Back to Journals » Clinical Epidemiology » Volume 16
Smoking is a Risk Factor for Autoimmune Hepatitis: An English Registry-Based Case–Control Study
Authors Grønbæk L , Omeife H, Ban L, Crooks CJ , Card TR, Jepsen P , West J
Received 29 September 2023
Accepted for publication 18 January 2024
Published 31 January 2024 Volume 2024:16 Pages 23—30
DOI https://doi.org/10.2147/CLEP.S439219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Laura Horsfall
Lisbet Grønbæk,1– 3 Harmony Omeife,3 Lu Ban,4 Colin J Crooks,5 Timothy R Card,3,4 Peter Jepsen,1,3 Joe West3,4,6
1Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Medicine, Regional Hospital Horsens, Horsens, Denmark; 3Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK; 4National Institute for Health Research (NIHR) Nottingham Biomedical Research Centre, the Nottingham University Hospitals NHS Trust and University of Nottingham, Nottingham, UK; 5Translational Medical Sciences, School of Medicine, University of Nottingham, Nottingham, UK; 6Department of Clinical Medicine, Aarhus University, Aarhus, Denmark
Correspondence: Lisbet Grønbæk, Department of Hepatology and Gastroenterology, Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, Aarhus, 8200, Denmark, Tel +45 78450000, Fax +45 78453897, Email [email protected]
Purpose: Smoking is a risk factor for some autoimmune diseases, but its association with autoimmune hepatitis remains unknown. We conducted a population-based matched case–control study to examine the association between tobacco smoking and the risk of autoimmune hepatitis in England.
Patients and Methods: From the Clinical Practice Research Datalink and linked Hospital Episode Statistics, 2005– 2017, we included 987 cases diagnosed with autoimmune hepatitis after age 18 years and up to 10 frequency-matched population controls per case. We used multiple logistic regression to estimate the odds ratio of autoimmune hepatitis in ever-smokers vs never-smokers, adjusting for sex, age, general practice, calendar time of registration with the general practice, and socioeconomic status.
Results: The autoimmune hepatitis cases were more likely to be ever-smokers than the controls (44% vs 37%). The ever-smokers had an increased risk of autoimmune hepatitis compared with the never-smokers (adjusted odds ratio = 1.20, 95% confidence interval 1.03– 1.39).
Conclusion: Smoking was associated with an increased risk of autoimmune hepatitis.
Plain Language Summary: Autoimmune hepatitis is a chronic liver disease associated with genetic variants and environmental exposures, but the causes of autoimmune hepatitis remain unknown. Using registry data, we evaluated the association between tobacco smoking and the risk for autoimmune hepatitis. We found that tobacco smoking was associated with an increased risk of autoimmune hepatitis.
Keywords: epidemiology, chronic hepatitis, risk factors, tobacco
Introduction
Autoimmune hepatitis (AIH) is a rare, female-predominated chronic inflammatory liver disease with an increased mortality rate.1,2 AIH may present heterogeneously from indolently to cirrhosis or acute hepatitis. The diagnosis is based on characteristic autoantibodies, biochemistry, histology, and the exclusion of other causes of liver disease.3 The incidence of AIH is increasing with a doubling in less than 20 years, 1997–2015.2 Diagnostic approaches might have changed in this time period, but changing risk factor exposure could be a more likely explanation. AIH has a multifactorial aetiology,3,4 and associations with genetic variants, particularly human leucocyte antigen (HLA)-DRB1*03,5 and environmental exposures, such as infectious agents, medicines, vaccines, and psychological stress, have been suggested.3 Tobacco smoking is a highly prevalent behaviour and a known risk factor for some autoimmune diseases with a 1.5 to 2 times increased risk of Crohn’s disease,6 primary biliary cholangitis (PBC), rheumatoid arthritis, multiple sclerosis, and hyperthyroidism;7 but smoking appears to exert a protective effect on other autoimmune diseases by halving the risk for ulcerative colitis6 and celiac disease.8 Smoking carries numerous exposures that may result in a hyperactive and dysregulated immune system: free radicals causing DNA alterations, increased activity of inflammatory cells and pro-inflammatory factors, and disruption of immune clearance.7,9 In genetically predisposed persons such exposure may trigger autoimmune disease development, and this could also be the case with AIH. Smoking may be a risk factor for AIH development – a hypothesis supported by the case of a male smoker who developed AIH, and no potential risk factor other than smoking could be identified.10 One study found a higher prevalence of current smoking in patients with AIH but not of previous smoking,11 and yet another study evaluated the effect of smoking on AIH and found indications of a slightly higher AIH incidence rate among smokers.12 The association between smoking and AIH remains unsettled. Our aim was to conduct a population-based matched case–control study examining the association between tobacco smoking and the risk of AIH. We hypothesised that tobacco smoking is a risk factor for AIH development.
