Back to Journals » Pathology and Laboratory Medicine International » Volume 15
Serum Lipids, Insulin-Like Growth Factor Binding Protein-3 and Treatment Outcomes in Women with and without Cervical Lesions in South Western Uganda: A Cohort Study
Authors Ssedyabane F , Randall TC, Tusubira D, Castro CM, Najjuma JN, Okeny C , Nuwashaba D , Mudondo H, Kajabwangu R , Muhumuza J, Namuli A, Ngonzi J
Received 8 November 2023
Accepted for publication 22 December 2023
Published 29 December 2023 Volume 2023:15 Pages 91—105
DOI https://doi.org/10.2147/PLMI.S447545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Paul Zhang
Frank Ssedyabane,1 Thomas C Randall,2 Deusdedit Tusubira,3 Cesar M Castro,4,5 Josephine Nambi Najjuma,6 Christopher Okeny,1 Doreen Nuwashaba,1 Hope Mudondo,1 Rogers Kajabwangu,7 Joy Muhumuza,7 Alexcer Namuli,7 Joseph Ngonzi7
1Department of Medical Laboratory Science, Mbarara University of Science of Science and Technology, Mbarara, Uganda; 2Department of Global Health and Social Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 3Department of Biochemistry, Mbarara University of Science of Science and Technology, Mbarara, Uganda; 4Centre for Systems Biology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 5Cancer Center, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 6Department of Nursing, Mbarara University of Science of Science and Technology, Mbarara, Uganda; 7Department of Obstetrics and Gynecology, Mbarara University of Science of Science and Technology, Mbarara, Uganda
Correspondence: Frank Ssedyabane, Email [email protected]
Purpose: There is a documented association between cervical cancer and metabolic syndrome. In this study, we determined the association between lipids, insulin-like growth factor binding protein-3 (IGFBP-3) and treatment outcomes for cervical lesions at Mbarara Regional Referral Hospital (MRRH) in south western Uganda.
Patients and Methods: In this prospective cohort study, we recruited 94 cases and 94 controls at the cervical cancer clinic of MRRH, and followed them for 12 months. Cases were confirmed with cytology and/or histology. With consent, we collected demographic data and measured lipids and IGFBP-3 both at baseline and after 12 months. The lipid profile was measured using a Cobas 6000 Chemistry Analyzer, whereas IGFBP-3 was measured using a MAGLUMI fully automated chemiluminescence immunoassay analyzer. Abnormal values for lipids were defined using WHO recommended cut-offs. IGFBP-3 concentrations were divided into two categories of low concentration (< 3.29 μg/mL) and raised concentration (≥ 3.29 μg/mL). Statistical analyses were conducted in STATA version 17 using logistic regression analysis. A p-value of < 0.05 was taken to be statistically significant.
Results: The average (mean ± SD) age of our participants was 38.7± 8.2 years for controls and 34.5± 7.8 years for cases. The average serum IGFBP-3 concentration was 3.769± 1.098 μg/mL among cases with cleared lesions and 3.505± 0.979 μg/mL among cases whose lesions persisted. A serum IGFBP-3 concentration of less than 3.291 μg/mL was likely to be associated with clearance of cervical lesions (AOR 1.65, p=0.67), although this was not statistically significant. A serum triglyceride concentration of 35– 135 mg/dL was also likely to be associated with clearance of cervical lesions (AOR 2.41, p=0.46), although this was also not statistically significant.
Conclusion: Although not statistically significant, reduced serum concentrations of IGFBP-3 and triglyceride may be associated with clearance of cervical lesions. Lipid management may be of benefit in the treatment of cervical lesions.
Keywords: cervical intraepithelial neoplasia, cervical lesions, clearance, persistence, IGFBP-3
Background
By the year 2020, 770,828 incident cases of cervical cancer were being registered globally per year.1 Moreover, cervical cancer was reported to be the second most common of all cancers in women aged between 15 and 44 years worldwide.1,2 Cervical cancer accounts for more than 270,000 deaths annually. Worryingly, over 85% of these deaths occur in less developed countries,3,4 particularly in sub-Saharan Africa.5,6 In East Africa, there were over 43 new cervical cancer cases per 100,000 in the year 2014.7 Furthermore, the age-standardized incidence rate of cervical cancer in Uganda is said to be higher than global estimates (56.2 per 100,000 women).8
The cervical cancer screening program in Uganda employs a “screen and treat” approach, in which all those screened and found positive are immediately initiated onto a treatment pathway. Screening involves performing visual inspection with acetic acid (VIA) and Papanicolaou (Pap) smear cytological confirmation.9 For women who test negative on VIA or Pap smear cytology, the interval for rescreening recommended by the World Health Organization (WHO)10 ranges from 3 to 5 years. For women who test negative for human papilloma virus (HPV), screening should be repeated after 5 years. After repeated screening with negative results, even for older women, it is recommended that the screening interval can be prolonged to at least 5 years. The current treatment modalities for cervical cancer, including chemotherapy, radiotherapy, single-agent treatment, concurrent chemoradiotherapy, neoadjuvant chemotherapy before surgery and radiotherapy, combination treatment, and platinum and non-platinum-based therapies, are able to target the early stage of cervical cancer, advanced stages and recurrent cervical cancer, but the survival rates are highest for women with early-stage disease.11 After undergoing treatment for cervical precancerous lesions, women should receive post-treatment follow-up testing after 12 months, as recommended by the WHO.10 At Mbarara Regional Referral Hospital (MRRH), patients who receive treatment for either precancerous or cancerous cervical lesions are asked to return for a post-treatment review visit after 6 weeks. They are also asked to return for a follow-up visit at 12 months post-treatment, although there are no published data on whether these women actually return.
Several factors play multiple roles in HPV infection, its persistence, the development of cervical lesions and their clearance or persistence following treatment.12 Lipid profiles in patients with cervical cancer and healthy patients differ during the development and pathogenesis of the disease.13 The different lipid profiling is therefore a useful tool in monitoring cervical cancer patients. In the early stages of the disease, total cholesterol, triglycerides and low-density lipoprotein (LDL) are usually significantly higher, whereas high-density lipoprotein (HDL) is significantly lower compared with the levels in healthy women.14 In cervical cancer, the expression and activity of insulin-like growth factor binding protein-3 (IGFBP-3(is often altered. Elevated levels of IGFBP-3 in cervical cancer cells can promote tumor growth by enhancing cell survival and proliferation. In addition, IGFBP-3 has been found to interact with various signaling pathways involved in cancer progression.15–20
Results from earlier studies indicate that reduced or increased levels of IGFBP-3 could be associated with cancers, especially those originating from epithelial and glandular tissues.21–24 Therefore, in this study our aim was to describe the association between lipids and IGFBP-3 and clearance or persistence cervical lesions among women attending and receiving care at the cervical cancer clinic of MRRH.
