Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Short-Term Oxygen Therapy Outcomes in COPD
Authors Soumagne T , Maltais F, Corbeil F, Paradis B, Baltzan M, Simão P, Abad Fernández A, Lecours R, Bernard S, Lacasse Y
Received 30 March 2022
Accepted for publication 21 July 2022
Published 28 July 2022 Volume 2022:17 Pages 1685—1693
DOI https://doi.org/10.2147/COPD.S366795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Thibaud Soumagne,1 François Maltais,1 François Corbeil,2 Bruno Paradis,3 Marc Baltzan,4 Paula Simão,5 Araceli Abad Fernández,6 Richard Lecours,7 Sarah Bernard,1 Yves Lacasse1 for the INOX Trial Group
1Quebec Heart and Lung Institute, Laval University, Quebec, Canada; 2Trois-Rivières Affiliated Hospital, Trois-Rivières, Canada; 3Laval Integrated Center of Health and Social Services, Laval, Canada; 4Mount Sinai Hospital, McGill University, Montreal, Canada; 5Pedro Hispano Hospital, Matosinhos, Portugal; 6Getafe University Hospital, Getafe, Spain; 7Hôtel-Dieu de Lévis Affiliated Hospital, Lévis, Canada
Correspondence: Yves Lacasse, Quebec Heart and Lung Institute - Laval University, 2725 Ste-Foy Road, Québec, P, Québec, G1V 4G5, Canada, Tel +1 418-656-4747, Fax +1 418-656-4762, Email [email protected]
Rationale: Short-term oxygen therapy (STOT) is often prescribed to allow patients with chronic obstructive pulmonary disease (COPD) to be discharged safely from hospital following an acute illness. This practice is widely accepted without being based on evidence.
Purpose: Our objective was to describe the characteristics and outcomes of patients with COPD who received STOT.
Patients and Methods: The study was a secondary analysis of the INOX trial, a 4-year randomised trial of nocturnal oxygen in COPD. The trial indicated that nocturnal oxygen has no significant effect on survival or progression to LTOT, allowing our merging of patients who received nocturnal oxygen and those who received placebo into a single cohort to study the predictors and outcomes of STOT regardless of the treatment received during the trial.
Results: Among the 243 participants in the trial, 60 required STOT on at least one occasion during follow-up. Patients requiring STOT had more severe dyspnoea and lung function impairment, and lower PaO2 at baseline than those who did not. STOT was associated with subsequent LTOT requirement (hazard ratio [HR]: 4.59; 95% confidence interval [CI]: 2.98– 7.07) and mortality (HR: 1.93; 95% CI: 1.15– 3.24). The association between STOT and mortality was confounded by age, disease severity and comorbidities. Periods of STOT of more than one month and/or repeated prescriptions of STOT increased the probability of progression to LTOT (OR: 5.07; 95% CI: 1.48– 18.8).
Conclusion: Following an acute respiratory illness in COPD, persistent hypoxaemia requiring STOT is a marker of disease progression towards the requirement for LTOT.
Keywords: oxygen therapy, short-term, long-term, mortality, chronic obstructive pulmonary disease
Plain Language Summary
Short-term oxygen therapy (STOT) is often prescribed to allow patients with COPD to be discharged safely from hospital following an acute illness. Persistent hypoxaemia after 1 month of STOT or more than 1 episode of STOT increase by 5 folds the probability of progression to long-term oxygen therapy.
