Back to Journals » Journal of Blood Medicine » Volume 15
Severe Cytokine Release Syndrome and Hemophagocytic Lymphohistiocytosis (HLH)-Like Syndrome Following Administration of Combined Brentuximab Vedotin and Nivolumab for Recurrent Classical Hodgkin Lymphoma: A Case Report
Authors Mosalem O , Pai T , Alqawasma M, Shaikh M, Li KD, Alhaj Moustafa M
Received 11 October 2023
Accepted for publication 18 January 2024
Published 24 January 2024 Volume 2024:15 Pages 29—34
DOI https://doi.org/10.2147/JBM.S444004
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Osama Mosalem,1 Tanmayi Pai,1 Mohammed Alqawasma,2 Marwan Shaikh,1 K David Li,3 Muhamad Alhaj Moustafa1
1Department of Hematology/Oncology, Mayo Clinic, Jacksonville, FL, USA; 2Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Jacksonville, FL, USA; 3Department of Laboratory Medicine and Pathology, Mayo Clinic, Jacksonville, FL, USA
Correspondence: Tanmayi Pai, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL, USA, Tel +1 904 953 2000, Email [email protected]
Abstract: Brentuximab vedotin (BV) and nivolumab are increasingly utilized as a novel regimen in patients with relapsed/refractory classical Hodgkin lymphoma (cHL). A 26-year-old male presented to the hospital with refractory diabetic ketoacidosis and multiple electrolyte abnormalities, 9 days after the first dose of brentuximab vedotin and nivolumab for recurrent classical Hodgkin lymphoma. During his hospitalization, he developed multi-organ failure. His workup showed elevated cytokine levels concerning severe cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis (HLH)-like syndrome. Despite treatment with CRS- and HLH-directed therapies, his clinical status deteriorated due to ongoing multifactorial shock and worsening multi-organ dysfunction, and comfort care measures were eventually pursued. To our knowledge, there have been no other cases reported of HLH-like syndrome after the combination of BV and nivolumab in patients with cHL. This case of a fatal adverse event following one dose of BV and nivolumab underscores the vital need for close monitoring of patients receiving this treatment regimen.
Keywords: immunotherapy, immune-related adverse event, antibody drug conjugate, CRS
Introduction
The combination of brentuximab vedotin (BV) and nivolumab has emerged as a compelling regimen with a good safety profile for older or unfit patients with classical Hodgkin lymphoma (cHL) who cannot tolerate intensive chemotherapy and patients with relapsed/refractory (R/R) cHL. BV plus nivolumab is therefore now utilized as a therapeutic option in R/R cHL.1–4 Commonly associated toxicities include peripheral neuropathy and immune-related adverse events such as rash and endocrinopathies. Here, we describe a fatal adverse event following the first dose of BV and nivolumab in a young patient, highlighting the need for careful safety monitoring with the use of this regimen.
Case Report
A 21-year-old Caucasian male was diagnosed with stage IIB unfavorable-risk cHL, nodular sclerosing type, and achieved a complete response (CR) after four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by involved-site radiation therapy (ISRT) targeting disease in the chest. Five years later, surveillance positron emission tomography-computed tomography (PET-CT) revealed hypermetabolic anterior mediastinal soft tissue masses involving the left side of the sternum and left pectoral muscle as well as multiple hypermetabolic lymph nodes (Deauville score of 5); no disease was noted elsewhere (Figure 1A). A biopsy of one of the chest wall masses confirmed recurrent cHL. As he had no significant history of autoimmune disease other than atopic dermatitis, he was a candidate for immunotherapy. He was recommended to complete 4–6 cycles of BV plus nivolumab followed by consolidation with high-dose chemotherapy and autologous stem cell transplantation. Baseline blood counts prior to starting treatment revealed mild anemia with hemoglobin of 11.5 g/dL (normal, 13.2–16.6), normal platelet count of 351×10(9)/L (normal, 150–450×10(9)/L), and normal white blood cell count (WBC) of 8×10(9)/L (normal, 4–10×10(9)/L). Comprehensive metabolic panel showed mild hyponatremia of 134 mmol/L (normal, 135–145 mmol/L), normal creatinine of 0.84 mg/dL, normal glucose of 91 mg/dL (normal, 70–140 mg/dL), and normal liver enzymes. The patient started treatment with BV (1.8 mg/kg) and nivolumab (3 mg/kg).