Materials and Methods
Study Design
This was a prospective cohort type of study, conducted from April 2022 to June 2023. Each patient was followed for 12 months. This cohort started with cases and respective unmatched controls. The cases were defined as those women with a positive VIA who were then confirmed, with either Pap smear cytology or histology, to have cervical intraepithelial neoplasia (CIN) (atypical squamous cells of undetermined significance [ASCUS], low-grade squamous intraepithelial lesion [LSIL] and high-grade squamous intraepithelial lesion [HSIL]). The controls were women who were negative for cervical intraepithelial lesions or malignancy. Our outcomes of interest were clearance or persistence of cervical intraepithelial lesions, while the exposures were serum concentrations of IGFP-3 and lipids.
Study Setting
We conducted this study among women attending the cervical cancer clinic of Mbarara Regional Referral Hospital, a referral hospital located in rural south western Uganda. This health facility serves a population of approximately four million people.25 This referral clinic receives an average of 300 women per month and operates five days every week. The clinic serves over 13 districts of the whole of south western Uganda plus nearby countries, including Rwanda, Burundi, Tanzania and the Democratic Republic of Congo. The clinic staff include several nursing staff, a gynecologist and senior residents. This team is supervised by a gynecological oncologist. Screening tests routinely done at the clinic include VIA, colposcopy, conventional cytology and HPV DNA. Women diagnosed with premalignant lesions are treated immediately with cryotherapy and thermocoagulation; those with confirmed cervical cancer undergo gynecological cancer surgery or are referred to a higher level health facility (Uganda Cancer Institute) for radiotherapy and chemotherapy, according to their cancer stage and clinical findings. Following treatment, the patients are advised to return for review visits at 6 weeks and 1 year.
Sampling Method
We recruited cases using a purposive sampling method. Thereafter, we used the incidence density sampling method to obtain a control each time a case was identified. This process went on prospectively until the required sample size was obtained.
Sample Size Determination
For the prospective cohort, we used a study power of 80%, a two-sided confidence level (1 − alpha) of 95%, and a case to control ratio of 1. We also considered a proportion of cases with metabolic syndrome of 40.99%26 and a proportion of controls with metabolic syndrome of 18.8%.27 These were fed into an online sample size calculator with the least extreme odds ratio of 3.0, a prevalence ratio of 2.2 and a prevalence difference of 22 (OpenEpi: Sample Size for X-Sectional, Cohort, and Clinical Trials). This gave a total of 76 cases and 76 controls after considering the module of Fleiss with continuity correction.28 Factoring in an expected 15% attrition rate, the resultant sample size came to a minimum of 86 cases and 86 controls.
Data Collection
Demographic Data Collection
We collected demographic data using a validated questionnaire which was administered by a trained research assistant, a midwife at the cervical cancer clinic. After receiving their routine care, participants who consented were helped by the research assistants to fill in the questionnaire. Thereafter, venous blood was drawn. Data collected included variables such as age, region of residence, family planning use and type, human immunodeficiency virus (HIV) status, highest level of education, marital status, and history of blood pressure and diabetes. The study’s principal investigator performed random daily supervisory checks on the procedures to ensure conformity to the study requirements and standards. This ensured validity as well as completeness of data.
Blood Collection
After provision of written informed consent and completion of the questionnaire, we collected 4 mL of venous blood aseptically by venipuncture from the mid-cubital vein, into plain Vacutainers (without anticoagulant). We labeled specimens with unique identification numbers (codes) and then allowed them to clot at room temperature, after which they were taken to the laboratory for centrifugation at 3000 rpm for 5 min, to separate serum from blood cells. The serum was then transferred into cryovial tubes using a micropipette.
Measurement and Interpretation of Serum Lipids
Non-fasting lipid concentrations were measured using a fully automated analyzer (Cobas 6000 Clinical Chemistry Analyzer; Roche Diagnostics International, Rotkreuz, Switzerland). All testing was performed following standard operating procedures. Serum lipid concentrations were interpreted as follows, using standard cut-off values: abnormal total cholesterol was defined as a concentration above 200 mg/dL, abnormal LDL was defined as a concentration above 100 mg/dL, abnormal triglyceride was defined as a concentration outside the range of 35–135 mg/dL and abnormal HDL was defined as a concentration outside the range of 35–55 mg/dL.
Measurement and Interpretation of Serum IGFBP-3 Concentration
IGFBP-3 was measured using MAGLUMI fully automated chemiluminescence immunoassay analyzer (MAGLUMI 2000; SNIBE Diagnostic, Shenzhen, China). The human IGFBP-3 solid-phase sandwich enzyme-linked immunosorbent assay (ELISA) is designed to quantitatively measure the target protein bound between a matched antibody pair. A target-specific antibody is precoated in the wells of the microplate. When samples, standards or controls are added into these wells, they bind to the immobilized antibody. The sandwich is then formed by the addition of the second antibody. When a substrate solution is added, it reacts with the enzyme–antibody-target complex, which eventually produces a measurable signal. The intensity of this signal is directly proportional to the concentration of target protein present in the specimen.
MAGLUMI kits and samples were thawed to room temperature, and serum samples were carefully transferred into sample tubes. Tubes were then loaded into sample racks and the fully automated testing was started. All this was done while strictly adhering to the operating instructions of the MAGLUMI fully automated chemiluminescence immunoassay analyzer. We ran samples along with quality control materials, which included Control 1 (lot 15622031Q1) and Control 2 (lot 15622031Q2). We created two categories of IGFBP-3 concentration, divided along the mean concentration in controls. The first category was named reduced concentration (<3.29 µg/mL) while the second category was named raised concentration (≥3.29 µg/mL).
Data Management and Analysis
The principal investigator collected the data together with research assistants. Data were entered into an Excel spreadsheet (Microsoft Office Professional Plus 2013, version 15.0.4675.1003; Microsoft, USA). The data were then imported into STATA 17 (StataCorp, College Station, Texas, USA) software. Descriptive statistics were used to describe the population using frequencies, means ± standard deviations (SDs) and median values for continuous variables. We used frequencies and proportions to describe categorical variables. We employed bivariate and multivariate logistic regression analysis to derive associations between serum IGFBP-3 and lipid concentrations and treatment outcomes for cervical lesions. Multivariate analysis was conducted after controlling for age, region of residence, family planning use and type, HIV status, highest level of education, marital status, and history of blood pressure and diabetes. We used odd ratios as measures of association with respective 95% confidence intervals. A p-value of <0.05 was considered to be statistically significant.