Introduction
Patients with chronic obstructive pulmonary disease (COPD) may temporarily become severely hypoxemic during an acute exacerbation of the disease requiring hospitalization.1 In such circumstances, oxygen therapy for at least 15 to 18 hours per day may be prescribed for a short period of time (usually up to 3 months before reassessment)2 to allow patients to be discharged safely from hospital. This is referred to as “short-term oxygen therapy” (STOT)2 and appears to be a common medical practice, although it is not based on evidence.3,4
Close follow-up of patients who are prescribed STOT is important. Studies in patients with COPD who received STOT in the course of an acute exacerbation indicate that up to 60% of such individuals will remain severely hypoxemic at 3-month follow-up and will require that supplemental home oxygen be continued.5–9 STOT then becomes long-term oxygen therapy (LTOT) delivered in most cases for the remainder of the patient’s life. It is, however, recommended that oxygen be discontinued upon re-evaluation in those whose partial pressure in oxygen in arterial blood (PaO2) improves to the point that it exceeds the criteria for LTOT prescription.4 Unfortunately, formal reassessment of these patients is often neglected. Supplemental home oxygen following hospitalisation for an acute illness is often renewed without assessing patients for ongoing hypoxaemia.10–12 This situation has been identified by the Choosing Wisely initiative as one of the top five areas of improvement in adult pulmonary medicine.13
Characteristics of patients who received STOT in convalescence from an exacerbation, predictors of persisting severe hypoxaemia and long-term prognosis following STOT have not been well studied. This information is important in the development and testing of strategies to securely discontinue home oxygen in patients who recover sufficiently after an exacerbation.4 Our objectives were therefore to describe the characteristics and outcomes of patients with COPD who received STOT and to determine factors associated with the requirement of LTOT after STOT.
Material and Methods
Study Subjects
Patients were recruited from November 2010 to January 2015 in 28 community and university affiliated hospitals in Canada, Portugal, Spain and France.14 Patients with a diagnosis of COPD (defined by a postbronchodilator forced expiratory volume in 1 second [FEV1] <70% of the predicted value, a ratio of the FEV1 to the forced vital capacity [FVC] <0.70, and a total lung capacity as measured by body plethysmography >80% of the predicted value, concurrently with a history of smoking) were included. All had isolated nocturnal oxygen desaturation (defined on the home oximetry as ≥30% of the recording time (time in bed) with a transcutaneous arterial oxygen saturation <90%).15 Also, all had stable disease for at least 6 weeks before enrolment and had not smoked for at least 6 months.
Exclusion criteria were severe daytime hypoxaemia according to the Nocturnal Oxygen Therapy Trial (NOTT) criteria (PaO2 ≤55 mmHg while breathing ambient air, or a PaO2 56–59 mmHg with evidence of cor pulmonale or erythrocytosis),16 sleep apnoea (apnoea/hypopnea index of ≥15 events/hour), current use of nocturnal oxygen and significant respiratory or cardiovascular diseases other than COPD that may influence survival.
Study Design
This study was a secondary analysis of the INOX trial (ClinicalTrials.gov ID: NCT01044628), a 4-year, multi-centre, randomised, double-blind, placebo-controlled trial assessing nocturnal oxygen therapy in COPD.14,17 The INOX trial received full approval from the Ethics Committee of the principal investigator’s institution (Institut universitaire de cardiologie et de pneumologie de Québec; CER-20490) and from the Ethics Committee of all the participating centers. The INOX trial was conducted in accordance with the Declaration of Helsinki. All participants gave informed consent. The INOX trial indicated that nocturnal oxygen has no significant effect on the primary outcomes of survival or progression to LTOT in patients with isolated nocturnal desaturation. These results allowed our merging of patients who received nocturnal oxygen during the trial and those who received placebo (sham oxygen therapy with a modified O2 concentrator delivering room air) into a single cohort to study the predictors and outcomes of STOT regardless of the treatment received during the trial.
Short-Term Oxygen Therapy
As per protocol, STOT could be provided during the course of the trial in case of acute respiratory illness (eg, acute exacerbation of COPD or pneumonia) necessitating or not hospitalisation. Patients’ evaluation and treatment were then under the responsibility of the treating physician. STOT prescription criteria were not protocol-based. We assumed that STOT was only provided to those who were found to be severely hypoxemic during the day (eg, SpO2 ≤88% or paO2 ≤55 mmHg), and that it was prescribed for at least 15–18 hours per day at a flow that increased SpO2 to at least 90%. During STOT, patients were asked not to use their study concentrator (to avoid those on placebo to receive ambient air) and were provided with another oxygen concentrator that was effective. However, protocol-based follow-up was mandatory within 90 days following STOT prescription. Upon re-evaluation, patients who remained severely hypoxemic according to the NOTT criteria were continued on LTOT. Otherwise, oxygen therapy was discontinued and patients were returned to their original treatment assignment within the trial (nocturnal oxygen or placebo).
