Back to Journals » Journal of Inflammation Research » Volume 17
Selenium Yeast Alleviates Dextran Sulfate Sodium-Induced Chronic Colitis in Mice by Reducing Proinflammatory Cytokines and Regulating the Gut Microbiota and Their Metabolites
Authors Wu Z, Li Y , Jiang M, Sang L, Chang B
Received 1 December 2023
Accepted for publication 29 February 2024
Published 30 March 2024 Volume 2024:17 Pages 2023—2037
DOI https://doi.org/10.2147/JIR.S449335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Zeyu Wu,1,* Yan Li,1,* Min Jiang,1 Lixuan Sang,2 Bing Chang1
1Department of Gastroenterology, First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China; 2Department of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lixuan Sang, Department of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang, People’s Republic of China, Email [email protected] Bing Chang, Department of Gastroenterology, First Affiliated Hospital of China Medical University, Shenyang, People’s Republic of China, Email [email protected]
Background: Inflammatory bowel disease (IBD) is a chronic recurrent gastrointestinal inflammatory disease. Selenium has been reported to have therapeutic potential in IBD. Selenium yeast is a common selenium supplement that is convenient to access. This study explored the effect of selenium yeast on dextran sulfate sodium- (DSS-)induced chronic colitis in mice.
Methods: Mice were randomly divided into four groups: the control group, selenium yeast group, chronic colitis group, and chronic colitis+selenium yeast group (n=6). Mice were killed on the 26th day. The disease activity index (DAI) score and histological damage score were calculated. Cytokines, serum selenium, colonic tissue selenium, gut microbiota and their metabolites short-chain fatty acids (SCFAs) were evaluated.
Results: Selenium yeast lowered IL-1β, IL-6, TNF-α, IL-17A, IL-22 and IFN-γ (P< 0.05). In addition, selenium yeast significantly elevated Turicibacter, Bifidobacterium, Allobaculum, Prevotella, Halomonas, Adlercreutzia (P< 0.05), and butyric acid (P< 0.05).
Conclusion: Selenium yeast could improve DSS-induced chronic colitis in mice by regulating cytokines, gut microbiota and their metabolites.
Keywords: selenium yeast, chronic colitis, gut microbiota, metabolism
Introduction
Inflammatory bowel disease (IBD) is a chronic recurrent gastrointestinal inflammatory disease that mainly includes ulcerative colitis (UC) and Crohn’s disease (CD).1 The pathogenesis of IBD is not fully understood. Genes, immunity, diet, environment, gut microbiota and their metabolites are all related to the pathogenesis of IBD.2–4
Cytokines play an important role in the occurrence and persistence of IBD inflammation.5,6 Drugs targeting certain cytokines, such as infliximab, the first tumor necrosis factor-(TNF-)αblocker, can alleviate IBD.7
Changes in the gut microbiota are also crucial for IBD. Some gut microbiota producing butyric acid in IBD patients decrease, such as Clostridium clusters IV and XIVa and Faecalibacterium prausnitzii.8 The gut microbiota could be used to distinguish CD from non-CD. Pascal et al reported that an unknown Peptostreptococcaceae, Faecalibacterium, Anaerostipes, Methanobrevibacter, and an unknown Christensenellaceae, were abundant in healthy people and UC patients. Fusobacterium and Escherichia were abundant in CD patients. Collinsella was abundant in UC patients.9 The change in gut microbiota can also be used to predict whether anti-TNF-α is effective for IBD patients. It was reported that patients with a high abundance of F. prausnitzii and Ruminococcus and a low abundance of Methanobrevibacter smithii have a good response to anti-TNF-α treatment.10 In addition, gut microbiota dysbiosis exists not only in patients themselves but also in brothers and sisters of Crohn’s disease patients.11 Metabolites of the gut microbiota, such as short-chain fatty acids (SCFAs), also play an important role in maintaining intestinal homeostasis. Studies have found that SCFAs can regulate the homeostasis of T cells in the colon and promote the production of IL-22 by innate lymphocytes and CD4+ T cells to prevent intestinal inflammation and maintain intestinal homeostasis.12,13
Selenium is an important trace element in the human body that has anti-inflammatory and antioxidant effects.14–16 It can be divided into inorganic selenium and organic selenium. Inorganic selenium mainly includes selenite and selenate, and organic selenium mainly includes selenomethionine and selenocysteine.17 Selenium can alleviate inflammation-mediated by cyclooxygenase (COX), which plays an important role in IBD.18 Selenium can also relieve intestinal inflammation by inhibiting the excessive immune response induced by Th1 cells.19 In addition, selenium can affect the composition and colonization of gut microbiota.20 Sodium selenite has been reported to alleviate dextran sulfate sodium- (DSS-) induced chronic colitis in mice.21 Selenium deficiency in IBD patients has been reported. A Japanese study found that 10.9% of children with IBD have selenium deficiency, and there is more selenium deficiency in CD patients than in UC patients.22 A Korean study also found that 30.9% of patients with IBD have selenium deficiency.23 Above all, selenium may play an important role in intestinal homeostasis.
Compared with inorganic selenium, organic selenium is better absorbed.24 Selenium yeast is produced by fermenting yeast in a selenium-rich environment to combine selenium with components in yeast to form organic selenium,25 in which the main form of selenium is selenomethionine.26 An animal experiment found that the specific bioavailability of selenium yeast was higher than that of sodium selenite.27 Therefore, we explored the effect of selenium yeast on mouse chronic colitis induced by DSS and its possible mechanism.