Eligibility Criteria
We included all women aged 21 years and above, who sought care at the cervical cancer clinic of MRRH during the time of the study, screened with either VIA or HPV DNA, who provided written informed consent to participate in the study, and were later confirmed positive or negative for a precancerous lesion (CIN). The current guidelines for cervical cancer screening dictate the minimum age for cervical cancer screening to be 21 years.
Results
We recruited a total of 188 participants, including 94 cases and 94 controls. The mean age of participants in the control group was 38.7±8.2 years, while that of the case group was 34.5±7.8 years. The difference in the mean age of cases and controls was statistically significant (p<0.001). Thirty-eight percent (35/94) of controls and 48% (45/94) of cases had attained primary education as their highest level of education. More than half (59%, 54/94) of cases were active contraceptive users, of whom 81.5% (44/54) used hormonal methods, while 41% (38/94) of controls were active contraceptive users, of whom 87% (33/38) used hormonal methods, as shown in Table 1.
 |
Table 1 Socio-Demographic Characteristics of Study Participants at Mbarara Regional Referral Hospital |
Distribution of Baseline Serum IGFBP-3 and Lipid Concentrations Across Grades of Cervical Lesions
The average serum IGFBP-3 concentration among controls was 3.29 (±1.01) while that of LSIL and HSIL cases was 3.49 (±0.96) and 3.22 (±0.72) respectively. More than half of all LSIL cases (64%, 52/81) had a raised serum IGFBP-3 concentration. Also, more than half of controls (55%, 52/94) had a raised serum IGFBP-3 concentration. This distribution was not statistically significant. A big proportion of LSIL cases (83%, 67/81) had normal serum triglyceride concentration compared with the 17% (14/81) who had abnormal triglyceride concentration. The overall distribution of serum triglyceride concentration across grades of cervical lesions was statistically significant (p=0.009), as shown in Table 2.
 |
Table 2 Distribution of Baseline Serum IGFBP-3 and Lipid Concentrations Across Grades of Cervical Lesions of Study Participants at Mbarara Regional Referral Hospital |
Treatment Outcomes at 12 Months Post-Treatment
Out of the 94 cases at baseline, only 50 returned for follow-up at 12 months, making a 12 months’ loss to follow-up rate of 46.8%. Close to 90% (88%, 44/50) of all cases who returned for 12 months’ follow-up were declared to have cleared their cervical lesions. A small proportion (12%, 6/50) had persistent lesions. The majority of cases with cleared cervical lesions (90%, 38/44) had been treated with cryotherapy, while 50% (3/6) of those with persistent lesions had also been treated with cryotherapy. This observation was statistically significant (p=0.03). More than half (64%, 28/44) of all cases with cleared lesions were married. More than half (67%, 4/6) of all participants with persistent lesions were residing in other districts of south western Uganda, 50% (3/6) were single and 50% were contraceptive users (3/6). More than half (59%, 26/44) of all cases with cleared lesions were HIV positive, as shown in Table 3.
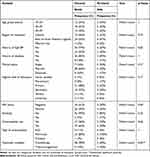 |
Table 3 Treatment Outcomes for Cervical Lesions at 12 Months Post-Treatment of Study Participants at Mbarara Regional Referral Hospital |
Distribution of Follow-Up Serum IGFBP-3 and Lipid Concentrations Across Treatment Outcomes
The mean serum IGFBP-3 concentration among cases with cleared lesion was 3.77±1.09 µg/mL, while it was 3.51±0.98 µg/mL among those cases whose lesions persisted after 12 months of post-treatment follow-up. More than half (68%, 30/44) of cases whose lesions cleared had raised IGFBP-3 serum concentrations after 12 months of post-treatment follow-up. An equally large proportion (67%, 4/6) of cases whose lesions persisted had raised IGFBP-3 serum concentrations after 12 months of post-treatment follow-up. This observation was, however, not statistically significant. Half (50%, 22/44) of all participants who had cleared cervical lesions had an abnormal total cholesterol concentration. The same proportion (50%, 3/6) of all participants who had persistent cervical lesions had an abnormal total cholesterol concentration. More than half (67%, 4/6) of participants with persistent cervical lesions had abnormal LDL concentration as well as an abnormal triglyceride concentration. However, none of the lipids showed any statistically significant distribution across treatment outcomes, as shown in Table 4.
 |
Table 4 Distribution of Follow-Up Serum IGFBP-3 and Lipid Concentrations Across Treatment Outcomes of Cervical Lesions of Study Participants at Mbarara Regional Referral Hospital |
Association Between Follow-Up Serum IGFBP-3 and Lipid Concentrations and Clearance of Cervical Lesions
After controlling for age, region of residence, family planning use and type, HIV status, highest level of education, marital status, and history of blood pressure and diabetes, we found that a serum IGFBP-3 concentration of less than 3.291 µg/mL is likely to be associated with clearance of cervical lesions (adjusted odds ratio [AOR] 1.65, p=0.67, 95% confidence interval [CI] 0.17–16.57), although this was not statistically significant. We also found that a serum triglyceride concentration of 35–135 mg/dL is likely to be associated with clearance of cervical lesions (AOR 2.41, p=0.46, 95% CI 0.23–25.69), although this was also not statistically significant, as shown in Table 5.
Discussion
We present results showing a trend towards a likely association between reduced serum IGFBP-3 and triglyceride concentrations and clearance of cervical lesions among our study population, although this was not statistically significant. To our knowledge, this is the first study in Uganda to examine the possible association between serum lipids and IGFBP-3 concentrations and treatment outcomes for cervical lesions.