Data Collection and Follow-Up
Baseline clinical measurements included the Charlson comorbidity index,18 spirometry, lung volumes measured by plethysmography, carbon monoxide diffusion capacity (TLCO) measured by the single-breath method and arterial blood gas measurements. Patients were followed for 3 to 4 years. They were contacted by telephone every 2 months in order to collect adverse events. On-site visits took place every 4 months for clinical assessment, including pulse oximetry. Arterial blood gas and lung function tests were obtained every 12 months or more frequently is required. To describe lung function at the time of STOT, we used the data obtained in stable condition at the closest point in time. The primary outcomes of this secondary analysis were mortality and progression to LTOT in the course of the trial.
Statistical Analysis
Data are presented as number (percent) and mean ± standard deviation (or median with interquartile range) for qualitative and quantitative variables, respectively. Quantitative variables were compared with Student’s t-tests or Wilcoxon tests, as appropriate whereas qualitative variables were compared using chi-square or Fisher exact tests. In case of multiple episodes of STOT during the trial, the analysis considered only its first occurrence. The mean numbers of exacerbations and hospitalisations per patient-year were compared between groups with the use of a Poisson distribution with overdispersion correction.19 We constructed survival curves using Kaplan–Meier estimates and used the Log rank test to compare the outcome of those who received STOT during the trial and those who did not. Hazard ratios and 95% confidence intervals for death or requirement for long-term oxygen therapy were estimated with the use of Cox regression models. Finally, to identify factors associated with progression to LTOT, multivariate logistic regression analysis with backward stepwise selection was performed among patients requiring STOT. In this analysis, duration of STOT was transformed into a dichotomous variable by using receiver-operating characteristics (ROC) analysis to determine the threshold at which STOT duration best predicted progression to LTOT with minimal false prediction rate. We also computed the area under the ROC curve to indicate the probability that a random pair of patients, one progressing to LTOT but not the other, will be correctly classified as to their disease state.20 All variables associated with progression to LTOT in the univariate analysis with a p value of less than 0.20 were included. Statistical analysis was performed with R version 4.0.3 and RStudio version 1.4.1103 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
A total of 243 patients were enrolled in the INOX trial; 60 (25%) required STOT on at least one occasion during follow-up (Figure 1). The main reason for initiating STOT was an acute exacerbation of COPD in 53 patients (88%). Nine patients received STOT more than once during the trial: STOT was prescribed in 2 and 3 occasions in 7 and 2 patients, respectively. Baseline characteristics of patients who received STOT are compared with those who did not in Table 1. Patients requiring STOT during follow-up were significantly older and had higher dyspnoea, more severe lung function impairment, and lower PaO2 at baseline than those who did not. The proportion of patients who received nocturnal oxygen or placebo (sham nocturnal oxygen therapy) was similar in the STOT and the non-STOT groups.
 |
Table 1 Baseline Characteristics of Patients |
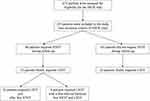 |
Figure 1 Patient flow diagram. See14 for more details. |
Progression to LTOT
During the trial, 84 patients progressed to LTOT, including 42 of the 60 (70%) patients who previously required STOT and 42 of the 183 (23%) who did not. Among the 42 patients who received STOT and who finally required LTOT, 34 (81%) were prescribed LTOT immediately after STOT (ie, upon re-evaluation while still receiving STOT), and 8 (19%) had an oxygen-free interval (excluding nocturnal oxygen or placebo) between STOT and LTOT (median interval of 240 days). Survival analysis indicated that STOT was significantly associated with subsequent LTOT requirement (Figure 2A). This association remained significant after adjusting for age, FEV1, TLCO, dyspnoea score and PaO2 (adjusted hazard ratio 3.79; 95% CI 2.29–6.27).