Materials and Methods
Animal and Ethical Matters
Twenty-four 8-week-old specific pathogen-free C57BL/6 male mice weighing 24±2 g were purchased from Liaoning Changsheng Biology and bred under specific pathogen-free conditions (a 12 h light/12 h dark-light regimen, temperature 23±2 °C, humidity 50–60%). Selenium yeast (selenium>2000 ppm) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. The research protocol was approved by the Animal Ethics Committee and Animal Care Committee of China Medical University. Ethical batch number: cmu2021321. The research followed Laboratory animals—Guideline ethical review of animal welfare (GB/T 35892-2018).
Experimental Design
Twenty-four mice were divided randomly into four groups: 6 in the control group (Group A), 6 in the selenium yeast group (Group B), 6 in the chronic colitis group (Group C), and 6 in the chronic colitis + selenium yeast group (Group D). The control group was given tap water and a normal diet. The selenium yeast group was given tap water and a normal diet, with selenium yeast (100 mg/kg) gavage once a day. The chronic colitis group was induced colitis by 1.5% DSS and given a normal diet. The chronic colitis + selenium yeast group was induced colitis by 1.5% DSS and given a normal diet with selenium yeast (100 mg/kg) gavage once a day. Weight and disease activity index were recorded every day.
Induction of Chronic Colitis by DSS
Oral administration of 1.5% DSS (molecular mass 36–50 kDa; MP Biomedicals, Solon, OH,
United States) was used to induce colitis on Days 0–5, 10–15, and 20–25 d and tap water on the other days.28 The mice were sacrificed on the 26th day.
Disease Activity Index
The severity of colitis in mice was assessed by the disease activity index (DAI), which consists of the percentage of weight loss (0–4 points), stool consistency (0–4 points), and intestinal bleeding (0–4 points),28 as shown in Table 1.
 |
Table 1 Disease Activity Index (DAI) Score |
Histological Injury Score
After the mice were sacrificed, we used 4% paraformaldehyde to fix colon tissues and embedded the colon tissues in paraffin. The colon tissues were stained with hematoxylin and eosin after being cut into 4-µm sections and scored for histological damage. Two pathologists assessed histological scores independently in a blinded fashion. The sum of scores of inflammation severity, degree of mucosal damage, percentage of crypt damage, and pathological change range were calculated. None, mild, moderate, or severe inflammation was quantified as the percentage involvement by inflammation (none, 0–33%, 33–67%, 67–100%). The depth of inflammation (none, mucous layer, submucosa, muscularis, and serosa) represented mucosal damage, as shown in Table 2.28
 |
Table 2 Histological Injury Score |
Cell Preparation, Culture, and Activation
The large intestine of each mouse was cut into 1–2 mm pieces. The pieces were stirred in PBS containing 3 mmol/L EDTA twice (15 min each time) and twice in RPMI 1640 (HyClone) containing 1 mmol/L EGTA (20 min each time) at 37 °C to eliminate the epithelium. The remaining pieces were stirred at 37 °C for 90 min in RPMI 1640 (HyClone) containing 20% fetal bovine serum, 100 U/mL collagenase (C2139; Sigma‒Aldrich Corp., St. Louis, MO, United States) and 5 U/mL DNase1 (Sigma‒Aldrich Corp). The suspensions were centrifuged, and the pellets were cleaned. Lamina propria lymphocytes (LPLs) were isolated from lamina propria (LP) cell preparations by centrifugation with a 45–66.6% discontinuous Percoll (Solarbio) gradient at 2500 rpm for 20 min.
In an atmosphere containing 5% CO2, 96-well plates coated with anti-CD3 (10 µg/mL e-Bioscience, San Diego, CA, United States) and soluble anti-CD28 (1 µg/mL, e-Bioscience) monoclonal antibodies (mAbs) were used to culture LPLs (1 × 105/well in 0.2 mL of RPMI 1640 medium containing 10% fetal bovine serum, 1% penicillin, and 1% streptomycin) at 37 °C for 48 h. The supernatants were collected, and the cytokines and myeloperoxidase (MPO) were assessed by enzyme-linked immunosorbent assay after 48 h.28
Enzyme-Linked Immunosorbent Assay
The cell culture supernatants were collected after centrifugation at 1000 rpm for 10 min according to the manufacturer’s instructions. Mouse immunoassay kits (R&D Systems Inc., Minneapolis, MN, United States) were used to measure cytokine concentrations. MPO kits were used to measure the activity of MPO in the colon tissue. The levels of IL-6, IL-1β, TNF-α and MPO were measured in the supernatants. The levels of IFN-γ, IL-17A, IL-21, IL-22 and IL-10 were measured in the supernatants with or without anti-CD28/anti-CD3 mAbs stimulation.28
Determination of Selenium in Serum and Colon Tissue
Fluorescence atomic absorption spectrometry was used to determine the selenium content in colon tissue. The concentrations of serum selenium were detected in duplicate by inductively coupled plasma‒mass spectrometry (ICP‒MS, Perkin-Elmer SCIEX ElAN 6000, US).21
DNA Extraction and Amplification
Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China) performed DNA extraction, sequencing and analysis. The sample microbial DNA was extracted using the OMEGA Soil DNA Kit (M5635-02) (Omega Bio Tek, Norcross, GA, USA) according to the instructions. The total DNA quantity and quality were tested by a NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and 0.8% agarose gel electrophoresis respectively. The forward primer 338F (5’-ACTCCTACGGGAGGCAGCA-3’) and the reverse primer 806R (5’-GGACTACHVGGGTWTCTAAT-3’) were used to perform PCR amplification of the bacterial 16S rRNA genes V3–V4 region. PCR amplicons were purified by Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China) and quantified by the Quantit PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA).
