Back to Journals » Cancer Management and Research » Volume 14
Role of Consolidative Stereotactic Body Radiation Therapy in Oligoresistant/Oligoprogressive Pulmonary Parenchymal Metastases
Received 10 February 2022
Accepted for publication 29 July 2022
Published 31 August 2022 Volume 2022:14 Pages 2597—2607
DOI https://doi.org/10.2147/CMAR.S360766
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Tanju Berber,1 Abdullah Sakin2
1Department of Radiation Oncology, Okmeydani Training and Research Hospital, Istanbul, Turkey; 2Department of Medical Oncology, Medipol University, Bahçelievler Medipol Hospital, Istanbul, Turkey
Correspondence: Tanju Berber, Department of Radiation Oncology, Okmeydani Training and Research Hospital, Istanbul, 34307, Turkey, Tel +0905324111202, Email [email protected]
Aim: To extend the survival of patients by providing local control of metastases in oligoresistance/oligoprogressive disease.
Methods: We retrospectively evaluated the efficacy of stereotactic body radiotherapy (SBRT) applied to 30 lesions in the lungs of 19 patients who were considered inoperable by the tumor board upon the development of oligoresistance/oligoprogressive lung metastasis while undergoing chemotherapy between January 2016 and December 2017. Each patient had one to five metastases in their lungs. The median SBRT biologic effective dose at α/β of 10 (BED10) was 180.0 (IQR: 115.5– 180.0) Gy.
Results: We obtained effective, low-toxicity results. The rates of local control were 89.4%, 84.2%, and 78.9% for the 1st, 2nd, and 3rd years, respectively. The median local control time was 4 (IQR: 3– 6) months. The median overall survival (OS) was 36.3 (IQR: 29.7– 42.9) months. The rates of OS for the 1st, 2nd, and 3rd years were 89.5%, 73.7%, and 61.4%, respectively. Despite the nonoccurrence of grade 4– 5 toxicity in the lungs, six (31.6%) patients had grade 1– 3 pulmonary pneumonia, one patient had a grade 4 skin ulceration, and two patients had increased chronic obstructive pulmonary disease in the follow-up period.
Discussion: In patients with oligometastatic lung tumors, SBRT is very effective in terms of progression-free survival and OS.
Keywords: lung metastasis, stereotactic body radiation therapy, stereotactic ablative radiotherapy, oligometastatic, oligoprogressive, oligoresistance, pallation
Introduction
Although different consortiums have moved to establish a guideline for local consolidative local therapy, there is still no established guideline for the ablative treatment of any oligo disease. Oligoresistance/oligoprogressive disease is generally considered to be the presence of no more than 3 to 5 deposits unresponsive to previous chemotherapy or that have progressed under chemotherapy, excluding the primary tumor.
Today, various treatment approaches are applied for curative purposes in oligometastatic diseases. Longer survival rates can be achieved with improvements in systemic treatments, molecular-targeted therapies, and immune checkpoint inhibitors.1,2 In patients with lung metastases without extrapulmonary disease, metastasectomy is still seen as the standard treatment. However, surgery is not always an option for patients with poor performance status. Moreover, as oligoresistance/oligoprogressive patients have the worst prognosis among patients with oligometastatses, surgical treatment is generally avoided in this group of patients. Patients with a limited number of persistent or recurrent/growing metastases, as well as a more extensive presentation who experience relative disease stability on “mostly effective” systemic therapy, are considered oligoresistant (induced oligometastases) and oligoprogressive disease, respectively. Oligoprogression is enlargement of one or more metastatic sites in stable disease. This finding indicates a subpopulation of potentially therapy-resistant clonogens. In particular, oligoresistaces that were unresponsive to previous treatments may be considered to have more resistant clones.3,4 Currently, in patients with oligoprogressive/oligoresistance lung metastases, targeted therapy or chemotherapeutics are switched with different agents as standard. However, the agents to which we can switch are quite limited. Moreover, even in second-line treatments, the contribution of these agents to progression-free survival (PFS) can often be limited to only 4–9 months, and in subsequent treatments, this is further reduced.5 In addition, it is known that the first systemic treatments are more tolerable, and subsequent regimens are much more toxic.
