Back to Journals » Biologics: Targets and Therapy » Volume 17
Significance of Interleukin 23 in Systemic Lupus Patients: Relation to Disease Activity and Damage Indices
Authors Haroon MM , Hegazy GA , Hassanien MA , Shaker O , Hussein WH
Received 7 October 2022
Accepted for publication 27 December 2022
Published 18 January 2023 Volume 2023:17 Pages 1—9
DOI https://doi.org/10.2147/BTT.S389021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Maysa M Haroon,1 Gehan A Hegazy,2,3 Mohammed A Hassanien,4,5 Olfat Shaker,6 Wafaa H Hussein1
1Department of Rheumatology, Faculty of Medicine, Cairo University, Cairo, Egypt; 2Clinical Biochemistry Department, Faculty of Medicine, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia; 3Medical Division, National Research Centre, Giza, Egypt; 4Vice Presidency for Educational Affairs and Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia; 5Medical Biochemistry Department, Faculty of Medicine, Tanta University, Tanta, Egypt; 6Departments of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, Cairo, Egypt
Correspondence: Maysa M Haroon, Department of Rheumatology, Faculty of Medicine, Cairo University, 71 El Kasr El Aini Street, P.O.Box 11562, Cairo, Egypt, Tel +201025868370, Email [email protected]
Background: Dysregulation of both cellular and humoral immune responses is central in systemic lupus erythematosus (SLE) pathogenetic mechanisms. Proinflammatory cytokines, such as interleukin 23 (IL23), and their roles in promoting such dysregulation have recently been highly considered. This research compared IL23 serum levels in 85 Egyptian SLE patients and 85 healthy controls. Then, IL23 level was correlated to various SLE disease parameters, disease activity, and damage indices.
Results: IL23 serum levels were significantly elevated in SLE patients versus healthy individuals. Furthermore, IL23 levels were positively correlated with SLE disease activity index (SLEDAI) and were positively correlated with arthritis, seizures, consumption of complements (C3, C4), and with parameters of nephritis (hematuria, pyuria, casts, and proteinuria). A positive correlation was also found between IL23 levels and oral prednisolone dose.
Conclusion: IL23 has higher levels in the serum of SLE patients, and is correlated to activity of the disease, especially lupus nephritis. Further researchis needed to explore its exact role in SLE pathogenesis and whether it can be considered a potential biomarker or therapeutic target in SLE.
Keywords: interleukin 23, proinflammatory cytokines, systemic lupus erythematosus, SLE disease activity index, lupus nephritis
The Plain Language Summary
Autoimmune diseases occur when your immune system attacks your own body. Systemic lupus erythematosus (SLE) is one of these autoimmune diseases which can affect different tissues, causing great damage. SLE affects more than 5 million people worldwide, mostly women of childbearing age. What makes your immune system behave like this is not fully clear, but it is likely an interaction between your genetics and your environment. The function of your immune system primarily depends on secreted proteins and signal molecules called interleukins (ILs). Some interleukins may be linked to the autoimmune inflammation in SLE, eg, IL23.
We compared the amount of IL23 in SLE patients and in normal people. We also checked the link between IL23 and various symptoms of lupus. Interestingly, we found IL23 is higher in SLE patients. Moreover, there was a link between IL23 and serious SLE symptoms. These results are important because they revealed some information about this protein in SLE. We hope that future studies can discover the precise role of IL23 in SLE. In addition, we predict that, in the near future IL23 might be used as a marker for serious SLE symptoms, or a target for new therapy for this challenging disease.
