Back to Journals » Risk Management and Healthcare Policy » Volume 17
Risk Factors of Tuberculosis Destroyed Lung in Patients with Pulmonary Tuberculosis and Structural Lung Diseases: A Retrospective Observational Study
Authors Liu L, Wang X, Luo L, Liu X, Chen J
Received 8 November 2023
Accepted for publication 19 March 2024
Published 28 March 2024 Volume 2024:17 Pages 753—762
DOI https://doi.org/10.2147/RMHP.S448765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Haiyan Qu
Linlin Liu,1,* Xiufen Wang,2,3,* Li Luo,2,3 Xuhui Liu,2,3 Jingfang Chen1– 4
1Hengyang Medical School, School of Nursing, University of South China, Hengyang, People’s Republic of China; 2Department of the Third Pulmonary Disease, The Third People’s Hospital of Shenzhen, Shenzhen, People’s Republic of China; 3National Clinical Research Center for Infectious Diseases, Shenzhen, People’s Republic of China; 4Faculty of Medicine, Macau University of Science and Technology, Macau, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuhui Liu; Jingfang Chen, Email [email protected]; [email protected]
Background: Tuberculosis destroyed lung constitutes a significant worldwide public health challenge, little is known about its associated risk factors and prognosis. Our study aimed to identify the risk factors of tuberculosis destroyed lung among pulmonary tuberculosis and structural lung diseases.
Methods: Between January 2019 and December 2021, a case-control study was conducted at the Third People’s Hospital of Shenzhen in China. We collected the clinical data among patients with pulmonary tuberculosis and structural lung diseases. Cases were defined as patients with tuberculosis destroyed lung. Controls were not diagnosed with the tuberculosis destroyed lung. A binary logistic regression was performed.
Results: In our study, a total of 341 patients met the inclusion criteria, including 182 cases and 159 controls. We found that age ranges of 46– 60 years (aOR: 4.879; 95% CI: 2.338– 10.180), > 60 years (aOR: 3.384; 95% CI: 1.481– 7.735); history of TB treatment (aOR: 2.729; 95% CI: 1.606– 4.638); malnutrition (aOR: 5.126; 95% CI: 1.359– 19.335); respiratory failure (aOR: 5.080; 95% CI: 1.491– 17.306); and bronchiarctia (aOR: 3.499; 95% CI: 1.330– 9.209) were the independent risk factors for tuberculosis destroyed lung. Conversely, having a normal (aOR: 0.207; 95% CI: 0.116– 0.371) or overweight BMI (aOR: 0.259; 95% CI: 0.090– 0.747) emerged as a protective factor against tuberculosis destroyed lung.
Conclusion: This study indicated that tuberculosis destroyed lung is a common condition among patients with pulmonary tuberculosis and structural lung diseases. The independent risk factors for tuberculosis destroyed lung were identified as being within the age groups of 46– 60 and over 60 years, having a previous history of TB treatment, malnutrition, respiratory failure, and bronchiarctia. It is essential to closely monitor patients possessing these risk factors to prevent the progression towards tuberculosis destroyed lung.
Keywords: tuberculosis, pulmonary, tuberculosis destroyed lung, structural lung diseases, risk factors
Introduction
Tuberculosis (TB) is a major global health challenge, with approximately 10 million new cases reported each year.1 From 1980 to 2019, among 363 million TB patients, yet only 172 million received treatment.2 In 2019, an estimated total of 122 million disability-adjusted life years (DALYs) was caused by TB, with 58 million DALYs were attributed to post-TB sequelae, accounting 47% of total burden.3 In low and middle income countries, TB constitutes a substantial risk factor for chronic respiratory diseases.4 In large population-based cross-sectional studies, abnormal airway physiology has been observed following anti-TB treatment.5–7 Even after achieving microbiological cure, there is a considerable burden for post tuberculosis lung disease (PTLD), marked by spirometry decline and reduced quality of life.8–10 The prevalence of PTLD varies widely, ranging from 18% to 87%, depending on the population under investigation and the pulmonary function tests (PFTs) utilized.11 Previous research has unveiled several risk factors associated with PTLD, such as extensive lung involvement, prior anti-TB treatment, prolonged treatment duration, delayed initiation of anti-TB therapy, drug-resistant tuberculosis, multiple tuberculosis infections, and the female.12,13 A retrospective cohort study conducted in South Korea from 2005 to 2011 involving 595 patients with tuberculosis destroyed lung (TDL) revealed that decreased lung function with exacerbation, and progressive decline of FEV1 were typical characteristics. TDL, representing the severe PTB and structural lung disease, is characterized by widespread structural damage to the lungs and a fundamental loss of respiratory function, with a high likelihood of developing extensive multidrug-resistant tuberculosis.14 Despite the global focus on TB, research specific to TDL, especially in high-incidence regions like China, remains underexposed.