In parallel with the technological developments in radiotherapy devices in recent years, stereotactic body radiotherapy (SBRT) has also become widespread. The most important feature that distinguishes SBRT from conventional radiation therapy is that it is given in larger doses in fewer fractions, resulting in a high biologically effective dose (BED10). When calculating lung doses with SBRT, biologically equivalent doses are considered to be α/β=10 for lung cancer and acute toxicity (BED10) and α/β=3 for late toxicity (BED3). Serious adverse events with SBRT are rarely seen because they occur after at least a year, later than the expected survival of patients with oligo disease. With SBRT, high doses can be given to target tissues by protecting normal tissues with a very high conformally.6 The fact that there are promising studies with SBRT, in which high local control and overall survival (OS) rates have been obtained using SBRT as an alternative to surgery in early-stage inoperable diseases, and in non-small-cell lung carcinoma (NSCLC) in recent years, was one of the main drivers for the initiation of this study.7 Even inoperable disease results similar to those obtained via surgery have been achieved, thus SBRT could also be a promising alternative to surgery.8 There were a few published studies on oligoprogressive or even oligometastatic disease at the time of the study, but there was no other option for these patients. There are some assumptions that SBRT can contribute to PFS, which is the primary endpoint, in oligoprogressive or oligoresistance lung metastases. It is suggested to start clinical trails in this direction.
In 2019, after the results of the Phase 2 SABR-COMET study were published, which showed that local therapies in oligometastases could prolong survival, treatment approaches were used for curative purposes in oligometastatic diseases.9 Based on the SABR-COMET findings, we expect to be able to contribute to the treatment of these patients using SBRT. However, although SBRT seems to be beneficial in oligometastatic disease, it remains unclear whether it is effective in patients with oligoresistance/oligoprogressive disease because there are currently insufficient studies. In addition to local control of metastatic disease, SBRT may affect the immune response, as seen in some sporadic cases,10 a striking example of which was observed in one of our patients anecdotally.1
There were 158 publications on oligometastses in PubMed alone in 2021 (oligometases SBRT, stereotactic ablative radiotherapy – SABR). Unfortunately, however, there is little research on the worst-progressing group including patients with oligoresistant/oligoprogressive pulmonary parenchymal metastases, let alone a phase 2 study.
In oligoprogression/resistant cases that develop during and/or immediately after first-line systemic treatments, the continuation of treatment is usually switched to new agents. However, second-line treatments are more toxic for the patient and less effective for the tumor when compared with first-line treatments. Therefore, we aimed to extend the survival and PFS of patients by providing local control of metastases in oligoresistance/oligoprogressive disease, which is known to have a very poor prognosis, with limited toxicity. In doing so, we could reserve a group of drugs for later use.
Materials and Methods
Patient Characteristics
In our clinic at Okmeydani Training and Research Hospital, radiosurgery treatment techniques have been applied to treat various malignant and benign tumors since 2011. We retrospectively evaluated 19 patients (1–5 metastases) who had pulmonary metastases and were medically inoperable and who had 30 pulmonary metastases that were exposed to radiosurgery between January 2016 and December 2017. The treatment characteristics and toxicities of these patients were investigated retrospectively and included in the study. First-line chemotherapy is relatively effective and less toxic, and systemic agents used in the progression of the disease are more toxic to the body and less effective against the disease. By contributing to PFS with SBRT, which is a local ablative method, the toxic effects of these systemic agents can be avoided temporarily by delaying the use of these systemic agents, and it can also contribute to OS.
All patients with pulmonary metastases undergoing pulmonary SBRT therapy were included, regardless of whether they had metastases in other regions. The inclusion criteria were as follows: all patients who underwent SBRT because of intrapulmonary metastases under systemic treatment but did not respond to these treatments or even progressed despite systemic treatment. The exclusion criteria included the presence of other primary malignancies in the same region and undergoing locoregional radiosurgery for the primary disease. Only those who received SBRT as relapse treatment were included in the treatment. Those who received another treatment methodology such as chemotherapy or immunotherapy after relapse were not included in the study. Patients whose lung lesions regressed after chemotherapy were also excluded from the study. All patients were evaluated by tumor boards, and it was decided to administer radiosurgery instead of surgery due to poor performance of surgery on their tumor types. Ethical approval was received for this study from the Ethics Committee of Prof. Dr. Cemil Taşçıoğlu Şehir Hastahanesi (AKA: Okmeydani Training and Research Hospital) (Decision number: 48670771–514.99, Date: November 8th, 2021; Decision number 382).