Materials and Methods
This is a population-based case–control study of English patients registered with an AIH diagnosis in primary or secondary care, 2005–2017, and their matched control persons from the English general population. The study is registry-based, and no patient enrolment was involved. The study was approved by the Independent Scientific Advisory Committee for MHRA Database Research, United Kingdom (protocol 18_022R).
We extracted data from the Clinical Practice Research Datalink (CPRD) (www.cprd.com)13 and person-level linked data from Hospital Episode Statistics (HES) (www.data.gov.uk) to identify cases with AIH and population controls. The AIH cases were defined by a first time CPRD registration of a Read code for AIH or by HES registration of an International Classification Code (ICD) 10th edition for AIH. We excluded cases with alcoholic liver disease, viral hepatitis B or C, primary sclerosing cholangitis (PSC), or PBC (Supplementary Table 1 shows Read codes and ICD-10 codes for inclusion and exclusion). Patients from primary and secondary care were included using the same inclusion and exclusion criteria as in our previous study and previously validated.2 We included 987 cases diagnosed with AIH after age 18 years between 1 January 2005 and 31 July 2017 and up to 10 frequency-matched population controls per case (matching variables: sex, 20-year age categories, general practice, and calendar year for registration with general practice).
We categorised the AIH cases and the controls as to exposure of smoking based on the last CPRD record of smoking status before the AIH diagnosis. We defined ‘ever-smokers’ as patients recorded with indicators of current or previous smoking and ‘never-smokers’ as patients recorded as non-smokers and never recorded as a current or previous smoker. A patient, who was previously recorded as a smoker but later as a non-smoker, would be categorised as an “ex-smoker.” Information on smoking status was obtained by a validated method of using three sources from the CPRD dataset:14 Read codes related to smoking in clinical and referral files, records of smoking status in additional files, and prescriptions for smoking cessation in therapy files (Supplementary Table 2 shows Read codes, product codes, and product names used for categorising cases and controls as to smoking status).
We used a multiple logistic regression model to estimate the odds ratio (OR) of AIH in “ever-smokers” vs “never-smokers” including the frequency matching variables, while also adjusting for age as a continuous variable and for socioeconomic status (expressed by quintiles of Index of Multiple Deprivation [IMD]). We adjusted for sex and age, as these factors may affect the possibility of being a smoker and at the same time may affect the possibility of developing AIH; we adjusted for IMD as socioeconomic status may affect the probability of being a smoker and may be used as a proxy for unknown environmental factors. We repeated analyses in stratified groups of sex, age, and quintiles of IMD. We also performed likelihood ratio tests (significance level of 0.05) to assess the presence of interaction effects in the multiple logistic regression models. These tests were conducted to determine whether the inclusion of interaction terms significantly improved the model fit compared to a model without interaction terms. To deal with missing data, we conducted complete-case analyses and multiple imputation analyses.15 To evaluate if ascertainment bias in “ever-smokers” (more and earlier AIH diagnoses resulting from more general practitioner visits) could explain any higher risk of having an AIH diagnosis, we estimated the cumulative mortality after AIH diagnosis in “ever-smokers” and “never-smokers” using the Kaplan Meier estimator of survival: If “ever-smokers” were seen more regularly by their general practitioner and thereby were diagnosed at an earlier stage of AIH, they could have a better survival than would be otherwise expected. For a more detailed description of methods and statistical analyses, see Supplementary Material. All data management and analyses were carried out using MonetDB SQL and Stata software version 14.2.
Results
We identified 1213 cases with AIH, and 8524 matched controls. We excluded 226 cases (37 with alcoholic liver disease, 61 with viral hepatitis, 58 with PSC, and 70 with PBC) and their matched controls and another 67 controls (19 with alcoholic liver disease, 22 with viral hepatitis, 18 with PSC, and 8 with PBC). We included the remaining 987 cases and 6767 matched controls.