We did not find any statistically significant associations between baseline IGFBP-3 concentration or its categories and cervical intraepithelial lesions among study cases and controls. Previous work by Wu et al29 demonstrated that serum IGFBP-3 was significantly higher in groups with CIN compared to controls. This difference could be explained by the fact that our IGFBP-3 concentrations were not necessarily raised above normal ranges (0.63–10.2 µg/mL). Our categorization was based on the mean serum concentration in controls. In agreement with our finding, Lee et al30 found no association between CIN and serum IGFBP-3 levels. IGFBP-3 as a binding protein is said to have the ability to play a dual role. IGFBP-3 can inhibit cell proliferation and accelerate apoptosis in a manner that is insulin-like growth factor (IGF)-dependent or IGF-independent. However, it is worth noting that in situations of high IGF-1 concentration, as it could be in our case, IGFBP-3 can synergize the effects of IGF. It can present and release IGF-1 for interaction with its receptor, while at the same time protecting the receptor from down-regulation by exposure to high IGF-1 concentrations.31
The distribution of triglyceride concentrations across grades of cervical lesion was statistically significant (p=0.009). In our earlier studies in the same population,32 we demonstrated that dyslipidemia, especially triglyceride dyslipidemia, is a prevalent condition among this population. In agreement with our finding, studies on metabolic syndrome components have hypothesized that increased triglyceride concentrations can increase a person’s risk of developing not only cardiovascular diseases but also other non-communicable diseases, including cervical cancer.33 Research by Bumrungthai et al34 indicates that an abnormal serum triglyceride concentration is associated with an increased risk of development of cervical precancerous lesions. Dyslipidemia, in common with other components of metabolic syndrome such as obesity, elevates estrogen levels,35 which, in turn, can increase the risk of HPV infection.36,37
We observed a 12 months’ loss to follow-up rate of 46.8%. This is comparable to our previous finding of 76.2% at the same clinic.38 Close to 90% of all cases had cleared cervical lesions. The majority of cases with cleared cervical lesions had been treated with cryotherapy. This concurs with earlier reports on the success rates of cryotherapy for the treatment of cervical precancerous lesions,39 although more recent work by Randall et al40 shows otherwise. Cryotherapy has been described as a simple, cheap, accessible, applicable and effective treatment modality for cervical precancerous lesions in developing countries.41 Most of the cases in this study had been diagnosed with CIN and received treatment, mostly with cryotherapy, and after 12 months they were confirmed to have cleared their lesions. A previous study by Luciani et al42 reported that cryotherapy effectively cured 418 women (88%) with cervical precancerous lesions, of whom 49 (70%) had a baseline diagnosis of cervical intraepithelial lesion grade 3. Although thermal ablation treatment modalities have been argued to have better treatment outcomes,40 it is important to note that availability of a given treatment modality, and especially in the cervical cancer clinic of MRRH, is based on a number of factors, including equipment breakdown and supply shortages, as highlighted previously.43
More than half (64%, 28/44) of all cases with cleared lesions were married. This also highlights the importance of social support in the treatment of cervical lesions. Previous studies have demonstrated that social support by family members or peers, or any other form of social support, is key to achieving positive treatment outcomes for malignancies.38,44–47 Also, more than half (59%, 26/44) of all cases with cleared lesions were HIV positive. A systematic review published in 2020 found that adequate facilitation, as is currently available for women living with HIV, and high compliance with antiretroviral therapy have a positive impact on cervical cancer treatment response.48 It is worth noting that, in our setting, women living with HIV have more contact with the health system and benefit from more health information than do their HIV-negative peers.
We take note that more than half of cases with persistent lesions were residing in districts other than Mbarara and central Uganda. This reflects on the impact of socio-economic status49 and distance from health facility50,51 on treatment outcomes, since central Uganda and Mbarara city are periurban areas compared to the rest of south western Uganda. Also, half of participants with persistent lesions were single. This may be explained by their relative perceived absence of social support, which is known to influence treatment outcomes of various disease conditions in married people.38,44–47
We categorized IGFBP-3 along the mean concentration in controls (3.29 µg/mL) as raised (≥3.29 µg/mL) or reduced (<3.29 µg/mL). Although it was not statistically significant, we present a finding that more than one-third (32%, 14/44) of cases with cleared lesions had reduced IGFBP-3 serum concentrations at 12 months post-treatment. This underscores the biochemical roles of IGFBP-3. IGFBP-3 is the primary transport protein for IGF-1, called somatomedin C in humans,52 and it binds to and regulates the effects of IGF-1, thereby inhibiting cell division and inducing apoptosis in various cell types in an IGF-1-independent manner.53 This is because IGFBP-3 increases the ratio of proapoptotic to antiapoptotic proteins and/or causes the activation of the cascades involved in a death receptor-mediated pathway,54,55 and hence the arrest of the cell cycle at the G1/S phase of cytokinesis, subsequently inducing apoptosis.55 This is a negative response to the action of growth hormones and is beneficial in the arrest of unnecessary cytokinesis, such as in neoplasms.52 However, 68% of participants with cleared lesions also had raised IGFBP-3 levels. This elevation in post-follow-up serum IGFBP-3 could have inhibited the effects of IGF-1 (somatomedin C), thereby reverting the growth hormone effects and subsequently contributing to clearance of the cervical precancerous lesions.53,55
We report increased proportions of abnormal lipids, namely total cholesterol, triglycerides, HDL or LDL, across the treatment outcomes, although these differences were not statistically significant. Metabolic syndrome components, including abnormal lipids, are said to act as cofactors in cervical carcinogenesis, as seen in the positive correlation between estrogen and adipokine levels in cancer of the cervix.56,57 Abnormal serum triglyceride and HDL concentrations are said to be risk factors for cervical cancer58 and hence could be associated with poor treatment outcomes. They are known to accelerate the growth of cancer cells and induce antiapoptotic capacity via activation of the production of reactive oxygen species.59 A previous study by Jiang et al14 demonstrated that total cholesterol, triglyceride and LDL serum concentrations are significantly higher in cervical cancer patients compared to controls. However, the same study revealed that HDL concentrations are lower in cervical cancer patients compared to controls. Similar distributions have been reported by Raju et al in an Indian population60 and Tulinius et al in an Icelandic population.61 However, in agreement with our finding, Sun et al62 reported no significant differences in any lipid concentrations between cervical cancer patients and controls.
With a categorization of IGFBP-3 along the mean concentration in controls (3.29 µg/mL) as raised (≥3.29 µg/mL) or reduced (<3.29 µg/mL), we present findings that show that a serum IGFBP-3 concentration of less than 3.29 µg/mL is likely to be associated with clearance of cervical lesions, although this result was not statistically significant. IGFBP-3 is the dominant among several circulating binding proteins for IGF-1.63,64 It has been shown to induce apoptosis65 and its expression is involved in regulation of many tumors.66 IGF-1 binds to IGFR-1, thereby activating the P13K/AKt pathway, which stimulates cell growth and blocks apoptosis.65 Studies have suggested that increased concentrations of circulating IGF-1 are associated with an increased risk of cancer.63,67,68 However, significantly reduced IGFBP-3 concentrations have been shown to have an inverse relationship with the risk of cancers63,69,70 as well as tumor migration,71,72 leading to tumor growth and suppressed apoptosis.73,74 In addition, reduced IGFBP-3 concentrations could indicate a stronger immune system, which plays an important role in the suppression of tumors.