Treatment Details
Oligoprogression/resistance was determined as the group of patients who had previously received at least one series of systemic therapy, with ≤5 progressive/non-responsible tumors with solid lesions, and response evaluations or positron emission tomography-computed tomography (PET-CT). In non-progressed nodules, we did not determine any upper limit in such lesions. PFS was defined as the time from the initiation of treatment to disease progression or death. Disease free surival, referred to as regional disease recurrence, occurrence of distant metastasis to another, or time to death.
In our study, 19 medically inoperable patients who had undergone SBRT and had only metastasis to the lung between January 2016 and December 2017 were included. The total number of metastases was 30. The primary origin was the lung (NSCLC) in 14 patients, the gastrointestinal system in three patients (colon n=2, rectum n=1), and the genitourinary system in two patients (prostate n=1, bladder n=1). Fourteen patients had single metastases, and five patients had 2–5 metastases. In the follow-up period, only six patients received different chemotherapy treatments based on the decision of a medical oncologist.
The median follow-up period was 31 (IQR: 19–38) months. The median age was 65 (IQR: 62–71) years. Six patients were aged 70 years and older, and thus, there was a related decrease in performance and comorbid diseases. The median tumor diameter was 2.7 (IQR: 1.2–3.7) cm. The tumor diameter was larger than 4 cm in four patients. All except two patients were chronic smokers. The median Karnofsky Performance Status (KPS) was 70 (IQR: 60–80). All 19 patients had undergone CT, and 10 had undergone 18F fluorodeoxyglucose PET-CT (Table 1).
 |
Table 1 Patient and Treatment Characteristics |
Our patients underwent one or more of the following systemic treatments until at least 4 weeks before the SBRT treatment period. The patients may also have received concomitant chemoradiotherapy if their disease was primary before metastasis developed and they received systemic treatment, or metastasectomy before SBRT. However, our patients were unsuitable for surgery because they were old and had comorbid disease. All patients had a histologic tumor diagnosis. However, a histologic diagnosis could not be made from metastases due to poor performance. Recurrences were diagnosed using PET-CT and/or CT (according to the tumor board discretion) with a high predictive value (95%).11 In all cases, the disease was restaged according to the 8th (2018) edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.
Follow-Up and Primary and Secondary Endpoints
In patients with only CT before SBRT, the tumor response was evaluated using the Response Evaluation Criteria for Solid Tumors (RECIST) system.12 For patients with PET/CT before SBRT, if the tumor diameter and viability increased by 20% or more in PET-CT, recurrence was considered. Acute and late toxicities were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0).13 In accordance with the American College of Chest Physicians (ACCP) 2013 guidelines, the patients were first assessed through PET-CT or CT 3 months after the treatment.14 Until April 2020, all patients were evaluated with CT at least every 6 months and with PET-CT when an assessment could not be made with CT.
The primary end-points were the 1-year PFS, local control rate, and toxicity. The secondary end-point was OS.