Mortality
Sixty-one patients died during follow-up, including 23 of the 60 (38%) patients who previously required STOT and 38 of the 183 (21%) who did not. STOT requirement was significantly associated with mortality (Figure 2B). However, this association was not significant after adjusting for confounders associated with mortality including age, Charlson comorbidity index, FEV1, TLCO, dyspnoea score and PaO2 (adjusted hazard ratio 1.57; 95% CI 0.87–2.81).
Factors Associated with LTOT Among Patients Requiring STOT
Patients who progressed to LTOT had a significantly longer duration of STOT and more severe airway obstruction (post-BD FEV1/FVC) at the time of first STOT prescription (Table 2). Demographics, symptoms, quality of life, exacerbation rate, and hospitalisation rate did not differ at the time of first STOT prescription between patients who ultimately required LTOT and those who did not. ROC analysis indicated that a STOT duration of >32 days best predicted progression to LTOT with an area under curve of 0.69 (95% CI: 0.55–0.83), with a positive predictive value of 83%.
 |
Table 2 Characteristics of Patients at the Time of First Short-Term Oxygen Therapy (n = 60) |
Table 3 shows the results of the univariate and multivariate analyses in the subgroup of patient requiring STOT (n = 60) for the association between LTOT and several selected variables. Multivariate analysis showed that requirement for LTOT was significantly associated with a duration of STOT > 32 days and/or several prescriptions of STOT and a lower FEV1/FVC ratio.
 |
Table 3 Risk Factors for LTOT in Patients Requiring STOT (Univariate and Multivariate Analysis) |
Discussion
The results of this study indicate that patients with COPD who required STOT in the course of an acute respiratory illness had more severe disease than those who did not and progressed more frequently towards LTOT during follow-up. The association between STOT and increased mortality was confounded by age, poor lung function and comorbidities. Requirement for STOT for more than one month and/or repeated periods of STOT represent independent predictors for progression towards LTOT.
The requirement for LTOT should be determined when the patient is in stable condition according to prescription criteria that are derived from the inclusion criteria of the NOTT and the MRC trial and are well accepted worldwide.16,21,22 On the contrary, STOT is provided during a period of clinical instability and its prescription criteria are ill-defined. Most clinicians will consider providing their patients with home oxygen at hospital discharge if severe resting hypoxaemia persists. Previous studies of STOT used the same prescription criteria as for LTOT (PaO2 ≤55 mmHg at rest, or a PaO2 <60 mmHg with evidence of cor pulmonale or erythrocytosis).6,10 Although reasonable, these criteria are not based on evidence.
The prescription of STOT at hospital discharge is first and foremost a matter of safety. Important clinical outcomes include hospital readmission for respiratory failure, incidence of arrhythmia, myocardial ischemia or heart failure, quality of life and mortality. The real impact of STOT on these outcomes is unclear however. It is very unlikely that a clinical trial of STOT will ever be conducted for obvious reasons. Nevertheless, STOT is usually prescribed in the course of acute exacerbations of COPD that are themselves associated with increased morbidity and mortality, irrespective of the presence of severe hypoxaemia.23 The one-year mortality rate after a severe exacerbation requiring hospitalisation ranges from 20% to 43%.24–26 In one study, the most frequent causes of death at 1-year follow-up were respiratory and cardiovascular disorders.26 Hypoxaemia and requirement for oxygen therapy at discharge have also been reported as independent predictors of long-term mortality.27–29 We would consider these findings as justifications for STOT when severe hypoxaemia persists at hospital discharge.
In our opinion, the issue is not to determine whether or not STOT is truly indicated at hospital discharge. Rather, the question is whether or not STOT should be discontinued, and if so, when is the most appropriate duration of STOT prior to re-evaluation. Several national practice guidelines recommend reassessing the need for oxygen within 2 to 3 months.3,4,30 In previous studies, between 16% and 56% of patients who were prescribed STOT do not recover from severe hypoxaemia and progress toward LTOT.5–9 In the INOX trial, 70% of those who received STOT ultimately required LTOT. We also found that periods of STOT of more than one month and repeated prescriptions of STOT increased by 5 times the probability of progression to LTOT. Since LTOT improves survival,16,21 we submit that LTOT should be considered in patients who remain severely hypoxemic one month after being discharged from hospital or who have received more than one episode of STOT. Arterial blood gas measurement should be preferred to pulse oximetry to determine the need for LTOT.31 If severe hypoxaemia persisted, STOT would then become LTOT.