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease distinguished by its very heterogeneous and composite clinical picture. SLE pathogenesis is not completely elucidated but it is believed to be multifactorial, involving hereditary, environmental, as well as hormonal factors. Dysregulation of both cellular and humoral immune responses is central in SLE pathogenetic mechanisms. The result is disruption of self-tolerance and formation of autoantibodies with subsequent production and precipitation of immune complexes in various organs. This ultimately leads to complement activation and aggregation of neutrophils, monocytes, and lymphocytes.1–3
The role of T-lymphocytes in SLE pathogenesis is evident by signal transduction, aberrant cytokine secretion, in addition to activation and accumulation of B lymphocytes and dendritic cells (DC).4
A subtype of T cells, Th17 is implicated in SLE pathogenesis by producing proinflammatory cytokines, mainly interleukin 17 (IL17), depending on the presence of another cytokine, IL23. IL23 has a role in the growth and differentiation of Th17 as well as its sustained IL17 secretion.5
Interleukin-23 (IL-23) is one of the IL-12 cytokine family. It is a heterodimer that includes IL-12p40 and IL-23p19 subunits. The p19 subunit of IL-23 is formed by antigen-presenting cells (APC) as well as T lymphocytes and endothelial cells. The P40 subunit is formed only by antigen-presenting cells like macrophages, monocytes, and dendritic cells.6,7 Engagement of IL23 to its heterodimeric receptor complex composed of IL-12Rb1 and IL-23R is required to exert its biological activities.7,8 Interleukin-23 was found to be up-regulated in patients with several autoimmune disorders, such as Crohn’s disease, rheumatoid arthritis, and multiple sclerosis.9 Previous studies showed controversial data regarding the association of IL23 with disease activity of SLE through IL17 pathways.5
The current study was conducted to compare the serum levels of IL23 in a cohort of Egyptian SLE patients with those in healthy controls, and to study the correlation of IL23 levels with SLE manifestations, as well as activity and damage indices using the SLE–Disease Activity Index (SLE-DAI) and the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SLICC/ACR-DI), respectively.
Patients and Methods
Study Design and Participants Recruitment
The present case-control research was carried out on 85 adult Egyptian patients with SLE diagnosed according to The 2019 European League Against Rheumatism/American College of Rheumatology (EULAR/ACR) classification criteria for Systemic Lupus Erythematosus.10 Patients were regular attendees following up at the outpatient clinic and the inpatient unit of the Rheumatology department, Kasr Al-Ainy Faculty of Medicine, Cairo University, during the year 2022. Patients were excluded if they had any malignancy, other autoimmune disease, mixed connective tissue disease, overlap syndrome, or were pregnant at the time of study. Eighty-five healthy adults (age- and sex-matched) were included as controls. All individuals signed their informed consent before enrollment. The study has been approved by the Local Scientific Research Ethics Committee of the Faculty of Medicine, Cairo University, number (N-154-2022), and was carried out in accordance to the Declaration of Helsinki.
Data Collection
For all patients, detailed history taking and clinical and rheumatological examination were performed. Demographic data, disease duration, medication history, presence of concomitant chronic diseases, such as hypertension (HTN) and diabetes mellitus (DM), as well as clinical data of the domains for disease activity and damage indices: SLEDAI and SLICC/ACR-DI11,12 were collected from the case record forms (CRFs) of the patients. Laboratory investigations included erythrocyte sedimentation rate (ESR), complete blood count (CBC), full basic biochemical analysis (including 24 hours urinary protein), as well as immunological profiles (including immunofluorescence antinuclear antibodies (ANA) and anti-double stranded antibodies (ds-DNA), complement 3 (C3), and complement 4 (C4).
Determination of Human Interleukin 23 (IL-23) in Serum of SLE Patients
IL-23 level was estimated in sera of patients and controls using ELISA kit provided by SunLong Biotech Co., LTD (Catalogue Number: SL0989Hu), Gangzhou, China. This ELISA kit utilizes Sandwich-ELISA as the method. A pre-coating with IL-23-specific antibody has been applied to the Microelisa stripplate included in this kit. The correct Microelisa stripplate wells are filled with standards or samples, and the particular antibody is then added. Then, each Microelisa stripplate well receives an addition of an antibody that has been Horseradish Peroxidase (HRP)-conjugated and is specific for IL-23. Uncombined components are removed. After adding TMB substrate solution to each well, only the wells containing IL-23 and the HRP-conjugated IL-23 antibody will initially appear blue, which changes to yellow when the stop solution is added. At a wavelength of 450 nm, the optical density (OD) is measured spectrophotometrically. The relationship between the OD value and IL-23 concentration is linear. By comparing the OD of the samples to the standard curve, the concentration of IL-23 in the samples was determined.