Radiation Therapy Specifications
Most of the patients were treated using the CyberKnife® (Accuray Inc., Sunnyvale, CA, USA) radiosurgery system with 6-MV X-rays under a respiratory gating system; the remaining patients were treated on a Varian Trilogy (6–18 MV X-ray) linear accelerator platform under a 4DCT gating system. In patients (n=10) treated with Cyberknife®, images in inspiration and expiration were used to measure gross tumor volume (GTV). These were performed using wide window level settings [window level range, −700 to −500 Hounsfield units (HU); window width range, 1500–2000 HU];15 tumor monitoring was performed using an x-site spine without a fiducial marker, and an internal target volume (ITV) was created. Internal gross target volumes (iGTVs) were created using an x-site lung respiratory monitoring system with fiducial markers in two patients treated with Cyberknife® and with a 4D RPM system in four patients treated with the Varian® device. For imaging, guidance was used during the treatment of kilovoltage cone-beam CT (kVCBCT). The planning target volume (PTV) was determined to be 3–5 mm after the formation of the internal target volume according to GTV. No margin was given for the clinical target volume (CTV). In radiosurgery applications, we use a 1.0-mm CT slice thickness. The Varian® 4D RPM system with breath-hold was preferred in lung lower lobe tumors according to the protocol of our institute. Dose and fractionation were selected depending on the location of the tumor. Treatment was prescribed so that 95% of the PTV received 100% of the prescribed dose. In this study, we documented the tumor size, not the PTV, to prevent bias, because we employed very limited PTV margins due to the techniques we used. As a result, tumor size was used in the study, as in most studies and meta-analyses performed to date.16 We used a standard formula biologic effective dose (BED) calculation: BED=nd (1 +d/α/β) for comparison of different fractionation regimens. This formula is derived from the linear-quadratic (L-Q) model, where n and d are the number and size of the dose fractions, and the ratio of α/β is accepted as 3 and 10 Gy for normal lung tissue and tumor, respectively.
Statistical Design
All time-related events (failure or death) were calculated from the date of first SBRT to the date of death or censoring at the last clinical follow-up and analyzed using Kaplan–Meier and Cox proportional hazard methods. Significance was considered at p < 0.05, and all significance levels were 2-sided. The IBM® SPSS® Statistics version 23 software package was used for all statistical analyses. Univariate Cox regression analysis was conducted to identify clinical variables associated with end-points of interest, followed by multivariate models including all variables. In this study, local control and OS, whether tumor diameter affected these, and the statistical significance of the PET-CT responses in the 3rd month was tested.
Results
Treatment Information and Local Control
The median SBRT BED at α/β of 10 (BED10) was 180.0 (IQR: 115.5–180.0) Gy. The median BED3 was 460.0 (227.7–460.0). The majority of our patients had lung cancer (73.6%), and 42.1% had squamous cell lung cancer. All patients had undergone previous chemotherapy but remained oligoresistant/oligoprogressive. Five (26%) of our patients had other metastases during the treatment. The most commonly used dose was 60 Gy in three fractions (frx) (52.6%). One patient had five metastases, and 18 patients (95%) had three or fewer metastases. The patients had metastases in one or both lungs. Single metastasis was observed in 13 (63.8%) patients. Five patients had tumors with a low response to radiotherapy, such as colorectal, bladder, and prostate cancer (26.3%). Except for two patients, all were chronic smokers, and 10 (52.6%) continued to smoke despite all the recommendations and therapy during the treatment process. Twelve (63%) of our patients had previously received locoregional radiotherapy to the lung area. Two patients were ultracentral (touched the main bronchus) and were treated with 5 frx/55 Gray (Gy) and 10 frx/70 Gy. (Table 1).
Local control rates were 89.4%, 84.3%, and 78.9% for the 1st, 2nd, and 3rd years, respectively. The median local control time was 4 (IQR: 3–6) months. Four patients with local recurrence over time and one patient with no response at the beginning had lung cancer. Local control, regional control of regions of the lung and mediastinal lymph regions, and survival analyses are given in Table 2 as univariate and multivariate analyses (Table 2). Tumor diameter below or above 2 cm did not differ in terms of local control, regional control, or OS (Table 2).
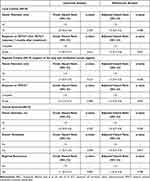 |
Table 2 Local Control, Regional Control, and Survival Analysis |
Overall Survival, Disease-Free Survival, and Progression-Free Survival
The median OS was 36.3 (IQR: 29.7–42.9) months (Figure 1). The rates of OS for the 1st, 2nd, and 3rd years were 89.5, 73.7%, and 61.4%, respectively.
 |
Figure 1 Overall Survival-Local Recurrence Relationship (months). |
The median PFS was 34.6 (IQR: 28.6–40.5) months (Figure 2). The rates of disease-free survival (DFS) for the 1st, 2nd, and 3rd years were 89.5, 73.7%, and 63.2%, respectively.