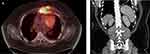 |
Figure 1 (A) Pre-treatment PET-CT. (B) Post-cycle 1 CT abdomen showing splenomegaly. |
Nine days after the first dose, he presented to the hospital with diffuse myalgias, back and abdominal pain, nausea, vomiting, dehydration, and low-grade fever (38°C), the latter of which had started approximately 5 days prior to his hospitalization. On admission, he was afebrile and normotensive with blood pressure of 128/83 but tachycardic to 130 bpm and mildly tachypneic. Physical examination showed mild right-sided abdominal tenderness without rebound or guarding. Laboratory testing was consistent with diabetic ketoacidosis (DKA) based on hyperglycemia with serum glucose level of 459 mg/dL, anion-gap metabolic acidosis with elevated lactate level of 4.5 mmol/L, and ketonuria. He also had grade 1 transaminitis with alanine aminotransferase (ALT) 87 U/L (normal, 7–55 U/L) and aspartate aminotransferase (AST) 105 U/L (normal, 8–48 U/L). Admission blood counts included hemoglobin 10.4 g/dL, new-onset thrombocytopenia with platelet count of 133×10(9)/L, and normal WBC, 5.8×10(9)/L. Coagulation studies were not available on admission. Computed tomography (CT) scan of the abdomen and pelvis was notable for splenomegaly measuring 16 cm that was not present on pre-treatment PET-CT scan (Figure 1B).
Despite initiation of appropriate DKA treatment, the patient’s acidosis worsened. He developed respiratory distress, distributive shock, and multiorgan dysfunction with acute renal injury and worsening transaminitis, necessitating transfer to the intensive care unit for intubation, vasopressor support, and continuous renal replacement therapy. In addition to transaminitis and splenomegaly, he was found to have several laboratory abnormalities suspicious for cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis (HLH)-like syndrome, including hyperferritinemia, hypertriglyceridemia, and pancytopenia (Table 1). The patient’s HScore was 181 points, correlating to a high score with 70–80% probability of hemophagocytic syndrome.
 |
Table 1 Laboratory Results Consistent with the Diagnosis of CRS and HLH-Like Syndrome |
Although he was treated empirically with broad-spectrum antibiotics, extensive infectious workup including bacterial, fungal, and viral cultures was negative. Specifically, viral evaluation was negative for viral hepatitis, influenza, respiratory syncytial virus, cytomegalovirus, Epstein Barr virus, and herpes simplex virus. HIV, adenovirus, enterovirus, HHV-6, and parvovirus B19 testing were not performed. Pneumocystis jiroveci pneumonia smear was negative. A repeat CT scan of the chest, abdomen, and pelvis showed no acute pathology, redemonstrating splenomegaly and the known mediastinal mass. A biopsy to identify tissue evidence of hemophagocytosis could not be obtained due to the acuity of the patient’s condition.
While awaiting the results of confirmatory HLH testing, the patient was started empirically on tocilizumab, etoposide, and glucocorticoids for treatment of both CRS and HLH. He also received therapeutic plasma exchange for management of hypertriglyceridemia. Cytokine panel results did eventually show elevated soluble IL-2 receptor alpha of >4000 pg/mL (normal, <959 pg/mL), elevated IL-18 of 2592 pm/mL (normal, <468 pg/mL) and elevated IL-6 level of >315 pg/mL (normal, <5.0 pg/mL). Unfortunately, the patient’s condition deteriorated as vasopressor requirements increased. Life support measures were withdrawn 2 days later in accordance with the patient’s previously stated wishes. On autopsy, there was no clear tissue evidence of hemophagocytes. Figure 2 illustrates the case timeline.
 |
Figure 2 Timeline of the case. Notes: Timeline created with BioRender.com. |
Discussion
Our case report describes a fatal case of severe CRS and HLH-like syndrome after the first dose of BV and nivolumab, generally thought to be a well-tolerated and safe regimen.