Pulmonary tuberculosis (PTB) and structural lung disease frequently emerge as the common symptom among PTLD. Persistent damage by Mycobacterium tuberculosis on lung tissues leads to irreversible destruction of the normal lung structure. This destruction is characterized by extensive caseous necrosis, cavitation, fibrosis, bronchiectasis, or bronchiarctia, affecting either pulmonary lobes or entire lung unilaterally, and is collectively referred to as structural lung diseases.15 The deformation of airways, along with the breakdown of elastic and bronchial wall muscle fibers, results in the abnormal lung architecture. These changes lead to substantial variations in lung volume and respiratory function. Patients suffering from PTB and structural lung disease exhibit significant decline in pulmonary function, making pharmacological treatments less effective and increasing their risk of recurrent bacterial or fungal infections. The most severe symptom is seen in cases of cavernous PTB and TDL, significantly diminishing the patients’ quality of life. Conventional drug treatments frequently fall short due to the extensive lung damage, making patients with TDL more susceptible to repeated bacterial or fungal infections, thus acting as potential reservoirs for continuous infection transmission. Cavernous PTB is characterized by the presence of bilateral or unilateral fibrous cavity with thick walls and extensive fibrous tissue growth, indicating a high bacterial load, enhanced infectivity, increased risk of spread and drug resistance.16 The fibrosis around the cavity walls severely limits the ability of anti-tuberculosis drugs to reach the target areas through the bloodstream, complicating treatment efforts.17 Thereby escalating healthcare costs and hastening the progression of the disease.18 TDL is particularly common in regions with a high prevalence of TB.19,20 TDL is characterized by the presence of extensive parenchymal damage after a PTB infection with or without anti-TB treatment.21–23 TDL heightens the susceptibility to hemoptysis, secondary bacterial infections, and the reactivation of TB, all of which are strongly linked to recurrent exacerbation.24,25
Despite the efficacy of anti-TB therapy, patients may continue to experience chronic physical and psychosocial impairments. As a result of persistent chronic inflammation leading to destroyed lung, TDL patients suffer from a heightened incidence of pulmonary adhesion, frequently affecting neighboring lung lobes or resulting in multi-lobe damage. Studies indicated that surgical treatment of TDL is effective, with rates of postoperative complications ranging from 9.6% to 45.7%.26–28 Traditional TB-related research predominantly concentrates on postoperative complications, mortality, and treatment outcomes, with limited attention given to the long-term sequelae of TB.18 Therefore, the objective of this study is to identify the risk factors associated with TDL in patients suffering from PTB and structural lung diseases.
Methods
Study Design and Participants
Between January 1, 2019 and December 31, 2021, we conducted a case-control study in Shenzhen, located in Guangdong province. The Third People’s Hospital of Shenzhen is the largest chest hospital in Shenzhen, offering specialized services for the diagnosis and treatment of TB. During the same period, a random selection of PTB and structural lung diseases patients within the Third People’s Hospital of Shenzhen was conducted on a 1:1 basis. Those who did not fulfill the study’s inclusion criteria were subsequently excluded to establish the present participants. We consecutively enrolled 182 patients with TDL, and 159 patients without TDL were included to the control group, all of whom were registered in the hospital information system (HIS). Furthermore, we followed up the post-discharge survival status of the 341 patients. The research protocol was granted ethical approval by the Ethics Review Committee of the Third People’s Hospital of Shenzhen, China (Approval Number: [2022-197-02]). This study complied with the Declaration of Helsinki. Given the observational nature of the study, which did not directly involve the participation by the patients, the Ethics Committee of The Third People’s Hospital of Shenzhen exempted the requirement for obtaining informed consent from the participants.