 |
Figure 2 Progression-Free Survival -Local Recurrence Relationship (months). |
Of the 19 patients, 10 are still alive and progression-free. Three patients were alive but had the disease, and six patients died. Considering the causes of death, two patients died of lung disease, three patients died of other metastases, and one patient died of age-related comorbid disease (Table 3). The last conditions of the patients in our study are presented in Table 4. Six patients receiving chemotherapy after SBRT and one patient receiving targeted therapy are still alive. Of the six patients who died, two had progressive lung cancer that could not achieve local control at the beginning. Before treating this patient, lung metastases appeared one year Dec, and each time these metastases were removed by surgery. When the fourth metastasis appeared, the patient presented to us for SBRT because his lung capacity was insuffiecient and he was no longer in a position to undergo further surgery after the three previous procedures. After undergoing SBRT, no new metastases were seen. This may indicate that SBRT has an immunologic effect.
 |
Table 3 Cause of Death |
 |
Table 4 The Final Conditions of the Patients |
Adverse Events
Despite the nonoccurrence of grade 4–5 toxicity in the lungs, six (31.6%) patients had grade 1–3 pulmonary pneumonia, one patient had a grade 4 skin ulceration, two patients had increased chronic obstructive pulmonary disease (COPD) in the follow-up period, and one patient developed a grade 2 myocardial ischemic event 2 years after SBRT treatment.
Two patients with peripheral lung metastasis and BED10 180 developed chest pain, but no rib fractures were observed in our patients.
Discussion
The theory behind ablating resistant/progressive disease clones to allow for continued response to current systemic therapy is oncologically valid. However, thus far, evidence for treating oligoresistant/progressive disease in this way has only been provided anecdotally. Overall, despite a lack of randomized data, evaluation of ablative therapies for oligoresistant/progressive disease is logical because it allows patients to delay next-line systemic therapy and may improve PFS and OS. In such patients, SBRT makes a significant contribution to disease control as a local treatment modality. This is one of the first studies to show the contribution of local therapy in oligoprogressive/resistant disease. Currently, in tumor oligoprogressive/resistant cases, another systemic treatment is generally used as a standard treatment or palliative treatments are used.17 However, by controlling these lesions with SBRT, which is a local treatment method, the PFS of patients can be contributed to and their OS can be extended.
Radiosurgery applications are used as a standard treatment in patients with early-stage medically inoperable lung cancer, and promising results in terms of local control and survival have been obtained.18–20 However, until now, only chemotherapy and surgery could be performed in metastases in the lungs in a limited number of cases. With the development of radiosurgery techniques in recent years, the applicability of radiotherapy techniques for a limited number of lung metastases has been investigated.21,22 In this study, the effects of SBRT on local control, PFS, and OS were retrospectively investigated in lung cancer metastases or other cancers in the lung parenchyma. Our median follow-up time was 3 years. This is comparable to the average in other studies with a median follow-up period of 3 years, where local control rates were 89.4%, 84.2%, and 78.9% for the 1st, 2nd, and 3rd years, and OS rates (median 36.3 months) for the 1st, 2nd, and 3rd years were 89.5–73.7% and 61.4%.23 Publications indicate that in oligometastatic disease, SBRT can increase the survival time up to 13 months and nearly double PFS and DFS, and treatment-related mortality has been reported as 5%. However, localized treatments may be futile for patients who are not under systemic control.24 In the interim analysis of an ongoing phase 2 study, the median follow-up was 51 weeks.25 Our 3-year follow-up is adequately long to determine efficacy and the early or late adverse effects of SBRT. As a result, when our toxicities are examined during this period, it is seen that this treatment method has a very reasonable toxicity profile.
No relationship with tumor size was detected in the absence of local control, PFS, locoregional and DFS, or OS. The reason for this may be due to relatively high doses of SBRT, or it may be related to the numerical or total volumetric size of the tumor because we did not look at the total tumor volume. It is a shortcoming that we did not design the study in this way.