DNA Sequencing and Analysis
QIIME2 2019.429 was used to perform microbiome bioinformatics with slight modification according to the official tutorials (https://docs.qiime2.org/2019.4/tutorials/). In brief, raw sequence data were demultiplexed using the demux plugin followed by primer cutting with the cutadapt plugin.30 Sequences were quality filtered, denoised, merged and chimera removed using the DADA2 plugin then.31 Taxonomy was assigned to non-singleton amplicon sequence variants (ASVs) using the classify-sklearn naïve Bayes taxonomy classifier in the feature-classifier plugin32 against SILVA Release 132.
Sequence data analyses were mainly performed using QIIME2 and R packages (v3.2.0). ASV-level alpha diversity indices, such as observed species, Chao 1 richness estimator, Shannon diversity index, Faith’s PD, Simpson index, Good’s coverage and Pielou’s evenness were calculated using the ASV table in QIIME2 and visualized as box plots. Beta diversity analysis was performed to investigate the structural variation in microbial communities across samples using Bray‒Curtis metrics33 and visualized via principal coordinate analysis (PCoA). The significance of differentiation of microbiota structure among groups was assessed by permutational multivariate analysis of variance (PERMANOVA)34 using QIIME2. Linear discriminant analysis effect size (LEfSe) was performed to detect differentially abundant taxa across groups using the default parameters.35 The correlation analysis was performed by the genescloud tools, a free online platform for data analysis (https://www.genescloud.cn).
Determination of SCFAs
The large intestinal content samples were placed into 2 mL EP tubes, extracted with 0.5 mL of distilled water, and vortexed for 10s. The samples were incubated in ice water, homogenized in a ball mill at 45 Hz for 4 min and then treated with ultrasound for 5 min. The samples were centrifuged at 4 °C at 12,000 rpm for 15 min. Then, 0.3 mL of supernatant was transferred into fresh 2 mL EP tubes, and the previous steps were repeated. A total of 0.8 mL of supernatant was collected. Then, 0.1 mL of 50% H2SO4 and 0.5 mL of 2-methylvaleric acid were added to the supernatant. The mixture was vortexed for 1 minute and centrifuged at 12,000 rpm for 15 min at 4 °C. Agilent 7890 gas chromatography coupled with an Agilent 7000D mass spectrometer (Agilent Technologies, Wilmington, DE, USA) was used for analysis. The system used an HP-FFAP capillary column. In split mode (5:1), a 1 μL aliquot of the analyte was injected. Helium was the carrier gas. The purge flow rate at the front inlet was 3 mL/min, and the gas flow rate was 1 mL/min. The initial temperature was 80 °C for 1 minute, raised to 150 °C at a speed of 5 °C/min, and then raised to 230 °C at a speed of 40 °C/min kept for 12 minutes. The temperatures of the injection port, transfer line, quadrupole and ion source were 240°C, 240°C, 150°C and 230°C, respectively. In the electron impact mode, the energy was −70 eV. After a solvent delay of 5 minutes, the mass spectrum data were recorded in full scan mode with a m/z range of 33–200.
Data Analysis
The data are expressed as the mean ± standard error. The Shapiro‒Wilk test was used for normality analysis. If the data conformed to normal distribution and homogeneity of variance, analysis of variance or t-test was used. If the data conformed to normal distribution and heterogeneity of variance, the Welch test or t- test was used. If the data did not conform to normal distribution, nonparametric test was used. DAI score was analyzed by generalized estimating equation (GEE). The difference was statistically significant when P<0.05. For SCFAs, P<0.05 and fold change < 0.5 or>2 indicated statistical significance. SPSS version 22.0 (SPSS, Inc., Chicago, IL, United States) was used for data analysis, and GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, United States) was used for drawing.
Result
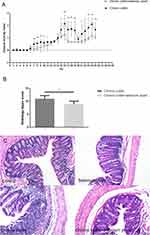
Selenium Yeast Could Alleviate DSS-Induced Chronic Colitis in Mice
The effect of selenium yeast on DSS-induced chronic colitis in mice was evaluated by comparing the difference between the chronic colitis group and the chronic colitis+selenium yeast group. The DAI score of the chronic colitis+selenium yeast group was significantly lower than that of the chronic colitis group on the 16th, 17th, 18th, 19th, 24th and 25th days (P<0.05). The histological injury score in the chronic colitis+selenium yeast group was significantly lower than that in the chronic colitis group (P<0.05) (Figure 1A–C).
Selenium Yeast Can Regulate Cytokine, MPO and Selenium Concentrations
The concentrations of cytokines detected by ELISA showed that IL-1β, IL-6, and TNF-α increased significantly in the chronic colitis group (P<0.001). Selenium yeast could significantly decrease the concentration of these cytokines (P<0.001). Compared with the control group, the activity of MPO in the chronic colitis group increased significantly, and selenium yeast significantly reduced the activity of MPO (P<0.05). Compared with the control group, in the supernatant without CD3/CD28 stimulation, IL-10, IL-17A, IL-21, IFN-γ, and IL-22 significantly increased in the chronic colitis group (P<0.01). Selenium yeast significantly decreased IFN-γ, IL-22 and IL-17A (P<0.05). Compared with the control group, in the supernatant stimulated by CD3/CD28, IL-10, IL-17A, IL-21, IFN-γ, and IL-22 increased significantly in the chronic colitis group (P<0.01). Selenium yeast significantly decreased IFN-γ, IL-22 and IL-17A (P<0.01). Compared with the control group, the serum selenium and colon tissue selenium in the chronic colitis group decreased significantly (P<0.01), while selenium yeast significantly elevated the serum selenium (P<0.01) (Figure 2A–D).
