that integrates readily accessible laboratory data and CGA outcomes. This nomogram effectively predicted the early imaging progression in elderly individuals infected with COVID-19 during the Omicron era.
According to the 10th edition of China’s Guidelines for the Diagnosis and Treatment of COVID-19 Infection, chest CT plays a pivotal role in clinical classification, early identification of severe/critical cases, and inpatient discharge criteria for COVID-19. Chest CT imaging, which is consistent with clinical findings, can reflect the severity of COVID-19 patients.10 Therefore, early detection of rapid chest CT imaging progression in older adult COVID-19 patients serves as an early warning sign for disease exacerbation, enabling timely interventions and appropriate treatments.
Aging contributes to the heightened severity and mortality observed in older adult COVID-19 patients.11 Cellular senescence and chronic inflammation are hallmark features of aging, while immune senescence is one of the important phenotypes of cell senescence.12 Emerging evidence suggested that changes in CD4+ T-LYM can drive chronic inflammation and accelerate the aging phenotype, rendering individuals more susceptible to infections and less responsive to vaccinations.13 On the other hand, considering COVID-19 as an acute inflammatory process, severely infected patients may experience the impact of inflammation storms, leading to inevitable trends such as fever, decreased appetite, and weight loss. In our study, immune indicators (CD4+ T-LYM) and inflammatory markers (CRP and IL-6) emerged as independent predictors of early progression among older adult COVID-19 patients. This association may be attributed to immune deficiencies and chronic inflammation, as described above.
Studies have consistently linked elevated levels of LDH with adverse outcomes in COVID-19 patients, with increased LDH levels showing a 6-fold increased chance of severe disease and a 16-fold increased mortality.14 LDH has been established as a reliable and specific biomarker for gauging disease progression in COVID-19 pneumonia,14 meanwhile, no individual LDH isoenzyme had better predictive utility than total LDH for discriminating COVID-19 severity.15 Similarly, elevated LDH levels emerged as an independent risk factor in our study.
Alongside the above laboratory indexes, we incorporated CGA into our nomogram to predict early-stage imaging progression in older adults infected with Omicron sub-variants of COVID-19 for the first time. CGA is a multidisciplinary approach to assess the physical health, functional status, mental health, and social adaptation of older adults, which offers vital information to formulate individualized protocols to enhance the health and ability of life. Previous studies have applied CGA during the COVID-19 pandemic in foreign countries,16,17 however, without combining with other clinical impact factors. To some extent, a lower CGA status exacerbates the impact of immunosenescence and chronic inflammation in older adult COVID-19 patients. In the present study, all recruited subjects were subjected to CGA, involving the assessment of nutritional status, ADL, and frailty. Significantly lower PNI, MNA-SF and ADL scores, and a significantly higher FRAIL score were detected in the early-stage imaging progression group, suggesting a poor nutritional status, decreased functional ability, and increased frailty.
Notably, our results highlight the substantial prognostic value of ADL and MNA-SF within CGA in predicting COVID-19 outcomes. Okoye et al assessed determinants of cause-specific mortality and loss of independence in daily life activities post-hospitalization due to COVID-19, establishing a correlation between specific mortality rates after discharge and at least one ADL functional impairment.18 Cangiano et al,19 supported by Covino et al,20 identified low ADL scores as a risk factor for COVID-19 mortality. On the other hand, malnutrition is prevalent among the elderly population, with research indicating malnutrition rates ranging from 35% to 65% among hospitalized older adult patients.21 Studies have shown that COVID-19 patients at nutritional risk, as assessed by NRS2002, MNA-SF, and NRI, experienced significantly worse clinical outcomes compared to their well-nourished counterparts.22 Similarly, an investigation of older adult COVID-19 patients admitted to hospitals in Kuala Lumpur demonstrated a correlation between poor nutritional status and various adverse outcomes.23 Additionally, an investigation revealed a significant association between ADL scores ≤40 points and malnutrition risk in COVID-19 patients.24 These findings align with our results and underscore the reliability of CGA in predicting recovery outcomes in elderly individuals affected by COVID-19.
Limitation
We acknowledge certain limitations, including the exclusion of severely ill COVID-19 patients and the relatively small sample size, which may impact the robustness of our nomogram.
Conclusion
In keeping with the evolving landscape of the COVID-19 pandemic, research focusing on Omicron variants is imperative. Our study introduces, for the first time, a nomogram that combines clinical and CGA data to predict early-stage imaging progression in older adult patients infected with Omicron sub-variants of COVID-19. This convenient prediction model, incorporating highly accessible factors, aligns with the specific challenges posed by the Omicron era and facilitates the early identification of risk factors, enabling swift intervention.
Ethical Approval
This study was approved by the Medical Ethics Committee of Nanjing Second Hospital (No. 2023-LS-ky-020) and conducted in accordance with the Declaration of Helsinki (2013). Informed consent was obtained from all subjects.