Statistical Analysis
The statistical analysis was performed by IBM SPSS Statistics for Windows, version 23 (IBM SPSS, IBM Corp., Armonk, NY). Shapiro–Wilk test was utilized to assess normal value distribution. Mean ± standard deviation (minimum–maximum) were used to present quantitative variables, while frequencies (number of cases) and relative frequencies (%) were used for categorical data. Statistical comparisons were made for independent parametric parameters by Mann Whitney test when data were not normally distributed and unpaired Student’s t-test for normally distributed parametric parameters. Pearson Chi square (χ2) test was used for comparing categorical data. Correlations were done between quantitative variables using Spearman correlation coefficient. P-values were statistically significant if less than 0.05.
Results
The demographic criteria of both patients and controls are presented in Table 1. The clinical and laboratory data within the patients group are presented in Table 2. Study group included 71 (83.5%) females and 14 (16.5%) males with mean age 31.45±7.66 years. The duration of the disease ranged from 1–17 years. The age at disease onset ranged from 19–54 years with the mean 25.95±6.62 years. Antinuclear antibodies were present in 100% of patients while anti ds-DNA antibodies were found in 50.6%. Lupus nephritis (LN) was present in 62 patients (72.9%). Among them, 20 patients (23.5%) had focal proliferative glomerulonephritis (class III), 28 patients (32.9%) had diffuse proliferative glomerulonephritis (class IV), and eight patients (9.4%) had membranous glomerulonephritis (class V). The comorbidities were hypertension in 30 (35.3%) patients and diabetes mellitus in six patients (7.1%). As regards medications: oral Steroids prednisolone (P5) were used in 72 (84.7%) patients at a mean dose of 24.07±14.77 mg daily. Intravenous steroids with methyl prednisolone (IV-MP) were administered in 25 (29.4%) patients. Other disease modifying and immunosuppressive medications were used with the following frequencies: hydroxychloroquine (HCQ), 65 (76.5%); azathioprine (AZA), 30 (35.3%); mycophenolate mofetil (MMF), 8 (9.4%); and cyclophosphamide (CYC), 12 (14.1%).
 |
Table 1 Demographic Characteristics of Patients and Controls |
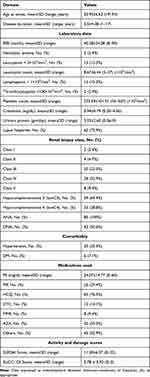 |
Table 2 Clinical and Laboratory Characteristics Within the Patients’ Group (N=85) |
IL23 serum levels were significantly elevated among patients versus controls (P<0.0001) (Table 3). A correlation study was performed between IL23 and various clinical and laboratory parameters as well as total activity and damage indices. IL23 showed a significant positive correlation with SLE-DAI score (r=0.840, P<0.0001), disease duration (r=0.291, P=0.007), seizures (r=0.219, P=0.044), arthritis (r=0.223, P=0.040), urinary casts (r=0.539, P<0.0001), hematuria (r=0.825, P<0.0001), pyuria (r=0.758, P<0.0001), proteinuria (r=0.683, P<0.0001), hypocomplementemia (r=0.060, P<0.0001), and prednisone dose (r=0.457, P<0.0001). Other parameters (including the SLICC/ACR-DI score) showed a positive but insignificant correlation. Interestingly, vasculitis showed a low negative correlation with IL 23, which was not significant (Table 4). In relation to LN, IL23 levels were significantly higher in patients with than without LN. Further analysis in relation to the class of glomerulonephritis by renal biopsy revealed an insignificant difference between classes, yet the higher level was among class III (focal proliferative glomerulonephritis) (Table 5).