Our study has limitations. First, the prescriptions of STOT were not based on protocol. We assumed that STOT was only provided to those who were found to be severely hypoxemic (eg, SpO2 ≤88% or paO2 ≤55 mmHg). This practice is widespread and well accepted.3,4 Second, our study is a secondary analysis of a clinical trial in patients with isolated nocturnal desaturation. It has been suggested that this phenomenon may represent an independent risk factor for the development of chronic hypoxaemia in patients with COPD.32 Whether our results apply to all patients with COPD is uncertain. However, the INOX trial indicated that nocturnal oxygen has no significant effect on survival or requirement to LTOT.14 In addition, we confirmed that nocturnal oxygen therapy had no effect on the requirement of STOT.
Conclusion
Our study emphasizes the recommendation to closely follow patients discharged from hospital with STOT. Requirement for STOT at hospital discharge in the course of an acute respiratory illness in COPD is a factor of poor prognosis. We also identified predictors of progression to LTOT in patients who received STOT. We suggest that continuing LTOT in patients who remain severely hypoxemic after a period of one month of home oxygen therapy and/or in those who have more than one episode of STOT may be considered.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. A complete list of the investigators in the International Nocturnal Oxygen (INOX) Trial Group is provided in the Online Supplement.
Funding
Funded by the Canadian Institutes of Health Research (Grant MCT99512). The sponsor was not involved in any of the stages of this study from the design to the submission of the manuscript for publication.
Disclosure
T Soumagne has no conflict of interest to disclose. F Maltais reports grants from GlaxoSmithKline, AstraZeneca, Sanofi, Novartis, Boehringer Ingelheim, and Grifols, and personal fees for serving on speaker bureaus and consultation panels from GlaxoSmithKline, Boehringer Ingelheim, Grifols, and Novartis; he is financially involved with OxyNov, a company which is developing an oxygen delivery system. F Corbeil, B Paradis, M Baltzan, P Simão, A Abad Fernández, R Lecours and S Bernard have no conflict of interest to disclose. Y Lacasse reports participation in Innovair, a company that holds shares in OxyNov, the owner of FreeO2, an automated oxygen delivery system.
References
1. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
2. Lacasse Y, Tan AM, Maltais F, Krishnan JA. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(10):1254–1264. doi:10.1164/rccm.201802-0382CI
3. Hardinge M, Annandale J, Bourne S, et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax. 2015;70(Suppl 1):i1–43. doi:10.1136/thoraxjnl-2015-206865
4. Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(10):e121–e141. doi:10.1164/rccm.202009-3608ST
5. Spece LJ, Epler EM, Duan K, et al. Reassessment of home oxygen prescription after hospitalization for chronic obstructive pulmonary disease. A potential target for deimplementation. Ann Am Thorac Soc. 2021;18(3):426–432. doi:10.1513/AnnalsATS.202004-364OC
6. Khor YH, Wong R, McDonald CF. Post-hospitalization short-term oxygen therapy: use of a clinical management pathway and long-term follow-up. Respir Care. 2019;64(3):272–278. doi:10.4187/respcare.06303
7. Levin K, Borg B, Miller B, Kee K, Dabscheck E. Characteristics of patients who progress from bridging to long-term oxygen therapy. Intern Med J. 2018;48(11):1376–1381. doi:10.1111/imj.13737
8. Eaton TE, Grey C, Garrett JE. An evaluation of short-term oxygen therapy: the prescription of oxygen to patients with chronic lung disease hypoxic at discharge from hospital. Respir Med. 2001;95(7):582–587. doi:10.1053/rmed.2001.1106
9. Chaney JC, Jones K, Grathwohl K, Olivier KN. Implementation of an oxygen therapy clinic to manage users of long-term oxygen therapy. Chest. 2002;122(5):1661–1667. doi:10.1378/chest.122.5.1661
10. Oba Y, Salzman GA, Willsie SK. Reevaluation of continuous oxygen therapy after initial prescription in patients with chronic obstructive pulmonary disease. Respir Care. 2000;45(4):401–406.