Immune evasion has been identified as the hallmark of carcinogenesis, and immunotherapy has revolutionized cancer therapies by enhancing the immune system and identifying cancer cells as “non-self”.5 Immune checkpoint inhibitors (ICIs) became a standard of care for many solid cancers and hematological malignancies (ie, melanoma, renal cell carcinoma, non-small cell lung carcinoma, and Hodgkin lymphoma).6
However, as ICIs activate the T-cell-mediated immune response, active T-cells can attack the healthy tissues due to cross-reactivity between the T-cells of the tumor and normal tissues.7 This phenomenon can give rise to a broad spectrum of immune-related adverse events (irAEs), of which the most common are endocrinopathies, gastrointestinal toxicity, pulmonary toxicity, arthritis, and skin rash.8
HLH is a severe, life-threatening disorder and has been rarely reported as an irAE secondary to ICIs. The pathophysiology of HLH with ICIs could be explained by immune system hyperactivation, which subsequently leads to increased levels of inflammatory cytokines and activation of the macrophages and reticuloendothelial system.9 In our literature search, we found 61 studies on PubMed and 17 studies across Embase database reporting secondary HLH after using ICIs. The most common malignancy associated with HLH was malignant melanoma, followed by non-small cell lung carcinoma.10
Pembrolizumab is the most frequently reported ICI causing HLH followed by nivolumab or nivolumab/ipilimumab.10 Moreover, Noseda et al reviewed the World Health Organization (WHO) global database of suspected drug reactions as of 2018; among 50,000 reported AEs from ICIs, HLH was reported in only 38 cases.11 The time from the initiation of an ICI to the development of HLH is highly variable across reports. While HLH mainly occurs after prolonged exposure to ICIs, with a median duration of ICI treatment of 9.9 months, it can arise at any point during the treatment, ranging from a few days following exposure to ICIs to 17 months after treatment initiation.10–13
BV is an antibody–drug conjugate that targets CD30 chimeric IgG1 antibodies and disrupts the microtubule network, leading to cell death.14 In 2018, the Food and Drug Administration (FDA) approved BV for use in the treatment of CD30-positive peripheral T-cell lymphomas (PTCL) based on the results of the ECHELON-2 trial, in which the most common AEs reported were peripheral neuropathy, GI toxicity, and myelosuppression.15 Cytokine release syndrome (CRS) has rarely been reported in the literature in association with the use of BV; in 2015, Alig et al reported the first case of CRS after the first dose of BV in a patient with anaplastic large cell lymphoma (ALCL).16 The activated T-cell response with BV can result in altered cytokine levels with increased levels of proinflammatory cytokines.15 Among 7542 AEs reported for BV in the FDA adverse events reporting system (FAERS) database, 72 were for hyperglycemia, 32 for HLH, and 13 for CRS.17 Although FAERS reported CRS and HLH with BV use, those incidents were not described previously on PubMed or Embase, making it impossible to determine whether these cases were genuinely associated with exposure to BV.
Distinguishing between primary HLH, secondary HLH, and CRS is challenging. CRS features can overlap with HLH.18 Both CRS and HLH are characterized by a hyperinflammatory state and cytokine storm. Severe forms of CRS can progress to organ failure and HLH-like presentation. Due to this hyperinflammatory state, the cytokine profile in severe CRS can be very similar to the one seen in true HLH.19 The presence of hepatosplenomegaly and histopathological evidence of hemophagocytosis would support the diagnosis of HLH and help distinguish between CRS and HLH. In our case, a tissue biopsy could not be pursued due to the patient’s hemodynamic instability. The patient did meet the HLH-2004 criteria.20 An autopsy showed splenomegaly with no definitive evidence of hemophagocytosis. We did not obtain an HLH gene panel, which would have been helpful to rule out genetic susceptibility to primary, adult-onset HLH and distinguish between primary and secondary HLH. Regardless, such testing can take several days to yield a result and would not have changed our treatment strategy in an acute setting. There is no history to suggest presence of familial HLH syndrome.
Our patient had evidence of hyperinflammatory state, CRS, and HLH-like syndrome with fever, splenomegaly, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia, and elevated soluble IL-2 receptor alpha.21 Other symptoms were consistent with treatment-related AEs, including refractory DKA, which can occur with exposure to ICIs or BVs.22–24 It is unclear whether BV, nivolumab, or the combination triggered the hyperinflammatory response in our patient. Moreover, we cannot exclude progression of cHL, given the new splenomegaly found on the patient’s admission, as a contributor to the HLH-like presentation. It could be posited that DKA itself might have contributed to HLH-like syndrome, but this possibility is unlikely as the patient’s clinical status worsened even after resolution of DKA. We suspect that the synergistic effect of BV and ICI on the immune system resulted in hyperactivation of immune-mediated cytokines and, ultimately, HLH-like syndrome.
Conclusion
With increasing applications of immunotherapeutic and targeted agents in many types of cancer, evidence continues to emerge about the potential toxicities of these agents. We aim to raise awareness among physicians and other medical providers about the possibility that even young patients receiving BV and nivolumab, generally regarded as a reasonably safe therapeutic combination, could develop life-threatening CRS and HLH-like syndrome. While ICIs have been linked to HLH previously, to our knowledge this case is the first in the literature to demonstrate a fatal event of HLH-like syndrome in cHL after the use of a combination of antibody–drug conjugate and ICI.