Selenium Yeast Could Regulate Gut Microbiota
The relative abundance of gut microbiota in different groups was different at the phylum level (Figure 3A). There was a significant difference in Faith’s PD between the control group and the chronic colitis group (P<0.05), and there was no significant difference in the Chao 1 index, Shannon index, Good’s coverage index, Simpson index, Pielou’s evenness index, and observed species (P>0.05) (Figure 3B). There was a significant difference in β diversity between the control group and the chronic colitis group (P<0.05). There was a significant difference in β diversity between the selenium yeast+chronic colitis group and the chronic colitis group (P<0.05) (Figure 3C and D).
At the phylum level, compared with the control group, Firmicutes in the chronic colitis group decreased significantly (P<0.05). Compared with the chronic colitis group, Firmicutes in the selenium yeast+chronic colitis group increased significantly (P<0.05). At the genus level, compared with the control group, Lactobacillus in the chronic colitis group decreased significantly (P<0.05), while Bacteroides, Akkermansia, Turicibacter, and Sutterella increased significantly (P<0.05). Compared with the chronic colitis group, Turicibacter, Bifidobacterium, Allobaculum, Prevotella, Halomonas, and Adlercreutzia increased significantly in the selenium yeast+chronic colitis group (P<0.05) (Figure 4A–D).
Selenium Yeast Could Regulate SCFAs, Metabolites of the Gut Microbiota
Selenium yeast could regulate SCFAs. Compared with the control group, butyric acid in the chronic colitis group decreased significantly (P<0.05), while selenium yeast significantly increased the amount of butyric acid in the intestine of mice with DSS-induced chronic colitis (P<0.05) (Figure 5).
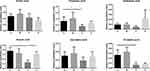 |
Figure 5 Relative quantitative value of SCFAs between different groups (*P<0.05). |
Correlation Analysis
To evaluate whether there are correlations between gut microbiota, cytokines and SCFAs, we conducted a correlation analysis. At the phylum level, Firmicutes was negatively correlated with INF-γ, IL-10, IL-17A, IL-21, IL-22, IL-1β, IL-6, TNF-α, and MPO. At the genus level, Akkermansia was positively correlated with IFN-γ, IL-10, IL-21, IL-1β and IL-6. Bacteroides and Turicibacter were positively correlated with INF-γ, IL-10, IL-17A, IL-21, IL-22, IL-1β, IL-6, and TNF-α. Butyric acid was negatively correlated with INF-γ, IL-17A, IL-21, IL-22, IL-1β, IL-6, TNF-α, and MPO. There were negative correlations between Bacteroides and acidic acid, N-valid acid, propionic acid, and butyric acid, while there were negative correlations between Turicibacter and acidic acid and N-valid acid, as shown in Figure 6.
Discussion
The protective effect of inorganic selenium, such as sodium selenite, on acute DSS-induced colitis has been reported.21 Selenium-containing amino acids could alleviate DSS-induced IBD in mice by reducing oxidative stress and the inflammatory response.36 Organic selenium can be better absorbed and utilized than inorganic selenium.24,27 Our research found that selenium yeast has a therapeutic effect on DSS-induced chronic colitis by increasing anti-inflammatory factors, reducing proinflammatory factors, and regulating gut microbiota.
The phylum Firmicutes in the intestine of patients with IBD decreased.37 In our study, we found that the phylum Firmicutes decreased in mice with DSS-induced chronic colitis, while selenium yeast increased intestinal Firmicutes. In addition, one study found that the abundance of Akkermansia increased in mice with DSS-induced acute colitis,38 which is consistent with our findings. However, a systematic review found that the abundance of Akkermansia in UC patients decreased,39 and some studies showed that polyphenol-rich cranberry extract and chlorogenic acid could alleviate colitis by increasing Akkermansia abundance.40,41 Another study reported that Akkermansia is positively correlated with IL-10,42 and IL-10 is an important anti-inflammatory factor.43 Mice with DSS-induced acute colitis had an increase in the abundance of Turicibacter.44 Our research also found that the abundance of Turicibacter increased in mice with DSS-induced chronic colitis. Interestingly, our research also found that selenium yeast can increase the abundance of Turicibacter. It has been reported that selenium has a regulatory effect on gut microbiota and can increase the relative abundance of Turicibacter, which has an anti-inflammatory effect.45 However, Rossi et al reported a decrease in the abundance of Turicibacter in the intestine of dogs with IBD.46 These findings showed that the increase in Turicibacter in chronic colitis might be a response to alleviate inflammation and that Turicibacter might have a protective effect on IBD, which requires further research.