The diagnostic standards for TDL in-accordance to “Tuberculosis Diagnostic Criteria WS288-2017”, Chest imaging indicating diminished lung tissue volume, secondary bronchiectasis, or the presence of multiple fibrotic cavities with thick walls and calcification. Additional indicators include chest wall collapse, the displacement of nearby hilar and mediastinal structures due to pulling forces, along with pleural thickening and adhesions. Structural lung diseases are a broad term that refer to the destruction of the normal architecture of the lungs, heightening an individual’s vulnerability to bacterial, fungal, viral, and other pathogen-related pulmonary infections. This category predominantly encompasses COPD, secondary bronchiectasis, interstitial lung disease, cystic pulmonary fibrosis, chronic lung abscess, pulmonary cavities, and other related pulmonary diseases. The identification of TDL or structural lung diseases within our study was established through a consensus reached by two pulmonologists, based on a comprehensive evaluation of image findings and clinical characteristics.
The inclusion criteria were as follows: (1) Individuals aged 18 years and older. (2) PTB cases adhered to the guidelines outlined in the Tuberculosis Classification Criteria WS196-2017 and Tuberculosis Diagnostic Criteria WS288-2017, as issued by the National Health Commission of the People’s Republic of China. (3) Structural lung diseases were confirmed through computed tomography and other imaging findings, including COPD, secondary bronchiectasis, interstitial lung disease, cystic pulmonary fibrosis, chronic lung abscess, pulmonary cavities, and other related disorders.29 (4) TDL were aligned with “Tuberculosis Diagnostic Criteria WS288-2017”, with chest imaging revealing indications of reduced lung tissue volume, secondary bronchiectasis, or the existence of multiple fibrotic cavities characterized by thick walls and calcification. The exclusion criteria included: (1) Pregnant or lactating women; (2) Patients diagnosed with malignant tumors, human immunodeficiency virus infection, or other wasting diseases. (3) Patients with incomplete clinical data.
Key Definitions
The definition of structural lung diseases: Mycobacterium tuberculosis continues to cause progressive damage to the pulmonary parenchyma and interstitium, which results in structural lung diseases, including COPD, secondary bronchiectasis, interstitial lung disease, cystic pulmonary fibrosis, chronic lung abscess, pulmonary cavities, and other related diseases.
The definition of TDL: TDL results from years of chronic progressive TB and inadequate treatment, and it leads to lymph node obstruction of the bronchi with a combination of distal collapse, necrosis and secondary infection.30 TDL presents a critical manifestation of structural lung diseases, characterized by profound destruction of the lung parenchyma and considerable impairments in lung function.
Data Collection
Patient data were collected from the HIS, encompassing demographics, clinical characteristics, routine laboratory data, and imaging findings. We identified the independent variables that corresponded to the predefined risk factors associated with TDL in previous research, as well as other potential factors not reported. The variables considered in this study encompassed age, gender, educational level, marital status, medical insurance, history of TB treatment, drug resistance, nutritional status, comorbidity, imaging findings, and laboratory data. A self-designed questionnaire was employed to gather medical records. And the data collection was undertaken by two researchers to ensure data quality and completeness (LLL and XFW). Throughout this process, patient identification numbers facilitated data entry while ensuring confidentiality. The study’s data were securely stored and accessible solely to authorized researchers for clinical analysis.
Statistical Analysis
In our study, the dependent variable was TDL, with socio-demographic and clinical characteristics of the 341 patients serving as explanatory variables. Quantitative data with normal distribution were presented as mean ± standard deviation (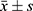 ), and non-normally distributed data were described by the median and interquartile range (IQR). Categorical variables were presented as frequencies and percentages (%). Variables with a normal distribution were compared by the Student’s t-test. Variables with a non-normal distribution were compared by the Mann–Whitney U-tests. Chi-square tests or Fisher’s exact tests were conducted to analyze differences in categorical data. Variables with a p value less than 0.20 in the univariate analysis were incorporated into a binary logistic regression (LR) analysis. The Forward LR was employed to filter independent variables, calculating the odds ratio (OR) and the confidence interval (CI) for each. To mitigate the effect of potential confounder, results from the binary LR analysis were presented as adjusted odds ratios (aORs). The results were statistically significant at p<0.05. All statistical analysis performed using SPSS software version 25.0 (IBM Corp, Armonk, NY, USA).