 |
Table 3 Comparison of IL23 (Pg/Ml) of Patients and Controls |
 |
Table 4 Correlations Between IL-23 and Measured Parameters |
 |
Table 5 Comparison of IL23 Levels in Patients with and without LN and Among Different Renal Biopsy Classes Within the LN Group |
Discussion
SLE is an inflammatory multisystem autoimmune disorder affecting numerous organs and distinguished by multifactorial etiology and pathogenesis. Immune dysregulation (innate and adaptive) is important in SLE pathogenesis. Autoantibodies and subsequent immune complex formation are crucial in SLE inflammatory pathways. Many cytokines are induced by immune complexes,13 and it has been shown that some cytokines are closely associated with pathogenesis of SLE.14 Since the definite pathogenetic pathways in SLE are still mysterious, the researchers tended to study several cytokines of the pro-inflammatory and anti-inflammatory categories, anticipating to discover their potential role. Among these cytokines are IL6, IL10, interferon-gamma (IFNγ), and tumor necrosis factor (TNF), which have been found to be helpful in the assessment of SLE serological and clinical activity.15 Another distinct one of these cytokines is the interleukin 23 (IL23), which is formed by monocytes and motivates the differentiation of T cells into Th17 cells.16
Our study involved 85 patients diagnosed as SLE, and 85 gender- and age-matched persons served as healthy controls. All participants were tested for IL23 serum level, and it was correlated with disease parameters within the SLE group.
The results of our study revealed that IL23 serum levels were significantly elevated in SLE patients versus healthy control individuals. These results were similar to many studies that had been done before and had shown that IL23 serum levels were elevated in SLE patients versus normal healthy controls.3,5,17
These results raised the possibility that IL23 might have a role in SLE pathogenesis and that it might be related to disease parameters.
IL23 has a key role in Th17 cells differentiation that forms IL17.18 IL17 induces the generation of many proinflammatory cytokines like tumor necrosis factor–alpha (TNF-α), IL6, some chemokines, and metalloproteinases, in addition to recruitment of neutrophils to tissues which are involved in SLE pathogenesis.19
Both IL23 and IL17 have vital roles in inflammation, they correspond to the IL23/IL17 axis, which promotes autoimmunity and chronic inflammation.20
Elevated IL17 serum levels have been revealed in SLE patients and were associated with increased SLE disease activity, indicating that the IL23/IL17 axis is implicated in SLE pathogenesis and disease activity.21 In lupus-predisposed mice, it had been found that a IL23 receptor lack leads to decreased IL17 production, and thus these mice were protected from developing the disease.22
In this research, IL23 levels were positively correlated with SLE disease activity index (SLEDAI) and also were positively correlated with arthritis, seizures, C3&C4 consumption, and with parameters of active nephritis (hematuria, pyuria, casts, and proteinuria). There was also a positive correlation between IL23 levels and oral prednisolone dose.
These results are comparable to other studies done in the same subject. One of these studies was the study done by Justiz Vaillant and Akpaka who had found that IL23 levels elevated in SLE patients versus normal controls and that IL23 levels were positively correlated with disease activity index (SLEDAI).23 In addition, our results were concomitant with the study done by Vukelic et al, who also showed that IL23 levels were positively correlated with SLEDAI, arthritis, and nephritis.24 Fischer et al3 had also found that IL23 levels were positively correlated with nephritis.
The role of IL23 in developing arthritis is obvious from its proved role in pathogenesis of synovitis in RA. The significance of the IL23/IL17 axis in RA pathogenesis, involving synoviocytes, osteoclasts, and immune cells, had been proved. Both IL17 and IL23 were absent in healthy joints, whereas their elevated levels were shown in the serum and synovial fluid of RA patients, corresponding to the IL23/IL17 axis in RA pathogenesis.24
The positive correlation between IL23 and oral steroid dose may be attributed to the associated increased oral steroids dose with increased disease activity. In addition, the correlation with C3 and C4 consumption could be explained by association of hypocomplementemia with SLE disease activity, especially in lupus nephritis. From these results, it could be postulated that IL23 plays a role in SLE activity, especially lupus nephritis.
In our study, we found that IL23 level was significantly higher among SLE patients with LN than in patients without LN. However, further analysis did not reveal a significant difference within different glomerulonephritis classes diagnosed by renal biopsy, yet the higher value was in the focal proliferative class III. These findings were agreed with Zickert et al,25 who had found that IL23 levels were higher in patients with biopsy proved lupus nephritis than in non-nephritic patients.