We also report that more than half of all participants with persistent cervical lesions had raised serum IGFBP-3. However, we were unable to perform statistical analyses between increased serum IGFBP-3 and the persistence of cervical lesions owing to the limited number of observations. There have been assertions that the overexpression of serum IGFBP-3 inhibits tumor growth and that it may be a laboratory marker of improved cancer survival.75 It has also been said that IGFBP-3 has an inverse association with death due to cancer, although that research was on male subjects.76
Previous work by Lee et al30 found no significant association between CIN and serum IGFBP-3 levels. However, levels of circulating insulin-like growth factors have been said to vary with age,77 increasing from birth to puberty and reducing thereafter. This could partly explain our finding. Most of our participants were beyond puberty and much older, and hence age may have affected this observation, as similarly reported previously.30 We hypothesize that a number of factors in this population could be influencing these observations. For example, factors such as obesity have been thought to cause variations in IGFBP-3.78 This could be true considering the fact that we demonstrated this population to have an increased body mass index.79 Also, IGFBP-3 could be working along with other factors associated with persistence of cervical lesions. Immortalization of HPV-infected cells is a major factor in cervical carcinogenesis. It may be activated by inactivation of P53, a cell cycle regulator, through the effects of E6 and E7 genes.80
We studied a population that was found to have comorbidities, including hypertension, HIV and diabetes. Some of these disease conditions have been thought to have an association with treatment outcomes for malignancies. For instance, patients with comorbidities such as diabetes tend to have poor treatment outcomes for any malignancy.81,82 Other population factors, including age, together with chronic diseases such as hypertension and diabetes, have been said to be associated with inflammation, which is associated with the secretion of numerous cytokines, most of which promote tumor growth, eventually leading to poor treatment outcomes including the persistence of lesions.83,84
We also observed a trend for reduced serum triglyceride concentrations to be associated with clearance of cervical lesions, although this observation was not statistically significant. This is in agreement with the findings of a number of previous studies. For instance, work by Lin et al85 showed that total cholesterol and triglycerides are not only independent prognostic factors for cervical cancer in its early stages but also associated with poor survival. It is emphasized that high triglycerides and high cholesterol lead to increased efflux of proinflammatory cytokines and chemokines, which results in reduced immunity and eventually tumor growth and poor treatment outcomes, including persistence of lesions.86 Another study, by Jiang et al,14 showed that a high triglyceride concentration is associated with worse treatment outcomes, especially in patients aged 50 years and above. However, only 1% of our participants were in the age bracket of 50–59 years.
Many of our participants with cleared cervical lesions also had abnormal lipids. This contradicts much of the available literature on the association between lipids and treatment outcomes. Because of this observation, we take note that carcinogenesis involves various pathways, mostly the catabolism to anabolism transition.87 Therefore, participants may have abnormal lipids but then experience better treatment outcomes, including clearance of cervical lesions.
Our study has several limitations. We did not derive associations between raised IGFBP-3 or lipids and the persistence of cervical lesions. This is because we had fewer than 10 participants with persistent lesions and this could not support the derivation of associations using logistic regression. However, high triglycerides being a predictor of poor treatment outcomes is consistent with many previous studies in other cancers, including prostate and breast. According to findings by Allott et al,88 increased serum triglyceride concentrations are associated with the recurrence of prostate cancer. A study of 5-year survival outcomes revealed that increased triglyceride concentrations are associated with decreased 5-year survival among breast cancer cases.89 Similar findings have also been reported in neuroendocrine tumors.90 Cancer cells are also known to require large amounts of lipids for a number of cellular functions, including cell membrane synthesis during increased cell growth and proliferation.91 In addition to the already known impact of HPV persistence on the recurrence of cervical lesions, especially CIN2+,92 there could be a potential role for lipids and IGFBP-3 in the persistence of cervical lesions.
We did not measure serum IGF-1, and thus we did not estimate the IGF-1 to IGFBP-3 molar ratio, which could also be used to derive associations with treatment outcomes, as used previously.30 We created categories of IGFBP-3 concentrations based on the mean concentrations in controls. This is because all of our IGFBP-3 concentrations were within the normal ranges as specified by the manufacturer of the measurement kit. In addition, all serum samples were stored at recommended temperatures and later run as a batch. This storage time may have led to reductions in the concentrations of IGFBP-3 and lipids. The fact that we used non-fasting lipid concentrations may have had an impact on triglyceride concentrations. We also experienced a high loss to follow-up rate, and this could have lowered the power of our study and also hindered derivations of associations. However, this study used internationally acceptable quantification methods for lipids and IGFBP-3. We also used standard cut-off values for categorizing abnormal lipids. The presence or absence of cervical lesions was confirmed with either a Pap smear or histology, the gold standard, at both baseline and follow-up.
Conclusion
Reduced serum concentrations of IGFBP-3 and triglycerides may be associated with clearance of cervical lesions, although this finding was not statistically significant among our study population. Larger prospective studies are recommended to further understand the biological mechanisms behind lipids, IGFBP-3, other insulin-like growth factors, and the persistence or clearance of cervical lesions. Lipid management may be beneficial in the treatment of cervical lesions.
Abbreviations
AOR, adjusted odds ratio; ASCUS, atypical squamous cells of undetermined significance; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HPV, human papilloma virus; HSIL, high-grade squamous intraepithelial lesion; IGF-1, insulin-like growth factor-1; IGFBP-3, insulin-like growth factor binding protein-3; IUD, intrauterine device; LDL, low-density lipoprotein; LSIL, low-grade squamous intraepithelial lesion; MRRH, Mbarara Regional Referral Hospital; NILM, negative for intraepithelial lesions or malignancy; Pap, Papanicolaou; SD, standard deviation; STATA, Statistical Software for Data Science; VIA, visual inspection with acetic acid.
Data Sharing Statement
All of the data that informed this article are available from the corresponding author upon reasonable request.
Ethical Considerations
We sought written informed consent from every participant before they took part in the study. We did not use participant names on any data collection tools or laboratory specimens. We did our best to delink all participants’ identifiable information before and during data analysis. All participant interaction with the research team took place in a separate, quiet and comfortable room, free from any disturbances and only accessible to one participant at any single time. This study was approved by the Mbarara University of Science and Technology Research Ethics Committee (MUST-2022-396) as well as the Uganda National Council for Science and Technology (HS2395ES). We also sought administrative clearance from the Hospital Director, Mbarara Regional Referral Hospital. All cases received the standard package of care as offered at the cervical cancer clinic. Participants received their VIA, Pap smear, histology, lipid profile and IGFBP-3 results through the nurses at the clinic. This study complied with the Declaration of Helsinki.
Acknowledgment
We acknowledge the clinical and support staff, research assistants and all patients of the cervical cancer clinic at Mbarara Regional Referral Hospital. The research reported in this publication was supported by the Fogarty International Centre of the National Institutes of Health under Award number D43TW011632. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
All authors made a significant contribution to the work reported in this paper, including conception of the idea, study design, execution, acquisition of data, data analysis and interpretation, in any or all of these areas; and took part in drafting, revising or critically reviewing the article. All authors gave final approval of the final version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Bruni L, Albero G, Serrano B, et al. ICO/IARC information centre on HPV and cancer (HPV information centre). Hum Papillom Rel Dis World Summ Rep. 2019;17:6.