11. Morrison D, Skwarski K, MacNee W. Review of the prescription of domiciliary long term oxygen therapy in Scotland. Thorax. 1995;50(10):1103–1105. doi:10.1136/thx.50.10.1103
12. Ringbaek TJ, Lange P. The impact of the Danish Oxygen Register on adherence to guidelines for long-term oxygen therapy in COPD patients. Respir Med. 2006;100(2):218–225. doi:10.1016/j.rmed.2005.04.023
13. Wiener RS, Ouellette DR, Diamond E, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: the Choosing Wisely top five list in adult pulmonary medicine. Chest. 2014;145(6):1383–1391. doi:10.1378/chest.14-0670
14. Lacasse Y, Series F, Corbeil F, et al. Randomized trial of nocturnal oxygen in chronic obstructive pulmonary disease. N Engl J Med. 2020;383(12):1129–1138. doi:10.1056/NEJMoa2013219
15. Levi-Valensi P, Weitzenblum E, Rida Z, et al. Sleep-related oxygen desaturation and daytime pulmonary haemodynamics in COPD patients. Eur Respir J. 1992;5(3):301–307.
16. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93(3):391–398. doi:10.7326/0003-4819-93-3-391
17. Lacasse Y, Bernard S, Sériès F, et al. Multi-center, randomized, placebo-controlled trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease: a study protocol for the INOX trial. BMC Pulm Med. 2017;17(1):8. doi:10.1186/s12890-016-0343-9
18. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
19. Aaron SD, Fergusson D, Marks GB, et al. Counting, analysing and reporting exacerbations of COPD in randomised controlled trials. Thorax. 2008;63(2):122–128. doi:10.1136/thx.2007.082636
20. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi:10.1148/radiology.143.1.7063747
21. Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1(8222):681–686.
22. Wijkstra PJ, Guyatt GH, Ambrosino N, et al. International approaches to the prescription of long-term oxygen therapy. Eur Respir J. 2001;18(6):909–913. doi:10.1183/09031936.01.00202301
23. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
24. Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–89. doi:10.1513/AnnalsATS.201208-043OC
25. Slenter RH, Sprooten RT, Kotz D, Wesseling G, Wouters EF, Rohde GG. Predictors of 1-year mortality at hospital admission for acute exacerbations of chronic obstructive pulmonary disease. Respiration. 2013;85(1):15–26. doi:10.1159/000342036
26. García-Sanz MT, Cánive-Gómez JC, Senín-Rial L, et al. One-year and long-term mortality in patients hospitalized for chronic obstructive pulmonary disease. J Thorac Dis. 2017;9(3):636–645. doi:10.21037/jtd.2017.03.34
27. Piquet J, Chavaillon J-M, David P, Martin F, Blanchon F, Roche N. High-risk patients following hospitalisation for an acute exacerbation of COPD. Eur Respir J. 2013;42(4):946. doi:10.1183/09031936.00180312
28. Connors AF, Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med. 1996;154(4 Pt 1):959–967. doi:10.1164/ajrccm.154.4.8887592
29. Matkovic Z, Huerta A, Soler N, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of chronic obstructive pulmonary disease. Respiration. 2012;84(1):17–26. doi:10.1159/000335467
30. McDonald CF, Whyte K, Jenkins S, Serginson J, Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76–78. doi:10.1111/resp.12678
31. Lacasse Y, Thériault S, St-Pierre B, et al. Oximetry neither to prescribe long-term oxygen therapy nor to screen for severe hypoxaemia. ERJ Open Res. 2021;7(4):00272–2021. doi:10.1183/23120541.00272-2021
32. Sergi M, Rizzi M, Andreoli A, Pecis M, Bruschi C, Fanfulla F. Are COPD patients with nocturnal REM sleep-related desaturations more prone to developing chronic respiratory failure requiring long-term oxygen therapy? Respiration. 2002;69(2):117–122. doi:10.1159/000056313
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