Our research also found that selenium yeast can increase Allobaculum. It has been reported that Allobaculum is positively correlated with tight junction (TJ) protein and negatively correlated with DAI score and histology score.47 The TJ protein plays an important role in the intestinal barrier.48 Moreover, Allobaculum can metabolize tryptophan, produce aryl hydrocarbon receptor (AHR) ligands, activate AHR, produce IL-22, and alleviate intestinal inflammation.49,50
Our research also showed that selenium yeast can increase the abundance of Bifidobacterium. One study pointed out that Bifidobacterium strains inhibit inflammation by regulating the inflammatory pathway and could be used as a supplementary treatment for IBD.51 The increase in Bifidobacterium was related to the success of anti-TNF-α treatment.52 However, some studies have different opinions. The abundance of Bifidobacterium increased in UC patients, and the abundance in active UC was higher than that in UC in continuous remission.53 It has also been reported that there was no significant difference in the abundance of Bifidobacterium between UC patients and healthy people.54 Meanwhile, our research found that selenium yeast increased the abundance of Prevotella. It has been found that Prevotella is abundant in UC patients with continuous remission.53 However, another study found that there is no significant difference in the abundance of Prevotella between UC patients and healthy people.54 These opposite findings indicate that the gut microbiota might play a complex and dynamic role in the occurrence and development of IBD, which needs further research.
SCFAs are metabolites of gut microbiota, including acetate, propionate, butyrate, pentanoic (valuable) acid, and hexanoic (caproic) acid.55 Butyric acid-producing bacteria mainly include Lachnospiraceae, Ruminococcaceae and some Bacteroides.56 Butyric acid is the energy source of colon epithelial cells.57 Our study found that butyric acid in the intestine of mice with DSS-induced chronic colitis decreased significantly (P<0.05). SCFAs in the intestine of IBD patients are different from those in normal people.58 The metabolism of butyric acid in UC patients was abnormal, and butyric acid intake and oxidation decreased.59 It has been reported that butyric acid decreases in active UC and increases in resting UC.60 Butyrate can promote the production of IL-10,61 inhibit the function of neutrophils, and alleviate the intestinal inflammation of mice with DSS-induced acute colitis.62 IL-10 plays an important role in regulating intestinal homeostasis.63 In addition, butyric acid could also regulate the repair of the intestinal mucus barrier.64 Our study found that selenium yeast can significantly increase the content of butyric acid, which may be related to the effect of selenium yeast in alleviating chronic colitis.
Selenium yeast also has an effect on cytokines. Studies have reported changes in cytokines in IBD. TNF-α, IFN-γ, and IL-1β were elevated in patients with IBD.65 Serum IL-17A was increased in active UC children when compared to those in remission.66 We found that selenium yeast could decrease the TNF-α concentration. TNF-α plays an important role in the pathogenesis of IBD, and anti-TNF-α treatment reduces the production of proinflammatory factors by neutrophils.67 Infliximab, an anti-TNF-α drug, has been widely used in the treatment of IBD. Neutralization of IL-6 and TNF-α decreased intestinal permeability and relieved DSS-induced acute colitis.68 A clinical study found that an IL-6 antagonist had effects on patients with moderate to severe CD who experienced anti-TNF-α treatment failure.69 IL-6 is an important proinflammatory factor.70
IL-1β plays an important role in the occurrence of intestinal inflammation. One study found that IL-1β can recruit immune cells (innate immune cells and CD4+ T cells) that secrete IL-17A.71 IL-1β can also increase intestinal permeability and then promote the occurrence and development of intestinal inflammation.72 Another study found that IL-1β upregulated MIR200c-3p, degraded occludin mRNA, and increased intestinal mucosal permeability.73 In addition, IL-1β can activate mitogen-activated protein kinase kinase kinase MEKK-1, thereby activating the canonical NF-κB pathway and increasing the permeability of Caco-2 intestinal epithelial TJs.74 IFN-γ can also affect the proliferation and apoptosis of intestinal epithelial cells and aggravate intestinal inflammation.75 IFN-γ can induce chemokine C-X-C motif ligand 10 (CXCL10).76 CXCL10 can promote the development of colitis.77 In addition, IFN-γ can also destroy the adherens junction protein vascular endothelial cadherin (VE-cadherin), damage the intestinal vascular barrier, and promote inflammation.78
Our study found that in mice with DSS-induced chronic colitis, IL-22 increased and selenium yeast significantly reduced IL-22. Different clinical studies have reported different changes in IL-22 in the serum of IBD patients. It has been reported that IL-22 in the serum of patients with active IBD was elevated.79 However, another study found that serum IL-22 in IBD patients was reduced compared with the control group.80 IL-22 has complex dual effects on intestinal homeostasis.81 On the one hand, IL-22 can promote endoplasmic reticulum stress in intestinal epithelial cells, thereby aggravating intestinal inflammation.82 In addition, IL-22 can promote intestinal fibrosis.83 IL-22 is also related to the nonresponse to ustekinumab in UC patients.84 On the other hand, IL-22 could enhance the integrity of the mucus barrier and promote the regeneration of intestinal epithelial cells.85 Similar to IL-22, IL-17A has both protective and pathogenic effects in the intestine,70 although our study found that selenium yeast can reduce the concentration of IL-17A. It has been reported that IL-17A plays an important role in DSS-induced acute colitis.86 Moreover, IL-17A can enhance the role of IL-22 in promoting endoplasmic reticulum stress in intestinal epithelial cells, thereby aggravating inflammation.82 IL-17A also has a protective effect on intestinal epithelial barrier function.87 Meanwhile, a clinical study found that anti-IL-17A did not contribute to the remission of CD.88 Therefore, the role of IL-17A and IL-22 in IBD needs further evaluation.