3. Ronco G, Montanari G, Aimone V, et al. Estimating the sensitivity of cervical cytology: errors of interpretation and test limitations. Cytopathology. 1996;7(3):151–158. doi:10.1046/j.1365-2303.1996.39382393.x
4. World Health Organization. Assessing National Capacity for the Prevention and Control of Noncommunicable Diseases: Report of the 2019 Global Survey. World Health Organization; 2020.
5. Anorlu RI. Cervical cancer: the sub-Saharan African perspective. Rep Health Matters. 2008;16(32):41–49. doi:10.1016/S0968-8080(08)32415-X
6. Denny L, Quinn M, Sankaranarayanan R. Screening for cervical cancer in developing countries. Vaccine. 2006;24:S71–S7. doi:10.1016/j.vaccine.2006.05.121
7. Sankaranarayanan R. Screening for cancer in low-and middle-income countries. Anna Global Health. 2014;80(5):412–417. doi:10.1016/j.aogh.2014.09.014
8. World Health Organization. Cervical cancer country profiles. World Health Organization; 2021. Available from: https://www.who.int/publications/m/item/cervical-cancer-country-profiles.
9. Nakisige C, Schwartz M, Ndira AO. Cervical cancer screening and treatment in Uganda. Gynecol Oncol Rep. 2017;20:37–40. doi:10.1016/j.gore.2017.01.009
10. World Health Organization. Screening as well as vaccination is essential in the fight against cervical cancer. Erişim adresi; 2014. Available from: http://www.who.int/reproductivehealth/topics/cancers/fight-cervical-cancer/en/Erişim.tarihi.
11. Yee PCG, de Souza P, Khachigian ML. Current and potential treatments for cervical cancer. Curr Cancer Drug Targets. 2013;13(2):205–220. doi:10.2174/1568009611313020009
12. Bogani G, Sopracordevole F, Ciavattini A, et al. HPV persistence after cervical surgical excision of high‐grade cervical lesions. Can Cytopathol. 2023. doi:10.1002/cncy.22760
13. Preetha A, Banerjee R, Huilgol N. Surface activity, lipid profiles and their implications in cervical cancer. J Cancer Res Ther. 2005;1(3):180–186. doi:10.4103/0973-1482.19600
14. Jiang Q, Wang L, Jin M, Shou Y, Zhu H, Li A. The clinical value of lipid abnormalities in early stage cervical cancer. Int J Gene Med. 2022;15:3903–3914. doi:10.2147/IJGM.S352934
15. Serrano M-L, Sánchez-Gómez M, Bravo -M-M. Cervical scrapes levels of insulin-like growth factor-II and insulin-like growth factor binding protein 3 in women with squamous intraepithelial lesions and cervical cancer. Hormone Metab Res. 2010;42(13):977–981. doi:10.1055/s-0030-1267175
16. Ingermann AR, Yang Y-F, Han J, et al. Identification of a novel cell death receptor mediating IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J Biol Chem. 2010;285(39):30233–30246. doi:10.1074/jbc.M110.122226
17. Mehta HH, Gao Q, Galet C, et al. IGFBP-3 is a metastasis suppression gene in prostate cancer. Cancer Res. 2011;71(15):5154–5163. doi:10.1158/0008-5472.CAN-10-4513
18. Mohanraj L, Kim H-S, Li W, et al. IGFBP-3 inhibits cytokine-induced insulin resistance and early manifestations of atherosclerosis. PLoS One. 2013;8(1):e55084. doi:10.1371/journal.pone.0055084
19. Min H-K, Maruyama H, Jang BK, et al. Suppression of IGF binding protein-3 by palmitate promotes hepatic inflammatory responses. THE FASEB Journal. 2016;30(12):4071. doi:10.1096/fj.201600427R
20. Kim M, Cai Q, Oh Y. Therapeutic potential of alpha-1 antitrypsin in human disease. Ann Pediatr Endocrinol Metab. 2018;23(3):131. doi:10.6065/apem.2018.23.3.131
21. Pollak M. Insulin-like growth factor physiology and cancer risk. Eur J Cancer. 2000;36(10):1224–1228. doi:10.1016/S0959-8049(00)00102-7
22. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11):3109S–3120S. doi:10.1093/jn/131.11.3109S
23. Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J National Cancer Inst. 2000;92(18):1472–1489. doi:10.1093/jnci/92.18.1472
24. Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J National Cancer Inst. 2002;94(13):972–980. doi:10.1093/jnci/94.13.972
25. Uganda Ministry of Health. Mbarara Regional Referral Hospital. Uganda Ministry of Health; 2016.
26. Ahn HK, Shin JW, Ahn HY, et al. Metabolic components and recurrence in early-stage cervical cancer. Tumor Biol. 2015;36(3):2201–2207. doi:10.1007/s13277-014-2831-y
27. Kim M, Kim I-H, Lim MK, Kim Y, Park B. Increased prevalence of metabolic syndrome in adult cancer survivors: asian first report in community setting. Cancer Epidemiol. 2019;58:130–136. doi:10.1016/j.canep.2018.12.006
28. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. john wiley & sons; 2013.
29. Wu X, Tortolero-Luna G, Zhao H, Phatak D, Spitz MR, Follen M. Serum levels of insulin-like growth factor I and risk of squamous intraepithelial lesions of the cervix. Clin Cancer Res. 2003;9(9):3356–3361.
30. Lee SW, Lee SY, Lee SR, Ju W, Kim SC. Plasma levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in women with cervical neoplasia. J Gynecol Oncol. 2010;21(3):174–180. doi:10.3802/jgo.2010.21.3.174
31. Conover CA, Powell DR. Insulin-like growth factor (IGF)-binding protein-3 blocks IGF-I-induced receptor down-regulation and cell desensitization in cultured bovine fibroblasts. Endocrinology. 1991;129(2):710–716. doi:10.1210/endo-129-2-710
32. Ssedyabane F, Ngonzi J, Kajabwangu R, Najjuma JN, Tusubira D, Randall TC. Association between obesity and cervical intraepithelial neoplasia: results from a case control study in south western Uganda. BMC Womens Health. 2023;23(1):1–8.
33. Cohen SS, Park Y, Signorello LB, et al. A pooled analysis of body mass index and mortality among African Americans. PLoS One. 2014;9(11):e111980. doi:10.1371/journal.pone.0111980
34. Bumrungthai S, Ekalaksananan T, Kleebkaow P, et al. Mathematical modelling of cervical precancerous lesion grade risk scores: linear regression analysis of cellular protein biomarkers and human papillomavirus E6/E7 RNA staining patterns. Diagnostics. 2023;13(6):1084. doi:10.3390/diagnostics13061084
35. Trabert B, Wentzensen N, Felix AS, et al. Metabolic syndrome and risk of endometrial cancer in the United States: a study in the SEER–medicare linked database. Cancer Epidemiol Biomarkers Prev. 2015;24(1):261–267. doi:10.1158/1055-9965.EPI-14-0923
36. Huang X, Zhao Q, Yang P, et al. Metabolic syndrome and risk of cervical human papillomavirus incident and persistent infection. Medicine. 2016;95:9.