), and non-normally distributed data were described by the median and interquartile range (IQR). Categorical variables were presented as frequencies and percentages (%). Variables with a normal distribution were compared by the Student’s t-test. Variables with a non-normal distribution were compared by the Mann–Whitney U-tests. Chi-square tests or Fisher’s exact tests were conducted to analyze differences in categorical data. Variables with a p value less than 0.20 in the univariate analysis were incorporated into a binary logistic regression (LR) analysis. The Forward LR was employed to filter independent variables, calculating the odds ratio (OR) and the confidence interval (CI) for each. To mitigate the effect of potential confounder, results from the binary LR analysis were presented as adjusted odds ratios (aORs). The results were statistically significant at p<0.05. All statistical analysis performed using SPSS software version 25.0 (IBM Corp, Armonk, NY, USA).
Results
Patient Characteristics
Table 1 showed demographic and clinical characteristics of the study participants. Of these 341 patients, the median age was 46 years, 250 (73.31%) being male, 234 (68.62%) possessed a secondary education background. Furthermore, 236 (69.21%) have married, 228 (66.86%) benefited from medical insurance, and the median length of hospitalization was 11 days. Regarding clinical characteristics, 192 (56.30%) had previously undergone TB treatment, 69 (20.23%) were diagnosed with drug-resistant TB, and 80 (23.46%) had reported incidents of hemoptysis. The median body mass index (BMI) was recorded at 19.06, 166 (48.68%) patients were underweight and 184 (53.96%) were at risk of malnutrition, 34 (9.97%) were malnourished, 45 (13.20%) suffered from hypoproteinemia, and 52 (15.25%) were anemic. Additionally, 86 (25.22%) patients had diabetes mellitus, 36 (10.56%) experienced respiratory failure, and another 36 (10.56%) had electrolyte disturbances. Imaging findings revealed that 278 (81.52%) patients had cavity, 151 (44.3%) had bilateral lung involvement, and 140 (41.1%) were diagnosed with concurrent pulmonary infections.
 |
Table 1 Baseline Characteristics of the Study Population |
In terms of clinical outcomes, significant statistical differences were observed at the time of discharge (p=0.014) and six months after hospitalization (p<0.001). Regarding long-term survival, patients with TDL exhibited a higher mortality risk compared to those with PTB and structural lung disease.
Univariate Analysis of Associated Factors involved in TDL
Based on the defined criteria for TDL, 182 patients were diagnosed as TDL. It is important to note that there was a statistically significant age difference between the case group and the control group (p<0.001), with the median age of TDL patients being older. There were notable differences in prior TB treatment history (p<0.001), drug-resistant TB (p<0.001), and hemoptysis (p=0.015). Concerning nutritional status, significant differences were observed in the TDL group regarding BMI (p<0.001), nutritional risk (p<0.001), malnutrition (p<0.001), hypoproteinemia (p<0.001), and anemia (p=0.034). Regarding comorbidity, significant differences were observed between the two groups in terms of cardiac insufficiency (p=0.014), respiratory failure (p<0.001), and electrolyte imbalances (p=0.002). And the two groups exhibited significant statistical differences in imaging finding, including bilateral lung involvement (p=0.001), cavity (p<0.001), bronchiarctia (p=0.014), pneumothorax (p=0.012), atelectasis (p=0.025), and pulmonary infection (p=0.015). As for laboratory indicators, the TDL group demonstrated significantly elevated levels of CRP (p=0.005), ESR (p=0.020), NEUT (p<0.001), PLT (p=0.011), WBC (p<0.001), and PCT (p=0.002), along with significantly lower levels of LYMPH (p=0.050), RBC (p=0.001), Cr (p=0.003), and ALB (p<0.001).