MPO is a kind of peroxidase containing hemoglobin, which is mainly expressed in neutrophils and less expressed in monocytes.89 Our study found that MPO increased in the chronic colitis group. Compared with normal people, MPO in the stool of patients with IBD increased.90 It has been found that fecal MPO can be used to assess the disease activity of UC and its response to treatment.91
Conclusion
In general, selenium yeast alleviated DSS-induced chronic colitis in mice by reducing proinflammatory factors and regulating the gut microbiota and metabolites. Cytokines, gut microbiota and their metabolites, such as SCFAs, play an important role in IBD. Drugs for these targets have been applied in treating IBD. In addition, selenium yeast is a common selenium supplement that is convenient to access. Therefore, selenium yeast might be a promising drug in the treatment of IBD.
Funding
Applied Basic Research Program of Liaoning Provincial Department of Science and Technology (2022JH2/101500063).
Disclosure
Zeyu Wu and Yan Li are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi:10.1146/annurev-immunol-030409-101225
2. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91–99. doi:10.3748/wjg.v20.i1.91
3. Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019:7247238. doi:10.1155/2019/7247238
4. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–237. doi:10.1038/s41575-019-0258-z
5. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–342. doi:10.1038/nri3661
6. Neurath MF. Targeting cytokines in inflammatory bowel disease. Sci Transl Med. 2022;14(675):eabq4473. doi:10.1126/scitranslmed.abq4473
7. Marafini I, Sedda S, Dinallo V, et al. Inflammatory cytokines: from discoveries to therapies in IBD. Expert Opin Biol Ther. 2019;19(11):1207–1217. doi:10.1080/14712598.2019.1652267
8. Wang W, Chen L, Zhou R, et al. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52(2):398–406. doi:10.1128/JCM.01500-13
9. Pascal V, Pozuelo M, Borruel N, et al. A microbial signature for Crohn’s disease. Gut. 2017;66(5):813–822. doi:10.1136/gutjnl-2016-313235
10. Busquets D, Oliver L, Amoedo J, et al. RAID prediction: pilot study of fecal microbial signature with capacity to predict response to Anti-TNF treatment. Inflamm Bowel Dis. 2021;27(Suppl 2):S63–S66. doi:10.1093/ibd/izab273
11. Hedin CR, van der Gast CJ, Stagg AJ, et al. The gut microbiota of siblings offers insights into microbial pathogenesis of inflammatory bowel disease. Gut Microbes. 2017;8(4):359–365. doi:10.1080/19490976.2017.1284733
12. Yang W, Yu T, Huang X, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):4457. doi:10.1038/s41467-020-18262-6
13. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–573. doi:10.1126/science.1241165
14. Avery JC, Hoffmann PR. Selenium, selenoproteins, and immunity. Nutrients. 2018;10(9):1203. doi:10.3390/nu10091203
15. Fairweather-Tait SJ, Bao Y, Broadley MR, et al. Selenium in human health and disease. Antioxid Redox Signal. 2011;14(7):1337–1383. doi:10.1089/ars.2010.3275
16. Jarmakiewicz-Czaja S, Piątek D, Filip R. The influence of nutrients on inflammatory bowel diseases. J Nutr Metab. 2020;2020:2894169. doi:10.1155/2020/2894169
17. Duntas LH, Benvenga S. Selenium: an element for life. Endocrine. 2015;48(3):756–775. doi:10.1007/s12020-014-0477-6
18. Kaur R, Thakur S, Rastogi P, et al. Resolution of Cox mediated inflammation by Se supplementation in mouse experimental model of colitis. PLoS One. 2018;13(7):e0201356. doi:10.1371/journal.pone.0201356
19. Huang LJ, Mao XT, Li YY, et al. Multiomics analyses reveal a critical role of selenium in controlling T cell differentiation in Crohn’s disease. Immunity. 2021;54(8):1728–1744.e7. doi:10.1016/j.immuni.2021.07.004
20. Kasaikina MV, Kravtsova MA, Lee BC, et al. Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. FASEB J. 2011;25(7):2492–2499. doi:10.1096/fj.11-181990
21. Sang L, Chang B, Zhu J, et al. Dextran sulfate sodium-induced acute experimental colitis in C57BL/6 mice is mitigated by selenium. Int Immunopharmacol. 2016;39:359–368. doi:10.1016/j.intimp.2016.07.034
22. Ishihara J, Arai K, Kudo T, et al. Serum zinc and selenium in children with inflammatory bowel disease: a Multicenter Study in Japan. Dig Dis Sci. 2022;67(6):2485–2491. doi:10.1007/s10620-021-07078-z
23. Han YM, Yoon H, Lim S, et al. Risk factors for vitamin D, zinc, and selenium deficiencies in Korean patients with inflammatory bowel disease. Gut Liver. 2017;11(3):363–369. doi:10.5009/gnl16333
24. Zhou Y, Zhu H, Qi Y, et al. Absorption and distribution of selenium following oral administration of selenium-enriched bifidobacterium longum DD98, selenized yeast, or sodium selenite in rats. Biol Trace Elem Res. 2020;197(2):599–605. doi:10.1007/s12011-019-02011-y
25. Rayman MP, Infante HG, Sargent M. Food-chain selenium and human health: spotlight on speciation. Br J Nutr. 2008;100(2):238–253. doi:10.1017/S0007114508922522
26. Schrauzer GN. Nutritional selenium supplements: product types, quality, and safety. J Am Coll Nutr. 2001;20(1):1–4. doi:10.1080/07315724.2001.10719007