Colorectal cancers are considered less responsive. Sharma et al found 2- and 3-year survival rates of 69% and 55%, respectively. However, in our study, one of our patients with five lung metastases and three patients with colorectal diseases died after 12 months of multiple liver and brain metastases that progressed 3 months after SBRT was performed on the lung. Another two patients were alive and DFS (66%), so we found local control as 100% and both 2- and 3-year OS as 66%. Local recurrence was not observed in our patients with prostate and bladder cancer, which can also be considered less responsive. One of our patients, whose primary disease origin was the bladder, died after 36 months of ischemic heart disease.
Tumor size is considered an important parameter in terms of local control for early-stage cancer.26 However, in our study, the median tumor size value was not found to be associated with local control, regional control, or survival. This may be due to the low number cases or that tumor size does not affect oligoresistant/oligoprogressive disease.
Three months after treatment in PET-CT, complete response or partial response did not affect local control or survival. The importance of PET CT response is emphasized in early-stage studies;27 however, in the present study, we found no relationship between the first response and local control or survival.
This group of patients is generally accepted as incurable and directed to palliative care.28 However, the median 36.3-month OS we found is indeed promising for this patient group. With SBRT, we observed a PFS, DFS, and OS in our patients that could compete with new systemic agents. Moreover, these results may encourage us to provide a therapeutic advantage by delaying the progression to new/different agents. Moreover, it should not be forgotten that our patient group consisted of oligoresistant/oligoprogressive disease and therefore considered the most resistant clones. New treatment agents such as tyrosin kinase inhibitors (TKI) and immunotherapy now offer very promising results in patients with metastasis, extending the life of patients. Despite the fact that none of our patients received these treatment methods, we received very good results.
As a result of preclinical studies, the abscopal effect is mentioned in some doses and fractions.29 Statistically, we did not find that it contributed to the development of intrapulmonary regional recurrence or distant metastases with or without local control. However, anecdotally, metastases were detected three times in the lung of a patient between 2008 and 2017, and these lesions were surgically removed each time. However, metastases recurred in different parts of the lung. When the metastases recurred for the fourth time, surgery was not considered due to the decrease in lung capacity, and the patient was referred to our clinic for SBRT. Although the patient, who subsequently received SBRT, did not receive any additional treatment, the patient was followed for another distant recurrence free for 3 years.
Of the six patients who died, three died of systemic disease, two of local disease, and one of cardiac ischemic disease after 3 years. The only common feature of the five patients who died of the disease is that they progressed rapidly after the first treatment they received. Three had a single metastasis to the lung, and two had multiple metastases. Since the number of our patients was low, it is not appropriate to make a judgment on these oligometastatic patients.
Despite the absence of grade 4–5 toxicities in the lung, six patients had grade 1–3 pulmonary pneumonia, and one patient had grade 4 skin ulceration. Two patients had deterioration in COPD later, and one patient developed a grade 2 myocardial ischemic event 2 years after SBRT treatment. These results are consistent with the SABR-COMET study in which toxicity of 30% and under was reported as grade 2 or worse adverse effects.9
In two phase 2 randomized trials on NSCLC, SBRT increased PFS approximately three times in metastatic disease (Gomez et al: 11.9 months vs 3.9 months; Iyengar et al: 9.7 months vs 3.5 months).30,31 In addition, an OS of 41 months was found in the studies of Palma and Gomez.9,30 The reason for not reaching these durations in terms of OS in our study might be that our patient population belonged to the more resistant clone group. Moreover, our patient group was older, and the lung cancer group for which we would expect a relatively poor prognosis was predominant. However, we think it is important to provide absolute local control with high-dose radiosurgery and prolong survival parallel to this. Moreover, the median PFS was 34.6 months, which is very promising for oligoresistant/oligoprogressive disease. The median PFS with palliation treatments has been reported as 10 weeks.25 Whether our DFS results were better than other studies in both locoregional and distant metastases, it may be that if there are other metastases other than lung metastases under control, that is, at the time of treatment, other metastases are under control. Another remarkable point in our study is that patients with local recurrence (Figures 1 and 2) after treatment had lower distant metastasis rates and longer PFS and OS times. This may indicate that some clones are prone to local replication only.