Funding
This study was supported by Nanjing Infectious Disease Clinical Medical Center; Innovation Center for Infectious Disease of Jiangsu Province (NO. CXZX202232), Nanjing, PR, China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wise J. Covid-19: symptomatic infection with omicron variant is milder and shorter than with delta, study reports. BMJ. 2022;377:0922. doi:10.1136/bmj.o922
2. Cheung PH, Chan CP, Jin DY. Lessons learned from the fifth wave of COVID-19 in Hong Kong in early 2022. Emerg Microbes Infect. 2022;11(1):1072–1078. doi:10.1080/22221751.2022.2060137
3. Hernandez Torres C, Hsu T. comprehensive geriatric assessment in the older adult with cancer: a review. Eur Urol Focus. 2017;3(4–5):330–339. doi:10.1016/j.euf.2017.10.010
4. Acar HC, Can G, Karaali R, et al. An easy-to-use nomogram for predicting in-hospital mortality risk in COVID-19: a retrospective cohort study in a university hospital. BMC Infect Dis. 2021;21(1):148. doi:10.1186/s12879-021-05845-x
5. Liu L, Xie J, Wu W, et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: a retrospective multicentre study. Lancet Digit Health. 2021;3(3):e166–e174. doi:10.1016/S2589-7500(20)30316-2
6. Zhou Y, He Y, Yang H, et al. Exploiting an early warning Nomogram for predicting the risk of ICU admission in patients with COVID-19: a multi-center study in China. Scand J Trauma Resusc Emerg Med. 2020;28(1):106. doi:10.1186/s13049-020-00795-w
7. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9
8. Hartigan I. A comparative review of the Katz ADL and the Barthel Index in assessing the activities of daily living of older people. Int J Older People Nurs. 2007;2(3):204–212. doi:10.1111/j.1748-3743.2007.00074.x
9. Pranata R, Henrina J, Lim MA, et al. Clinical frailty scale and mortality in COVID-19: a systematic review and dose-response meta-analysis. Arch Gerontol Geriatr. 2021;93:104324. doi:10.1016/j.archger.2020.104324
10. Yang Z, Shi J, He Z, et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging. 2020;12(7):6037–6048. doi:10.18632/aging.102999
11. Witkowski JM, Fulop T, Bryl E. Immunosenescence and COVID-19. Mech Ageing Dev. 2022;204:111672. doi:10.1016/j.mad.2022.111672
12. Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. 2023;186(2):243–278.
13. Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol. 2021;22(6):687–698. doi:10.1038/s41590-021-00927-z
14. Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi:10.1016/j.ajem.2020.05.073
15. Akay C, Orhan O, Sen V. Evaluation of clinical, biochemical, and demographic characteristics of paediatric COVID-19 patients admitted to Dicle University Hospital. Cureus. 2023;15(1):e34171. doi:10.7759/cureus.34171
16. Inzitari M, Arnal C, Ribera A, et al. Comprehensive geriatric hospital at home: adaptation to referral and case-mix changes during the COVID-19 pandemic. J Am Med Dir Assoc. 2023;24(1):3–9 e1. doi:10.1016/j.jamda.2022.11.003
17. Fernando C, Juan G, Louis F, Vg. J. Comprehensive geriatric assessment in a Mexican long-term care facility during a COVID-19 outbreak. Eur J Geriatr Gerontol. 2022;4(2):64–70. doi:10.4274/ejgg.galenos.2021.2021-11-3
18. Okoye C, Calsolaro V, Calabrese AM, et al. Determinants of cause-specific mortality and loss of independence in older patients following hospitalization for COVID-19: the gerocovid outcomes study. J Clin Med. 2022;11:19. doi:10.3390/jcm11195578
19. Cangiano B, Fatti LM, Danesi L, et al. Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging. 2020;12(24):24522–24534. doi:10.18632/aging.202307
20. Covino M, De Matteis G, Polla DAD, et al. Predictors of in-hospital mortality AND death RISK STRATIFICATION among COVID-19 PATIENTS aged >/= 80 YEARs OLD. Arch Gerontol Geriatr. 2021;95:104383. doi:10.1016/j.archger.2021.104383
21. Guyonnet S, Rolland Y. Screening for malnutrition in older people. Clin Geriatr Med. 2015;31(3):429–437. doi:10.1016/j.cger.2015.04.009
22. Liu G, Zhang S, Mao Z, Wang W, Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74(6):876–883. doi:10.1038/s41430-020-0659-7
23. Thiam CN, Mathavan S, Abdullah A, Chong EGM. Malnutrition among patients admitted to the subacute geriatric ward during the COVID-19 pandemic era: a cross-sectional study in a tertiary hospital in Malaysia. Med J Malaysia. 2022;77(3):313–319.
24. Liu A, Cong J, Wang Q, et al. Risk of malnutrition is common in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a cross-sectional study. J Nutr. 2021;151(6):1591–1596. doi:10.1093/jn/nxab009
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