37. Molokwu JC, Penaranda E, Lopez DS, et al. Association of metabolic syndrome and human papillomavirus infection in men and women residing in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(8):1321–1327. doi:10.1158/1055-9965.EPI-17-0129
38. Bergerot CD, Costas-Muñiz R, Lee D, Philip EJ. Social Support as a Protective Factor for Patients with Cancer During the Pandemic. Taylor & Francis; 2022:473–474.
39. Santesso N, Schünemann H, Blumenthal P, et al. World health organization guidelines: use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynecol Obstet. 2012;118(2):97–102. doi:10.1016/j.ijgo.2012.01.029
40. Randall TC, Sauvaget C, Muwonge R, Trimble EL, Jeronimo J. Worthy of further consideration: an updated meta-analysis to address the feasibility, acceptability, safety and efficacy of thermal ablation in the treatment of cervical cancer precursor lesions. Preventive Med. 2019;118:81–91. doi:10.1016/j.ypmed.2018.10.006
41. Jacob M, Broekhuizen FF, Castro W, Sellors J. Experience using cryotherapy for treatment of cervical precancerous lesions in low-resource settings. Int J Gynaecol Obstet off Organ Int Fed Gynaecol Obstet. 2005;89:S13–20. doi:10.1016/j.ijgo.2005.01.026
42. Luciani S, Gonzales M, Munoz S, Jeronimo J, Robles S. Effectiveness of cryotherapy treatment for cervical intraepithelial neoplasia. Int J Gynaecol Obstet off Organ Int Fed Gynaecol Obstet. 2008;101(2):172–177. doi:10.1016/j.ijgo.2007.11.013
43. Cremer ML, Conzuelo-Rodriguez G, Cherniak W, Randall T. Ablative therapies for cervical intraepithelial neoplasia in low-resource settings: findings and key questions. J Glob Oncol. 2018;4:1–10. doi:10.1200/JGO.18.00093
44. Pasek M, Suchocka L, Gąsior K. Model of social support for patients treated for cancer. Cancers. 2021;13(19):4786. doi:10.3390/cancers13194786
45. Usta YY. Importance of social support in cancer patients. Asian Pac J Cancer Prev. 2012;13:3569–3572. doi:10.7314/APJCP.2012.13.8.3569
46. Korotkin BD, Hoerger M, Voorhees S, Allen CO, Robinson WR, Duberstein PR. Social support in cancer: how do patients want us to help? J Psychosoc Oncol. 2019;37(6):699–712. doi:10.1080/07347332.2019.1580331
47. Hofman A, Zajdel N, Klekowski J, Chabowski M. Improving social support to increase QoL in lung cancer patients. Cancer Manage Res. 2021;13:2319–2327. doi:10.2147/CMAR.S278087
48. Mapanga W, Singh E, Feresu SA, Girdler-Brown B. Treatment of pre-and confirmed cervical cancer in HIV-seropositive women from developing countries: a systematic review. Syst Rev. 2020;9:1–16. doi:10.1186/s13643-020-01345-2
49. Arpey NC, Gaglioti AH, Rosenbaum ME. How socioeconomic status affects patient perceptions of health care: a qualitative study. J Prim Care Community Health. 2017;8(3):169–175. doi:10.1177/2150131917697439
50. Vetterlein MW, Löppenberg B, Karabon P, et al. Impact of travel distance to the treatment facility on overall mortality in US patients with prostate cancer. Cancer. 2017;123(17):3241–3252. doi:10.1002/cncr.30744
51. Ambroggi M, Biasini C, Del Giovane C, Fornari F, Cavanna L. Distance as a barrier to cancer diagnosis and treatment: review of the literature. oncologist. 2015;20(12):1378–1385. doi:10.1634/theoncologist.2015-0110
52. Fraenkel E, Lazurova I. IGF-1 and IGFBP3 as indirect markers of hepatic insulin resistance and their relation to metabolic syndrome parameters in liver steatosis patients. Endoc Regul. 2023;57(1):69–79. doi:10.2478/enr-2023-0009
53. Adachi Y, Nojima M, Mori M, et al. Insulin-like growth factor-1, insulin-like growth factor binding protein-3 and the incidence of malignant neoplasms in a nested case-control study. Cancer Prev Res (Phila). 2020;13(4):385–394. doi:10.1158/1940-6207.CAPR-19-0375
54. Kucera R, Topolcan O, Pecen L, et al. Reference values of IGF1, IGFBP3 and IGF1/IGFBP3 ratio in adult population in the Czech Republic. Clin Chim Acta. 2015;444:271–277. doi:10.1016/j.cca.2015.02.036
55. Pfeffer CM, Singh ATK. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018;19(2):448. doi:10.3390/ijms19020448
56. Chung S-H, Franceschi S, Lambert P. Estrogen and ERα: culprits in cervical cancer? Trends Endocrinol Metab. 2010;21(8):504–511. doi:10.1016/j.tem.2010.03.005
57. Baker R, Dauner JG, Rodriguez AC, et al. Increased plasma levels of adipokines and inflammatory markers in older women with persistent HPV infection. Cytokine. 2011;53(3):282–285. doi:10.1016/j.cyto.2010.11.014
58. Lee DY, Lee TS. Associations between metabolic syndrome and gynecologic cancer. Obstet Gynecol Sci. 2020;63(3):215–224. doi:10.5468/ogs.2020.63.3.215
59. Cowey S, Hardy R. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169(5):1505–1522. doi:10.2353/ajpath.2006.051090
60. Raju K, Punnayanapalya SS, Mariyappa N, Eshwarappa SM, Anjaneya C, Kai LJ. Significance of the plasma lipid profile in cases of carcinoma of cervix: a tertiary hospital based study. Asian Pac J Cancer Prev. 2014;15(8):3779–3784. doi:10.7314/APJCP.2014.15.8.3779
61. Tulinius H, Sigfússon N, Sigvaldason H, Bjarnadóttir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol. 1997;6(11):863–873.
62. Sun Y, Meng H, Jin Y, et al. Serum lipid profile in gynecologic tumors: a retrospective clinical study of 1550 patients. Eu J Gynaecol Oncol. 2016;37(3):348–352.