27. Zhang SQ, Shen S, Zhang Y. Comparison of bioavailability, pharmacokinetics, and biotransformation of selenium-enriched yeast and sodium selenite in rats using plasma selenium and selenomethionine. Biol Trace Elem Res. 2020;196(2):512–516. doi:10.1007/s12011-019-01935-9
28. Sang LX, Chang B, Zhu JF, et al. Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and γδT and increasing CD4(+)CD25(+) regulatory T-cell responses. World J Gastroenterol. 2017;23(21):3850–3863. doi:10.3748/wjg.v23.i21.3850
29. Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi:10.1038/s41587-019-0209-9
30. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. 2011;17(1). doi:10.14806/ej.17.1.200
31. Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi:10.1038/nmeth.3869
32. Bokulich NA, Subramanian S, Faith JJ, et al. Quality-filtering vastly improves diversity estimates from illumina amplicon sequencing. Nat Methods. 2013;10(1):57–59. doi:10.1038/nmeth.2276
33. Bray JR, Curtis JT. An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr. 1957;27(4):325–349. doi:10.2307/1942268
34. McArdle BH, Anderson MJ Fitting multivariate models to community data: a comment on distanceâbased redundancy analysis. Ecology. 2001;82(1):290–297. doi:10.2307/2680104
35. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi:10.1186/gb-2011-12-6-r60
36. Shi C, Yue F, Shi F, et al. Selenium-containing amino acids protect dextran sulfate sodium-induced colitis via ameliorating oxidative stress and intestinal inflammation. J Inflamm Res. 2021;14:85–95. doi:10.2147/JIR.S288412
37. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol. 2015;37(1):47–55. doi:10.1007/s00281-014-0454-4
38. Berry D, Schwab C, Milinovich G, et al. Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012;6(11):2091–2106. doi:10.1038/ismej.2012.39
39. Pittayanon R, Lau JT, Leontiadis GI, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology. 2020;158(4):930–946.e1. doi:10.1053/j.gastro.2019.11.294
40. Zhang Z, Wu X, Cao S, et al. Chlorogenic acid ameliorates experimental colitis by promoting growth of akkermansia in mice. Nutrients. 2017;9(7):677. doi:10.3390/nu9070677
41. Anhê FF, Roy D, Pilon G, et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872–883. doi:10.1136/gutjnl-2014-307142
42. Bian X, Wu W, Yang L, et al. Administration of Akkermansia muciniphila ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2019;10:2259. doi:10.3389/fmicb.2019.02259
43. Ouyang W, Rutz S, Crellin NK, et al. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29(1):71–109. doi:10.1146/annurev-immunol-031210-101312
44. Liu A, Lv H, Wang H, et al. Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. J Gerontol a Biol Sci Med Sci. 2020;75(7):1284–1292. doi:10.1093/gerona/glz263
45. Zhai Q, Cen S, Li P, et al. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ Sci Technol Lett. 2018;5(12):724–730. doi:10.1021/acs.estlett.8b00563
46. Rossi G, Pengo G, Caldin M, et al. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One. 2014;9(4):e94699. doi:10.1371/journal.pone.0094699
47. Chen Y, Yang B, Stanton C, et al. Bifidobacterium pseudocatenulatum ameliorates DSS-induced colitis by maintaining intestinal mechanical barrier, blocking proinflammatory cytokines, inhibiting TLR4/NF-κB signaling, and altering gut microbiota. J Agric Food Chem. 2021;69(5):1496–1512. doi:10.1021/acs.jafc.0c06329
48. Mehandru S, Colombel J-F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18(2):83–84. doi:10.1038/s41575-020-00399-w
49. Marsland BJ. Regulating inflammation with microbial metabolites. Nat Med. 2016;22(6):581–583. doi:10.1038/nm.4117
50. Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi:10.1038/nm.4102
51. Aghamohammad S, Sepehr A, Miri ST, et al. The potential role of Bifidobacterium spp. as a preventive and therapeutic agent in controlling inflammation via affecting inflammatory signalling pathways. Lett Appl Microbiol. 2022;75(5):1254–1263. doi:10.1111/lam.13793
52. Yilmaz B, Juillerat P, Øyås O, et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat Med. 2019;25(2):323–336. doi:10.1038/s41591-018-0308-z
53. Kitae H, Takagi T, Naito Y, et al. Gut microbiota associated with clinical relapse in patients with quiescent ulcerative colitis. Microorganisms. 2022;10(5):1044. doi:10.3390/microorganisms10051044
54. Ahmed EA, Ahmed SM, Zakaria NH, et al. Study of the gut microbiome in Egyptian patients with active ulcerative colitis. Rev Gastroenterol Mex. 2023;88(3):246–255. doi:10.1016/j.rgmxen.2022.07.006
55. Tan J, McKenzie C, Potamitis M, et al. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. doi:10.1016/B978-0-12-800100-4.00003-9
56. Vital M, Karch A, Pieper DH, Shade A. Colonic butyrate-producing communities in humans: an overview using omics data. mSystems. 2017;2(6):e00130–17. doi:10.1128/mSystems.00130-17
57. Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. doi:10.1016/j.cmet.2011.02.018
58. Zhuang X, Li T, Li M, et al. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(11):1751–1763. doi:10.1093/ibd/izz188
59. De Preter V, Arijs I, Windey K, et al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. 2012;18(6):1127–1136. doi:10.1002/ibd.21894
60. Xu HM, Zhao HL, Guo GJ, et al. Characterization of short-chain fatty acids in patients with ulcerative colitis: a meta-analysis. BMC Gastroenterol. 2022;22(1):117. doi:10.1186/s12876-022-02191-3