Ethics and Consent
The patient provided written informed consent for publication of the case report. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheson BD, Bartlett NL, LaPlant B, et al. Brentuximab vedotin plus nivolumab as first-line therapy in older or chemotherapy-ineligible patients with Hodgkin lymphoma (Accru): a multicentre, single-arm, Phase 2 trial. Lancet Haematol. 2020;7(11):e808–e815. doi:10.1016/S2352-3026(20)30275-1
2. Yasenchak CA, Bordoni R, Yazbeck V, et al. Phase 2 study of frontline brentuximab vedotin plus nivolumab in patients with Hodgkin lymphoma aged ≥60 years. Blood. 2019;134(Supplement_1):237. doi:10.1182/blood-2019-124866
3. Advani RH, Moskowitz AJ, Bartlett NL, et al. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood. 2021;138(6):427–438. doi:10.1182/blood.2020009178
4. Armand P, Engert A, Younes A, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm Phase II CheckMate 205 trial. JCO. 2018;36(14):1428–1439. doi:10.1200/JCO.2017.76.0793
5. Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front Pharmacol. 2022;13:868695. doi:10.3389/fphar.2022.868695
6. Klein BA, Shazib MA, Villa A, et al. Immune checkpoint inhibitors in cancer therapy: review of orofacial adverse events and role of the oral healthcare provider. Front Oral Health. 2022;3:968157. doi:10.3389/froh.2022.968157
7. Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20(1):e9. doi:10.4110/in.2020.20.e9
8. Martins F, Sofiya L, Sykiotis GP, et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi:10.1038/s41571-019-0218-0
9. Hakim NN, Chi J, Olazagasti C, Liu JM. Secondary hemophagocytic lymphohistiocytosis versus cytokine release syndrome in severe COVID-19 patients. Exp Biol Med. 2021;246(1):5–9. doi:10.1177/1535370220962043
10. Grewal US, Thotamgari SR, Shah PR, et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors: immune checkpoint inhibitor-related haemophagocytic lymphohistiocytosis. Eur J Cancer. 2021;153:270–271. doi:10.1016/j.ejca.2021.04.046
11. Noseda R, Bertoli R, Müller L, et al. Haemophagocytic lymphohistiocytosis in patients treated with immune checkpoint inhibitors: analysis of WHO global database of individual case safety reports. J Immunother Cancer. 2019;7:117. doi:10.1186/s40425-019-0598-9
12. Dupré A, Michot J, Schoeffler A, et al. Haemophagocytic lymphohistiocytosis associated with immune checkpoint inhibitors: a descriptive case study and literature review. Br J Haematol. 2020;189(5):985–992. doi:10.1111/bjh.16630
13. Liu LL, Skribek M, Harmenberg U, et al. Systemic inflammatory syndromes as life-threatening side effects of immune checkpoint inhibitors: case report and systematic review of the literature. J Immunother Cancer. 2023;11(3):e005841. doi:10.1136/jitc-2022-005841
14. Diefenbach CSM, Leonard JP. Targeting CD30 in Hodgkin lymphoma: antibody-drug conjugates make a difference. Am Soc Clin Oncol Educat Book. 2012;32:162–166. doi:10.14694/EdBook_AM.2012.32.83
15. Horwitz S, O’Connor OA, Pro B, et al. The ECHELON-2 Trial: 5-year results of a randomized, Phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol. 2022;33(3):288–298. doi:10.1016/j.annonc.2021.12.002
16. Alig SK, Dreyling M, Seppi B, et al. Severe cytokine release syndrome after the first dose of Brentuximab Vedotin in a patient with relapsed systemic anaplastic large cell lymphoma (s ALCL): a case report and review of literature. Eur J Haematol. 2015;94(6):554–557. doi:10.1111/ejh.12396
17. Qlik sense FDA Adverse Events Reporting System (FAERS) Public Dashboard - Brentuximab Vedotin. Available from: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/45beeb74-30ab-46be-8267-5756582633b4/state/analysis.
18. Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi:10.1186/s40425-018-0343-9
19. Maude SL, Barrett D, Teachey DT, Grupp SA. Managing cytokine release syndrome associated with novel t cell-engaging therapies. Cancer J. 2014;20(2):119–122. doi:10.1097/PPO.0000000000000035
20. Hines M, Bhatt N, Talano JAM. Diagnosis, treatment, and management of hemophagocytic lymphohistiocytosis in the critical care unit. Criti Care Pediat Immunocomp Hematol. 2018;159–182. doi:10.1007/978-3-030-01322-6_9
21. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465–2477. doi:10.1182/blood.2018894618
22. Thakkar K, Khurana S, Sun Y, Hembree TN. Diabetic ketoacidosis and profound insulin resistance from brentuximab vedotin. Cureus. 2023. doi:10.7759/cureus.35804
23. Quintas Joseph B, Mowatt KB, Mullally JA, et al. New onset persistent hyperglycemia with initiation of brentuximab treatment. Blood. 2021;138(Supplement 1):4540. doi:10.1182/blood-2021-148864
24. Tama E, Black M, Moustafa MA, Hurtado MD. Severe insulin resistance in a patient treated with nivolumab and brentuximab-vedotin for Hodgkin lymphoma. JCEM Case Rep. 2023;1(6):luad121. doi:10.1210/jcemcr/luad121
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