New studies showing that the disease is oligometastatic at the beginning have started to emerge,32 but few studies on oligoresistant/oligoprogressive tumors have been conducted. Our study has some limitations. First, we acknowledge that it was a retrospective cohort analysis with a relatively small population. Second, the study population included heterogeneously treated patients concerning the SBRT total dose, dose per fraction, and fractionation because of the location of the tumors and currently no standard treatment dose has been determined. Third, although the majority of patients had lung cancer, other tumor metastases were included in the study so the tumor population was not homogeneous.
The results we obtained in oligoresistant/oligoprogressive disease, which is the worst subgroup of oligometastatic disease are very promising and can encourage global prospective studies. The most important strength of this study is that because SBRT has not been performed in this group of patients before, the doses we administered may create a guide for subsequent SBRT applications. Currently, we largely have only anecdotal reports. Retrospective studies such as the present study can pave the way for prospective studies. Another advantage of our study is that applications and follow-ups were performed at a single center by a single physician.
The results of our study are important because the survival times of patients with tumors after resistant/progressive metastases are quite short. Riihimäki et al found a median OS of 5 months after metastasis in their study of 17,431 patients with lung cancer.33 In our study, the median OS was 36.3 months. Any oligometastatic disease that was previously considered for palliative care should now be controlled with high-dose SBRT.
Conclusion
When the contributions to local control, PFS, and survival are considered in our study and the studies in the literature, SBRT is promising in oligometastatic and controllable patients. However, in SBRT, its benefit may be limited in patients whose disease progresses in a very short time.
Institutional Review Board Statement
This study and all relevant procedures were performed in accordance with the Helsinki Declaration after obtaining ethical approval from the Ethics Committee of Prof. Dr. Cemil Taşçıoğlu Şehir Hastahanesi (AKA: Okmeydani Training and Research Hospital) (approval number: E-486707771-514.99). This is a retrospective study. Data were obtained from patient files.
Informed Consent Statement
The patients were not required to give informed consent for this study because anonymous retrospective data were obtained after each patient accepted the treatment by written consent.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bernstein MB, Krishnan S, Hodge JW, Chang JY. Immunotherapy and stereotactic ablative radiotherapy (ISABR): a curative approach? Nat Rev Clin Oncol. 2016;13(8):516–524. doi:10.1038/nrclinonc.2016.30
2. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw Open. 2019;2(5):e192535. doi:10.1001/jamanetworkopen.2019.2535
3. Bergsma DP, Salama JK, Singh DP, Chmura SJ, Milano MT. Radiotherapy for oligometastatic lung cancer. Front Oncol. 2017;7:210. doi:10.3389/fonc.2017.00210
4. Vrankar M. Challenges in the treatment of oligometastatic non-small cell lung cancer. In: Lung Cancer-Modern Multidisciplinary Management. IntechOpen; 2020.
5. Rheinheimer S, Heussel CP, Mayer P, et al. Oligoprogressive non-small-cell lung cancer under treatment with PD-(L)1 inhibitors. Cancers. 2020;12(4):Apr. doi:10.3390/cancers12041046
6. Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. doi:10.1118/1.3438081
7. Soldà F, Lodge M, Ashley S, Whitington A, Goldstraw P, Brada M. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol. 2013;109(1):1–7. doi:10.1016/j.radonc.2013.09.006
8. Timmerman RD, Paulus R, Pass HI, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: findings from the NRG oncology RTOG 0618 trial. JAMA Oncology. 2018;4(9):1263–1266. doi:10.1001/jamaoncol.2018.1251
9. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET Phase II randomized trial. J Clin Oncol. 2020;38(25):2830–2838. doi:10.1200/jco.20.00818
10. Maehata Y, Onishi H, Kuriyama K, et al. Immune responses following stereotactic body radiotherapy for stage I primary lung cancer. Biomed Res Int. 2013;2013:1–11. doi:10.1155/2013/731346