61. Chen L, Sun M, Wu W, et al. Microbiota metabolite butyrate differentially regulates Th1 and Th17 cells’ differentiation and function in induction of colitis. Inflamm Bowel Dis. 2019;25(9):1450–1461. doi:10.1093/ibd/izz046
62. Li G, Lin J, Zhang C, et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. 2021;13(1):1968257. doi:10.1080/19490976.2021.1968257
63. Neumann C, Scheffold A, Rutz S. Functions and regulation of T cell-derived interleukin-10. Semin Immunol. 2019;44:101344. doi:10.1016/j.smim.2019.101344
64. Liang L, Liu L, Zhou W, et al. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin Sci. 2022;136(4):291–307. doi:10.1042/CS20210778
65. Singh UP, Singh NP, Murphy EA, et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine. 2016;77:44–49. doi:10.1016/j.cyto.2015.10.008
66. Krawiec P, Pac-Kożuchowska E. Serum interleukin 17A and interleukin 17F in children with inflammatory bowel disease. Sci Rep. 2020;10(1):12617. doi:10.1038/s41598-020-69567-x
67. Zhang C, Shu W, Zhou G, et al. Anti-TNF-α therapy suppresses proinflammatory activities of mucosal neutrophils in inflammatory bowel disease. Mediators Inflamm. 2018;2018:3021863. doi:10.1155/2018/3021863
68. Xiao YT, Yan WH, Cao Y, et al. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine. 2016;83:189–192. doi:10.1016/j.cyto.2016.04.012
69. Danese S, Vermeire S, Hellstern P, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut. 2019;68(1):40–48. doi:10.1136/gutjnl-2017-314562
70. Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50(4):992–1006. doi:10.1016/j.immuni.2019.03.017
71. Coccia M, Harrison OJ, Schiering C, et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209(9):1595–1609. doi:10.1084/jem.20111453
72. Kaminsky LW, Al-Sadi R, Ma TY. IL-1β and the intestinal epithelial tight junction barrier. Front Immunol. 2021;12:767456. doi:10.3389/fimmu.2021.767456
73. Rawat M, Nighot M, Al-Sadi R, et al. IL1B increases intestinal tight junction permeability by up-regulation of MIR200C-3p, which degrades occludin mRNA. Gastroenterology. 2020;159(4):1375–1389. doi:10.1053/j.gastro.2020.06.038
74. Al-Sadi R, Ye D, Said HM, et al. IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NF-kappaB pathway. Am J Pathol. 2010;177(5):2310–2322. doi:10.2353/ajpath.2010.100371
75. Nava P, Koch S, Laukoetter MG, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32(3):392–402. doi:10.1016/j.immuni.2010.03.001
76. Walrath T, Malizia RA, Zhu X, et al. IFN-γ and IL-17A regulate intestinal crypt production of CXCL10 in the healthy and inflamed colon. Am J Physiol Gastrointest Liver Physiol. 2020;318(3):G479–G489. doi:10.1152/ajpgi.00208.2019
77. Hyun JG, Lee G, Brown JB, et al. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11(9):799–805. doi:10.1097/01.mib.0000178263.34099.89
78. Langer V, Vivi E, Regensburger D, et al. IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J Clin Invest. 2019;129(11):4691–4707. doi:10.1172/JCI124884
79. Nikolaus S, Schulte B, Al-Massad N, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153(6):1504–1516.e2. doi:10.1053/j.gastro.2017.08.028
80. Sakemi R, Mitsuyama K, Morita M, et al. Altered serum profile of the interleukin-22 system in inflammatory bowel disease. Cytokine. 2020;136:155264. doi:10.1016/j.cyto.2020.155264
81. Shohan M, Dehghani R, Khodadadi A, et al. Interleukin-22 and intestinal homeostasis: protective or destructive? IUBMB Life. 2020;72(8):1585–1602. doi:10.1002/iub.2295
82. Powell N, Pantazi E, Pavlidis P, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut. 2020;69(3):578–590. doi:10.1136/gutjnl-2019-318483
83. Mathur R, Alam MM, Zhao XF, et al. Induction of autophagy in Cx3cr1+ mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol. 2019;12(3):612–623. doi:10.1038/s41385-019-0146-4
84. Pavlidis P, Tsakmaki A, Pantazi E, et al. Interleukin-22 regulates neutrophil recruitment in ulcerative colitis and is associated with resistance to ustekinumab therapy. Nat Commun. 2022;13(1):5820. doi:10.1038/s41467-022-33331-8
85. Mizoguchi A, Yano A, Himuro H, et al. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53(4):465–474. doi:10.1007/s00535-017-1401-7
86. Ito R, Kita M, Shin-Ya M, et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377(1):12–16. doi:10.1016/j.bbrc.2008.09.019
87. Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43(4):727–738. doi:10.1016/j.immuni.2015.09.003
88. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. doi:10.1136/gutjnl-2011-301668
89. Aratani Y. Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys. 2018;640:47–52. doi:10.1016/j.abb.2018.01.004
90. Li Z, Long Y, Bai M, et al. Neutrophil and eosinophil granule proteins as potential biomarkers of assessing disease activity and severity in patients with ulcerative colitis. J Clin Lab Anal. 2016;30(5):776–778. doi:10.1002/jcla.21937
91. Masoodi I, Kochhar R, Dutta U, et al. Evaluation of fecal myeloperoxidase as a biomarker of disease activity and severity in ulcerative colitis. Dig Dis Sci. 2012;57(5):1336–1340. doi:10.1007/s10620-012-2027-5
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.