63. Yu H, Spitz MR, Mistry J, Gu J, Hong WK, Wu X. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case-control analysis. J National Cancer Inst. 1999;91(2):151–156. doi:10.1093/jnci/91.2.151
64. S-H O, Lee O-H, Schroeder CP, et al. Antimetastatic activity of insulin-like growth factor binding protein-3 in lung cancer is mediated by insulin-like growth factor–independent urokinase-type plasminogen activator inhibition. Mol Cancer Ther. 2006;5(11):2685–2695. doi:10.1158/1535-7163.MCT-06-0142
65. Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518. doi:10.1038/nrc1387
66. Kuhn H, Bräunlich J, Hammerschmidt S, Wirtz H. Candidate genes upregulated in density dependent growth inhibition of lung cancer cells. Int j Oncol. 2004;25(5):1481–1487.
67. Han J-Y, Choi BG, Choi JY, Lee SY, Ju SY. The prognostic significance of pretreatment plasma levels of insulin-like growth factor (IGF)-1, IGF-2, and IGF binding protein-3 in patients with advanced non-small cell lung cancer. Lung Cancer. 2006;54(2):227–234. doi:10.1016/j.lungcan.2006.07.014
68. Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet. 1998;351(9113):1393–1396. doi:10.1016/S0140-6736(97)10384-1
69. Chen B, Liu S, Xu W, Wang X, Zhao W, Wu J. IGF-I and IGFBP-3 and the risk of lung cancer: a meta-analysis based on nested case-control studies. J Exp Clin Cancer Res. 2009;28:1–6. doi:10.1186/1756-9966-28-89
70. Adachi Y, Nojima M, Mori M, et al. Insulin-like growth factor-related components and the risk of liver cancer in a nested case-control study. Tumor Biol. 2016;37:15125–15132. doi:10.1007/s13277-016-5360-z
71. Le HT, Lee HJ, Cho J, et al. Insulin-like growth factor binding protein-3 exerts its anti-metastatic effect in aerodigestive tract cancers by disrupting the protein stability of vimentin. Cancers. 2021;13(5):1041. doi:10.3390/cancers13051041
72. Luo Q, Shi W, Dou B, et al. XBP1-IGFBP3 signaling pathway promotes NSCLC invasion and metastasis. Front Oncol. 2021;11:654995. doi:10.3389/fonc.2021.654995
73. Luo -L-L, Zhao L, Wang Y-X, et al. Insulin-like growth factor binding protein-3 is a new predictor of radiosensitivity on esophageal squamous cell carcinoma. Sci Rep. 2015;5(1):17336. doi:10.1038/srep17336
74. Cheung SC, Long X, Liu L, et al. Inhibition of human MCF-7 breast cancer cells and HT-29 colon cancer cells by rice-produced recombinant human insulin-like growth binding protein-3 (rhIGFBP-3). PLoS One. 2013;8(10):e77516. doi:10.1371/journal.pone.0077516
75. Kuhn H, Frille A, Petersen MA, et al. IGFBP3 inhibits tumor growth and invasion of lung cancer cells and is associated with improved survival in lung cancer patients. Transl Oncol. 2023;27:101566. doi:10.1016/j.tranon.2022.101566
76. Friedrich N, Haring R, Nauck M, et al. Mortality and serum insulin-like growth factor (IGF)-I and IGF binding protein 3 concentrations. J Clin Endocrinol Metab. 2009;94(5):1732–1739. doi:10.1210/jc.2008-2138
77. Gomez J-M, Mourot B, Fostier A, Le Gac F. Growth hormone receptors in ovary and liver during gametogenesis in female rainbow trout (Oncorhynchus mykiss). Reproduction. 1999;115(2):275–285. doi:10.1530/jrf.0.1150275
78. Hoyo C, Grubber J, Demark-Wahnefried W, et al. Predictors of variation in serum IGFI and IGFBP3 levels in healthy African American and white men. J Natl Med Assoc. 2009;101(7):711–716. doi:10.1016/S0027-9684(15)30981-0
79. Ssedyabane F, Ngonzi J, Kajabwangu R, Najjuma JN, Tusubira D, Randall TC. Association between obesity and cervical intraepithelial neoplasia: results from a case control study in south western Uganda. BMC Women’s Health. 2023;23(1):1–8. doi:10.1186/s12905-023-02315-1
80. Dassonville O, Formento J, Francoual M, et al. Expression of epidermal growth factor receptor and survival in upper aerodigestive tract cancer. J clin oncol. 1993;11(10):1873–1878. doi:10.1200/JCO.1993.11.10.1873
81. Gillani SW, Zaghloul HA, Ansari IA, et al. Multivariate analysis on the effects of diabetes and related clinical parameters on cervical cancer survival probability. Sci Rep. 2019;9(1):1084. doi:10.1038/s41598-018-37694-1
82. Harding JL, Sooriyakumaran M, Anstey KJ, et al. Hypertension, antihypertensive treatment and cancer incidence and mortality: a pooled collaborative analysis of 12 Australian and New Zealand cohorts. J Hypertens. 2016;34(1):149–155. doi:10.1097/HJH.0000000000000770
83. Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–2465. doi:10.1158/0008-5472.CAN-10-3323
84. Bochet L, Lehuédé C, Dauvillier S, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657–5668. doi:10.1158/0008-5472.CAN-13-0530
85. Lin F, Zheng R, Yu C, Su Y, Yan X, Qu F. Predictive role of serum cholesterol and triglycerides in cervical cancer survival. Int J Gynecologic Cancer. 2021;31(2):171–176. doi:10.1136/ijgc-2020-001333
86. Porta C, Marino A, Consonni FM, et al. Metabolic influence on the differentiation of suppressive myeloid cells in cancer. Carcinogenesis. 2018;39(9):1095–1104. doi:10.1093/carcin/bgy088
87. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
88. Allott EH, Howard LE, Cooperberg MR, et al. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2349–2356. doi:10.1158/1055-9965.EPI-14-0458
89. Lofterød T, Mortensen ES, Nalwoga H, et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer. 2018;18:1–11. doi:10.1186/s12885-018-4568-2
90. Vernieri C, Pusceddu S, Fucà G, et al. Impact of systemic and tumor lipid metabolism on everolimus efficacy in advanced pancreatic neuroendocrine tumors (pNETs). Int J Cancer. 2019;144(7):1704–1712. doi:10.1002/ijc.32042
91. Solomon KR, Freeman MR. The complex interplay between cholesterol and prostate malignancy. Urol Clin. 2011;38(3):243–259. doi:10.1016/j.ucl.2011.04.001
92. Bogani G, Sopracordevole F, Ciavattini A, et al. Duration of human papillomavirus persistence and its relationship with recurrent cervical dysplasia. Eur J Cancer Prev. 2023;32(6):525–532. doi:10.1097/CEJ.0000000000000822
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