Binary Logistic Regression Analysis of Risk Factors involved in TDL
As shown in Table 2, the binary logistic regression analysis presented that the age ranges of 46–60 years (aOR: 4.879; 95% CI: 2.338–10.180), >60 years (aOR: 3.384; 95% CI: 1.481–7.735); history of TB treatment (aOR: 2.729; 95% CI: 1.606–4.638); normal BMI (aOR: 0.207; 95% CI: 0.116–0.371), overweight BMI (aOR: 0.259; 95% CI: 0.090–0.747); malnutrition (aOR: 5.126; 95% CI: 1.359–19.335); respiratory failure (aOR: 5.080; 95% CI: 1.491–17.306); and bronchiarctia (aOR: 3.499; 95% CI: 1.330–9.209) were associated with TDL.
 |
Table 2 Risk Factors of Tuberculosis Destroyed Lung |
Figure 1 showed the forest map of risk factors among TDL patients. Notably, patients aged 46–60 and those over 60 years experienced a significant risk in TDL compared to patients under 30 years of age. A history of TB treatment was found to be 2.73 times of developing TDL compared to those receiving the initial treatment. Malnutrition presented a 5.13-fold risk of TDL. Furthermore, patients experiencing respiratory failure and bronchiectasis were found to be a higher risk of developing TDL, with 5.08 times and 3.50 times, respectively. Conversely, a normal or overweight BMI were the protective factors, decreasing the risk of TDL by 79.3% and 74.1%, respectively, in comparison to those with an underweight BMI.
 |
Figure 1 The forest map of risk factors among TDL patients. |
Discussion
TDL represents one of the most devastating complications of TB, with a substantial proportion of patients experiencing a significant decline in lung function over time. In this study, we investigated the risk factors of TDL at the Third People’s Hospital of Shenzhen between 2019 and 2021. The TDL incidence rate of 53.37% was higher than other high TB prevalence regions, which stands at 1.3%.31 TDL patients presented a prolonged hospitalization period and an increased mortality in comparison to individuals with PTB and structural lung diseases. This phenomenon could be attributed to the frequent exacerbation and unfavorable prognosis associated with TDL, thereby imposing a significant influence on the duration of treatment and mortality rate. Furthermore, the findings unveiled that patients within the age range of 46–60 years and those aged over 60 years, a prior history of TB treatment, malnutrition, respiratory failure, and bronchiarctia were the risk factors for TDL. Conversely, maintaining a normal or overweight BMI was recognized as a protective factor against TDL.
The age-specific impact on the progression of TDL is particularly notable in individuals aged 46–60 and those over 60 years. Consistent with the finding, Ruan et al32 have also documented that individuals aged 40 years and older with TDL face an increased risk of severe postoperative complications. This vulnerability is primarily due to a weakened immune system and the presence of other coexisting conditions, leading to a greater likelihood of unfavorable outcome. Moreover, elderly patients frequently demonstrate inadequate medical awareness, neglect to promptly seek medical care, thereby delaying treatment, which culminates in the development of TDL.
The history of TB treatment is closely linked to the TB relapse after initial therapy, leading to adverse outcomes.33,34 Hnizdo et al35 found that the delayed diagnosis of PTB, non-standardized anti-TB treatment, and recurrent TB make individuals more susceptible to lung function impairment. Patients undergoing retreatment for TB are prone to experience treatment failures due to delayed diagnosis of PTB and non-standardized anti-tuberculosis treatment. Patients receiving retreatment for TB presented more extensive lung impairment and diminished pulmonary blood flow, hindering the effective delivery of anti-tuberculosis medications to the lung tissues. Moreover, the overall physical and mental health status of retreatment patients tends to be worse, culminating in sub-optimal therapeutic results. Commonly, patients requiring retreatment face prolonged therapy duration, diminished therapeutic efficacy, recurrent treatment disruptions, inadequate adherence to treatment regimens, severe adverse reactions to second-line drugs, and an escalated risk of developing drug resistance,36,37 subsequently increasing the risk of TDL.
Malnutrition stands as the leading risk factor for TB, impairing both innate and adaptive immune responses by weakening the body’s resistance to Mycobacterium tuberculosis infection.38 Additionally, malnutrition influences the pharmacokinetics and pharmacodynamics in TB patients. Our findings highlighted the pivotal role of malnutrition in heightening the risk of TDL. This phenomenon primarily occurs due to malnutrition, which results in an increased bacillary load in sputum, more frequent cavitation, and slower sputum conversion rates.39–42 Moreover, the reduced levels of serum albumin and other drug carriers in malnourished patients lower the effective concentrations of anti-TB drugs, thereby affecting treatment efficacy and possibly necessitating additional treatments. Meanwhile, we found that the normal or overweight BMI reduce the risk of TDL. Body weight is a prognostic indicator in the clinical course of TB.43 A higher BMI serves as a protective factor against TB.44 Individuals with a higher BMI may benefit from increased daily protein and energy intake, potentially fortifying immune function and consequently reducing both mortality rate and the incidence of TB. Thus, nutritional therapy ought to be recognized as a pivotal supplementary treatment in conjunction with the chemotherapeutic management of TB. It is essential to underscore the significance of nutritional interventions.