11. Lee DH, Kim SK, Lee HY, et al. Early prediction of response to first-line therapy using integrated 18F-FDG PET/CT for patients with advanced/metastatic non-small cell lung cancer. J Thorac Oncol. 2009;4(7):816–821. doi:10.1097/JTO.0b013e3181a99fde
12. Gehan EA, Tefft MC. Will there be resistance to the RECIST (response evaluation criteria in solid tumors)? J Natl Cancer Inst. 2000;92(3):179–181. doi:10.1093/jnci/92.3.179
13. Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer. 2016;24(8):3669–3676. doi:10.1007/s00520-016-3297-9
14. Win T, Miles KA, Janes SM, et al. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. Clin Cancer Res. 2013;19(13):3591–3599. doi:10.1158/1078-0432.Ccr-12-1307
15. Bankier AA, MacMahon H, Goo JM, Rubin GD, Schaefer-Prokop CM, Naidich DP. Recommendations for measuring pulmonary nodules at CT: a statement from the fleischner society. Radiology. 2017;285(2):584–600. doi:10.1148/radiol.2017162894
16. Li M, Yang X, Chen Y, et al. Stereotactic body radiotherapy or stereotactic ablative radiotherapy versus surgery for patients with T1-3N0M0 non-small cell lung cancer: a systematic review and meta-analysis. Onco Targets Ther. 2017;10:2885–2892. doi:10.2147/ott.S138701
17. Corrales L, Nogueira A, Passiglia F, et al. Second-line treatment of non-small cell lung cancer: clinical, pathological, and molecular aspects of nintedanib. Front Med. 2017;4:13. doi:10.3389/fmed.2017.00013
18. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi:10.1001/jama.2010.261
19. Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non–small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677–682. doi:10.1016/j.ijrobp.2008.11.042
20. Murray P, Franks K, Hanna GG. A systematic review of outcomes following stereotactic ablative radiotherapy in the treatment of early-stage primary lung cancer. Br J Radiol. 2017;90(1071):20160732. doi:10.1259/bjr.20160732
21. Sharma A, Baker S, Duijm M, et al. Prognostic factors for local control and survival for inoperable pulmonary colorectal oligometastases treated with stereotactic body radiotherapy. Radiother Oncol. 2020;144:23–29. doi:10.1016/j.radonc.2019.10.004
22. Pasalic D, Lu Y, Betancourt-Cuellar SL, et al. Stereotactic ablative radiation therapy for pulmonary metastases: improving overall survival and identifying subgroups at high risk of local failure. Radiother Oncol. 2020;145:178–185. doi:10.1016/j.radonc.2020.01.010
23. Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol. 2010;5(7):1091–1099. doi:10.1097/JTO.0b013e3181de7143
24. Macbeth F. Avoid futile therapy. Int J Radiat Oncol Biol Phys. 2017;99(4):768. doi:10.1016/j.ijrobp.2017.02.035
25. Tsai CJ, Yang J, Guttmann D, et al. Consolidative use of radiotherapy to block (CURB) oligoprogression―interim analysis of the first randomized study of stereotactic body radiotherapy in patients with oligoprogressive metastatic cancers of the lung and breast. Int J Radiat Oncol Biol Phys. 2021;111(5):1325–1326. doi:10.1016/j.ijrobp.2021.09.014
26. Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87(5):1064–1070. doi:10.1016/j.ijrobp.2013.08.020
27. Sheikhbahaei S, Mena E, Marcus C, Wray R, Taghipour M, Subramaniam RM. 18F-FDG PET/CT: therapy response assessment interpretation (Hopkins criteria) and survival outcomes in lung cancer patients. J Nucl Med. 2016;57(6):855–860. doi:10.2967/jnumed.115.165480
28. Tumati V, Iyengar P. The current state of oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Dis. 2018;10(Suppl 21):S2537. doi:10.21037/jtd.2018.07.19
29. Formenti SC. Optimizing dose per fraction: a new chapter in the story of the abscopal effect? Int J Radiat Oncol Biol Phys. 2017;99(3):677–679. doi:10.1016/j.ijrobp.2017.07.028
30. Gomez DR, Blumenschein JGR, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. doi:10.1016/S1470-2045(16)30532-0
31. Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non–small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncology. 2018;4(1):e173501. doi:10.1001/jamaoncol.2017.3501
32. Palma DA, Salama JK, Lo SS, et al. The oligometastatic state—separating truth from wishful thinking. Nat Rev Clin Oncol. 2014;11(9):549–557. doi:10.1038/nrclinonc.2014.96
33. Riihimaki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78–84. doi:10.1016/j.lungcan.2014.07.020
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