Zhang et al18 studied the role of IL23/IL17 in LN in mice and they found that IL23 promoted an autoimmune humoral response through autoantibodies deposition in kidneys and complement system activation causing nephritis.
Dedong et al26 found that IL17 was present in glomeruli of LN patients. Their findings showed that the IL23/IL17 axis has an important role in LN pathogenesis and that both cytokines might be useful as convenient biomarkers for development and assessment of renal disease.
It was found that there is increased glomerular expression of IL17, IL18, IL6, and IL23 in class IV lupus nephritis in comparison to normal controls indicating that there is more infiltration of Th17 cells in the glomeruli and an upregulation of IL23/IL17 and urinary cytokines obtained from this signaling pathway.27
Because of these observations, we can assume that the IL23/IL17 axis has a role in SLE disease manifestations and activity. Hence, antagonists of these cytokines have been tried as novel therapies for SLE, especially LN, with some promising results.28–34 Ustekinumab and Secukinumab were found to be effective in SLE treatment and gave a good response in comparison to placebo in a study done by Santacruz et al.35 Brodalumab and Ixekizumab do not have ongoing clinical studies to our knowledge.
Notwithstanding, our study has some limitations, which are being a single-center research with a relatively small cohort size and long disease duration. Of note, the confounding effect of medications patients received before IL23 quantification cannot be ignored, yet its serum level after treatment is far more than the normal populations and is found to be associated with disease activity. Hence, more studies, preferably multi-centric and on a larger scale are required to support our results.
Conclusion
According to the results of the current study supported by results of the previous studies in the same field, we can conclude that IL23 has higher levels in serum of SLE patients, and is correlated to activity of SLE, especially lupus nephritis. As a pro-inflammatory cytokine included in many inflammatory pathways, it could play an important role in SLE pathogenesis and disease activity. This role makes IL23 a potential biomarker or therapeutic target in SLE, so more studies are required to get benefits from these results in the management strategies of SLE.
Funding
No funding was received for conducting this study.
Disclosure
The authors report no competing interests in this work.
References
1. Pan L, Lu MP, Wang JH, Xu M, Yang SR. Immunological pathogenesis and treatment of systemic lupus erythematosus. World J Pediatr. 2020;16(1):19–30. doi:10.1007/s12519-019-00229-3
2. Larosa M, Zen M, Gatto M, et al. IL-12 and IL-23/Th17 axis in SLE. Exp Biol Med. 2019;244:42–51. doi:10.1177/1535370218824547
3. Fischer K, Przepiera-Będzak H, Sawicki M, Walecka A, Brzosko I, Brzosko M. Serum interleukin-23 in Polish patients with systemic lupus erythematosus: association with lupus nephritis, obesity, and peripheral vascular disease. Hindawi Mediators Inflamm. 2017;2017:9. doi:10.1155/2017/9401432
4. Comte D, Karampetsou MP, Tsokos GC. T cells as a therapeutic target in SLE. Lupus. 2015;24:351–363. doi:10.1177/0961203314556139
5. Yanti T, Yuliasih RLD. IL-23/IL-17 axis and disease activity in systemic lupus erythematosus patients. Eurasia J Biosci. 2020;14:2643–2649.
6. Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202::96–105. doi:10.1111/j.0105-2896.2004.00214.x
7. Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi:10.1146/annurev.immunol.22.012703.104758
8. Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12R b1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi:10.4049/jimmunol.168.11.5699
9. Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi:10.1084/jem.20041257
10. Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(9):1400–1412. doi:10.1002/art.40930
11. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi:10.1002/art.1780350606
12. Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi:10.1002/art.1780390303
13. Aringer M. Inflammatory markers in systemic lupus erythematosus. J Autoimmun. 2020;110:102374. doi:10.1016/j.jaut.2019.102374
14. Yap DYH, Lai KN. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: from basics to recent advances. J Biomed Biotechnol. 2010;2010:365083. doi:10.1155/2010/365083
15. Moreno-Torres V, Castejón R, Martínez-Urbistondo M, et al. Serum cytokines to predict systemic lupus erythematosus clinical and serological activity. Clin Transl Sci. 2022;15:1676–1686. doi:10.1111/cts.13283
16. Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi:10.1146/annurev.immunol.021908.132710
17. Vukelic M, Laloo A, Kyttaris VC. Interleukin 23 is elevated in the serum of patients with SLE. Lupus. 2020;29(14):1943–1947. doi:10.1177/0961203320952841
18. Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi:10.4049/jimmunol.0900385
19. Pan HF, Ye DQ, Li XP. Type 17 T-helper cells might be a promising therapeutic target for systemic lupus erythematosus. Nat Clin Pract Rheumatol. 2008;4:352–353. doi:10.1038/ncprheum0815
20. Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. doi:10.1038/nri3707
21. Abdel Galil SM, Ezzeldin N, El-Boshy ME. The role of serum IL-17 and IL-6 as biomarkers of disease activity and predictors of remission in patients with lupus nephritis. Cytokine. 2015;76(2):280–287. doi:10.1016/j.cyto.2015.05.007
22. Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. J Immunol. 2014;193(2):540–543. doi:10.4049/jimmunol.1400931
23. Vaillant AJ, Akpaka PE. Cytokines (IL-17, IL-23 and IL-33) in systemic lupus erythematosus in Trinidad and Tobago. medRxiv. 2020. doi:10.1101/2020.09.27.20202762
24. Yago T, Nanke Y, Kawamoto M, Kobashigawa T, Yamanaka H, Kotake S. IL-23 and Th17 disease in inflammatory arthritis. J Clin Med. 2017;6(9):81. doi:10.3390/jcm6090081
25. Zickert A, Amoudruz P, Sundström Y, Rönnelid J, Malmström V, Gunnarsson I. IL-17 and IL-23 in lupus nephritis - association to histopathology and response to treatment. BMC Immunol. 2015;16:7. doi:10.1186/s12865-015-0070-7
26. Dedong H, Feiyan Z, Jie S, Xiaowei L, Shaoyang W. Analysis of interleukin-17 and interleukin-23 for estimating disease activity and predicting the response to treatment in active lupus nephritis patients. Immunol Lett. 2019;210:33–39. doi:10.1016/j.imlet.2019.04.002
27. Kang HK, Ecklund D, Liu M, Datta SK. Apigenin. a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther. 2009;11:1–13. doi:10.1186/ar2682
28. van Vollenhoven R, Hahn BH, Tsokos GC, et al. Maintenance of efficacy and safety and reduction of BILAG flares with ustekinumab, an interleukin-12/23 inhibitor, in patients with active systemic lupus erythematosus (SLE): 2-year results of a Phase 2, randomized placebo controlled, crossover study.
29. van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330–1339. doi:10.1016/S0140-6736(18)32167-6
30. van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: 1-year results of a phase 2, randomized placebo-controlled, crossover study.
31. van Vollenhoven R, Hahn BH, Tsokos GC, et al. Maintenance of efficacy and safety of ustekinumab through one year in a phase 2 multicenter, prospective, randomized, double blind, placebo-controlled crossover trial of patients with active systemic lupus erythematosus. Arthritis Rheumatol. 2020;72(5):761–768. doi:10.1002/art.41179
32. van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab in patients with active systemic lupus erythematosus: results of a phase 2 open-label extension study. [published online ahead of print December 01 2021]. J Rheumatol. 2021;49:380–387. doi:10.3899/jrheum.210805
33. Costa R, Antunes P, Salvador P, et al. Secukinumab on refractory lupus nephritis. Cureus. 2021;13(8):e17198. doi:10.7759/cureus.17198
34. Farah Izati A, Wong KK, Che Maraina CH. IL-23/IL-17 axis in the pathogenesis and treatment of systemic lupus erythematosus and rheumatoid arthritis. Malays J Pathol. 2020;42(3):333–347.
35. Santacruz J, Pulido S, Arzuaga A, Juliana Mantilla M, Santos A, Londono J. Current evidence for IL-17/23 blockade for the treatment of lupus nephritis. Cureus. 2021;13(12):e20087. doi:10.7759/cureus.20087
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