Respiratory failure heightens the vulnerability to developing TDL. It is an irreversible condition characterized by progressive deterioration over time. Patients with TB frequently experience concurrent respiratory failure, likely stemming from significant impairment in lung function, disruptions in internal acid-base balance, and the widespread dissemination of tubercle bacilli infection throughout the body.
Bronchiarctia increases the risk of TDL. This association arises because bronchiarctia can induce irreversible pulmonary impairment, ultimately leading to respiratory failure and increased mortality risk. Prolonged bronchiarctia can trigger recurrent pulmonary infections, atelectasis, bronchiectasis, and pulmonary sequelae. These complications collectively diminish pulmonary function, potentially leading to respiratory failure or death by suffocation.
Limitation
This study had several limitations. Firstly, being a single-center retrospective analysis, it might lead to potential select bias and affect the generalizability of the results. The reliance on a single hospital for data collection can restrict the applicability of the findings to a wider demographic, failing to account for the diverse demographic, socioeconomic, and health condition variations found in broader populations. This limitation narrows the scope of the study’s conclusions. Future research should aim for a multi-center cohort, gathering data from various hospitals to enhance the diversity and representatives of the study.
Secondly, due to the retrospective nature of the study, it did not encompass parameters pertaining to mental health, pulmonary function tests, and the six-minute walk test, potentially resulting in restricted findings. Incorporating assessments of mental health and pulmonary function would contribute to a more comprehensive understanding of the risk factors of TDL.
Conclusion
This study has demonstrated that patients with PTB and structural lung diseases exhibit a relatively high prevalence of TDL. The risk factors for TDL encompass patients aged 46–60 years and over 60 years, prior history of TB treatment, malnutrition, respiratory failure, and bronchiarctia. It’s necessary to highlight the importance of early-stage screening for risk factors, including clinical symptoms, pulmonary function, radiographic changes, history of tuberculosis retreatment, as well as structural and functional pulmonary impairments. Prompt prevention and intervention are advantageous in slowing down the progression of TDL. These measures are crucial for enhancing patients’ quality of life and reducing the socioeconomic burden of TB.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Igbokwe V, Ruby LC, Sultanli A, Bélard S. Post-tuberculosis sequelae in children and adolescents: a systematic review. Lancet Infect Dis. 2023;23(4):e138–e150. doi:10.1016/S1473-3099(23)00004-X
2. Dodd PJ, Yuen CM, Jayasooriya SM, van der Zalm MM, Seddon JA. Quantifying the global number of tuberculosis survivors: a modelling study. Lancet Infect Dis. 2021;21(7):984–992. doi:10.1016/S1473-3099(20)30919-1
3. Menzies NA, Quaife M, Allwood BW, et al. Lifetime burden of disease due to incident tuberculosis: a global reappraisal including post-tuberculosis sequelae. Lancet Glob Health. 2021;9(12):e1679–e1687. doi:10.1016/S2214-109X(21)00367-3
4. Meghji J, Simpson H, Squire SB, Mortimer K, Hill PC. A systematic review of the prevalence and pattern of imaging defined post-TB lung disease. PLoS One. 2016;11(8):e0161176. doi:10.1371/journal.pone.0161176
5. Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76–85. doi:10.1159/000350917
6. Lam K, Bong H, Jiang CQ, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137(3):593–600. doi:10.1378/chest.09-1435
7. Ehrlich RI, Adams S, Baatjies R, Jeebhay MF. Chronic airflow obstruction and respiratory symptoms following tuberculosis: a review of South African studies. Int J Tuberc Lung Dis. 2011;15(7):886–891. doi:10.5588/ijtld.10.0526
8. Osman M, Welte A, Dunbar R, et al. Morbidity and mortality up to 5 years post tuberculosis treatment in South Africa: a pilot study. Int J Infect Dis. 2019;85:57–63. doi:10.1016/j.ijid.2019.05.024
9. Nightingale R, Chinoko B, Lesosky M, et al. Respiratory symptoms and lung function in patients treated for pulmonary tuberculosis in Malawi: a prospective cohort study. Thorax. 2022;77(11):1131–1139. doi:10.1136/thoraxjnl-2021-217190
10. Lin Y, Liu Y, Zhang G, et al. Is it feasible to conduct post-tuberculosis assessments at the end of tuberculosis treatment under routine programmatic conditions in China? Trop Med Infect Dis. 2021;6(3):164. doi:10.3390/tropicalmed6030164
11. Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. Eur Respir Rev. 2018;27(147):170077. doi:10.1183/16000617.0077-2017
12. Sarkar M, Srinivasa, Madabhavi I, Kumar K. Tuberculosis associated chronic obstructive pulmonary disease. Clin Respir J. 2017;11(3):285–295. doi:10.1111/crj.12621
13. Allwood BW, Byrne A, Meghji J, Rachow A, van der Zalm MM, Schoch OD. Post-tuberculosis lung disease: clinical review of an under-recognised global challenge. Respiration. 2021;100(8):751–763. doi:10.1159/000512531
14. Malherbe ST, Dupont P, Kant I, et al. A semi-automatic technique to quantify complex tuberculous lung lesions on 18F-fluorodeoxyglucose positron emission tomography/computerised tomography images. EJNMMI Res. 2018;8(1):55. doi:10.1186/s13550-018-0411-7
15. Stek C, Allwood B, Walker NF, Wilkinson RJ, Lynen L, Meintjes G. The immune mechanisms of lung parenchymal damage in tuberculosis and the role of host-directed therapy. Front Microbiol. 2018;9:2603. doi:10.3389/fmicb.2018.02603
16. Urbanowski ME, Ordonez AA, Ruiz-Bedoya CA, Jain SK, Bishai WR. Cavitary tuberculosis: the gateway of disease transmission. Lancet Infect Dis. 2020;20(6):e117–e128. doi:10.1016/S1473-3099(20)30148-1
17. Dorhoi A, Kaufmann SHE. Pathology and immune reactivity: understanding multidimensionality in pulmonary tuberculosis. Semin Immunopathol. 2016;38(2):153–166. doi:10.1007/s00281-015-0531-3
18. Han D, Lee HY, Kim K, Kim T, Oh YM, Rhee CK. Burden and clinical characteristics of high grade tuberculosis destroyed lung: a nationwide study. J Thorac Dis. 2019;11(10):4224–4233. doi:10.21037/jtd.2019.09.63
19. Bongomin F, Chowdhary A. Post-tuberculosis chronic pulmonary aspergillosis: an emerging public health concern. PLoS Pathog. 2020;16(8):e1008742. doi:10.1371/journal.ppat.1008742
20. Kosif Mısırlıoğlu A, Bayram S, Kıral H, et al. Factors affecting complication rates of pneumonectomy in destroyed lung. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26(2):272–278. doi:10.5606/tgkdc.dergisi.2018.14635
21. Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003;97(11):1237–1242. doi:10.1016/s0954-6111(03)00255-5
22. Rhee CK, Yoo KH, Lee JH, et al. Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis. 2013;17(1):67–75. doi:10.5588/ijtld.12.0351
23. Kim CJ, Yoon HK, Park MJ, et al. Inhaled indacaterol for the treatment of COPD patients with destroyed lung by tuberculosis and moderate-to-severe airflow limitation: results from the randomized INFINITY study. Int J Chron Obstruct Pulmon Dis. 2017;12:1589–1596. doi:10.2147/COPD.S128750
24. Kim SJ, Lee J, Park YS, et al. Effect of airflow limitation on acute exacerbations in patients with destroyed lungs by tuberculosis. J Korean Med Sci. 2015;30(6):737–742. doi:10.3346/jkms.2015.30.6.737
25. Jo YS, Park JH, Lee JK, Heo EY, Chung HS, Kim DK. Risk factors for pulmonary arterial hypertension in patients with tuberculosis-destroyed lungs and their clinical characteristics compared with patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2433–2443. doi:10.2147/COPD.S136304
26. Blyth DF. Pneumonectomy for inflammatory lung disease. Eur J Cardiothorac Surg. 2000;18(4):429–434. doi:10.1016/s1010-7940(00)00526-1
27. Shiraishi Y, Nakajima Y, Koyama A, Takasuna K, Katsuragi N, Yoshida S. Morbidity and mortality after 94 extrapleural pneumonectomies for empyema. Ann Thorac Surg. 2000;70(4):1202–1206; discussion 1206–1207. doi:10.1016/s0003-4975(00)01612-x
28. Ruan H, Liu F, Han M, Gong C. Incidence and risk factors of postoperative complications in patients with tuberculosis-destroyed lung. BMC Pulm Med. 2021;21(1):273. doi:10.1186/s12890-021-01641-0
29. Wang X, Luo L, Zhang D, et al. Factors associated with nutritional risk in patients with pulmonary tuberculosis and structural lung disease: a hospital-based cross-sectional study. J Multidiscip Healthc. 2022;15:1799–1807. doi:10.2147/JMDH.S375441
30. Ryu YJ, Lee JH, Chun EM, Chang JH, Shim SS. Clinical outcomes and prognostic factors in patients with tuberculous destroyed lung. Int J Tuberc Lung Dis. 2011;15(2):246–250.
31. Fawibe AE, Salami AK, Oluboyo PO, Desalu OO, Odeigha LO. Profile and outcome of unilateral tuberculous lung destruction in Ilorin, Nigeria. West Afr J Med. 2011;30(2):130–135.
32. Ruan H, Gong C, Wang J. The efficacy and safety of surgical treatment for patients with tuberculosis destroyed lung with or without chronic pulmonary aspergillosis. World J Surg. 2021;45(5):1595–1601. doi:10.1007/s00268-021-05969-w
33. Middelkoop K, Bekker LG, Shashkina E, Kreiswirth B, Wood R. Retreatment tuberculosis in a South African community: the role of re-infection, HIV and antiretroviral treatment. Int J Tuberc Lung Dis. 2012;16(11):1510–1516. doi:10.5588/ijtld.12.0049
34. Cohen DB, Davies G, Malwafu W, et al. Poor outcomes in recurrent tuberculosis: more than just drug resistance? PLoS One. 2019;14(5):e0215855. doi:10.1371/journal.pone.0215855
35. Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55(1):32–38. doi:10.1136/thorax.55.1.32
36. Peltzer K, Louw J. Prevalence of suicidal behaviour & associated factors among tuberculosis patients in public primary care in South Africa. Indian J Med Res. 2013;138(2):194–200.
37. de Araújo GS, Pereira SM, Dos Santos DN, Marinho JM, Rodrigues LC, Barreto ML. Common mental disorders associated with tuberculosis: a matched case-control study. PLoS One. 2014;9(6):e99551. doi:10.1371/journal.pone.0099551
38. Ockenga J, Fuhse K, Chatterjee S, et al. Tuberculosis and malnutrition: the European perspective. Clin Nutr. 2023;42(4):486–492. doi:10.1016/j.clnu.2023.01.016
39. Putri FA, Burhan E, Nawas A, et al. Body mass index predictive of sputum culture conversion among MDR-TB patients in Indonesia. Int j Tuberc Lung Dis. 2014;18(5):564–570. doi:10.5588/ijtld.13.0602
40. Birlie A, Tesfaw G, Dejene T, Woldemichael K, Hill PC. Time to death and associated factors among tuberculosis patients in Dangila Woreda, Northwest Ethiopia. PLoS One. 2015;10(12):e0144244. doi:10.1371/journal.pone.0144244
41. Hoyt KJ, Sarkar S, White L, et al. Effect of malnutrition on radiographic findings and mycobacterial burden in pulmonary tuberculosis. PLoS One. 2019;14(3):e0214011. doi:10.1371/journal.pone.0214011
42. Sinha P, Ponnuraja C, Gupte N, et al. Impact of undernutrition on tuberculosis treatment outcomes in India: a multicenter, prospective, cohort analysis. Clinl Infect Dis. 2023;76(8):1483–1491. doi:10.1093/cid/ciac915
43. Badawi A, Gregg B, Vasileva D. Systematic analysis for the relationship between obesity and tuberculosis. Public Health. 2020;186:246–256. doi:10.1016/j.puhe.2020.06.054
44. Abdullah A, Peeters A, de Courten M, Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabet Res Clin Pract. 2010;89(3):309–319. doi:10.1016/j.diabres.2010.04.012
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