At the time of AIH diagnosis, the cases and the controls did not differ in terms of sex, age, IMD, or regional affiliation (Table 1). The AIH cases were more likely to be “ever-smokers” than the controls (52% vs 48% of those with registered smoking status), and the AIH cases were less likely to have missing information on their smoking status than the controls (18% vs 22%). Men were more likely to be “ever-smokers” than women (42% vs 37%), and men were more likely to have missing records on smoking than women (27% vs 20%) (Table 1).
 |
Table 1 Characteristics of Cases with Autoimmune Hepatitis (AIH) and Matched Controls at the Time of AIH Diagnosis and Characteristics Based on Their Smoking Status |
When we conducted complete-case analysis on the 808 AIH cases and 5262 controls with complete records, the odds of AIH were higher in “ever-smokers” than in “never-smokers” (adjusted OR = 1.19, 95% confidence interval [CI] 1.02–1.38.) The stratified analyses indicated a stronger association between smoking and AIH in women compared with men and in the younger compared with the older, but the confidence intervals were wide and overlapping (Table 2), and we found no statistically significant interaction between smoking, sex, and age (Likelihood ratio significance test, p-value=0.32). We found that the association between smoking and AIH was essentially the same in the deprived and the affluent (Table 2 and Figure 1). The result from the analysis on all 987 cases and 6767 matched controls with imputed data on missing information was similar to the result from the complete-case analysis (adjusted OR = 1.20, 95% CI 1.03–1.40). When we compared the mortality after AIH diagnosis in “ever-smokers” and “never-smokers” we found a slightly higher mortality in “ever-smokers” (5-year cumulative mortality in “ever-smokers” = 18.9%, 95% CI 13.9–25.4; “never-smokers” = 17.2%, 95% CI 12.0–24.3).
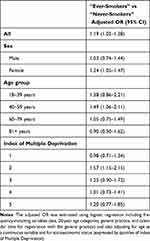 |
Table 2 The Odds Ratio (OR) of Autoimmune Hepatitis Among “Ever-Smokers” vs “Never-Smokers” in Stratified Groups of Sex, Age, and Quintiles of Index of Multiple Deprivation |
Discussion
This population-based case–control study evaluated the association between tobacco smoking and the risk for AIH. Our study suggests that smoking increases the risk for AIH. The main strengths of our study are that it is population-based, large, and includes patients with AIH from both primary and secondary care. AIH is a symptomatic disease eventually leading to a medical contact, and with cost-free access to diagnostic procedures in the UK our case ascertainment is most likely complete. We used the same inclusion and exclusion criteria for AIH cases as in our previous study, and we presume a high diagnostic validity, as previously argued.2 The systematic registration of smoking status in general practice in England allowed us to examine smoking as a risk factor for AIH development, and we used a validated method of categorising cases and controls as for smoking status. The validity of registry status of current- and non-smoking is presumed to be high, whereas ex-smoking might be underreported.14 We cannot rule out the possibility that some cases of ex-smokers were misclassified as never-smokers, and our result might be an underestimate of the true association between smoking and AIH. We could not evaluate any effect from the intensity of smoking because only 38% of those 809 cases with record of their smoking status had the smoking intensity recorded, and for controls it was only 32% of 5266. The linked data enabled us to adjust for confounding from sex, age, and IMD and thereby indirectly adjust for any unknown environmental factor associated with socioeconomic status. We considered adjustment for other autoimmune diseases or cancers for which smoking may be a risk factor. At the same time, AIH could be a risk factor for developing such comorbidities, and adjustment for common effects from exposure and outcome would introduce bias. We evaluated if ascertainment bias could explain our finding of a higher risk of AIH in “ever-smokers” vs “never-smokers”, but our finding of a slightly higher mortality in “ever-smokers” after an AIH diagnosis speaks against any noteworthy effect from ascertainment bias.
In this study, we found that a current or previous history of smoking was associated with an increased risk of having an AIH diagnosis. Our study supports the hypothesis that smoking could be an environmental risk factor of pathogenic significance in the complex aetiology of AIH. Tobacco smoke contains several chemicals that may trigger immunogenic changes leading to the development of autoimmune diseases.7 Smoking has been proposed to be a risk factor, particularly in those with HLA-DRB1*03, for rheumatoid arthritis16 and multiple sclerosis,17 and similar gene-environmental interactions might apply to AIH.5 One questionnaire-based study from the USA examining risk factors for AIH found a much higher prevalence of current-smoking in AIH patients than in controls (19% vs 7%), but a similar prevalence of ever-smoking (38% vs 41%).11 However, the AIH patients and the controls in that study were voluntarily recruited and may not be representative of AIH patients in general nor representative of the general population, and the questionnaire-based approach makes the study prone to recall bias. Another study evaluated the effect of smoking on AIH and found indications of a slightly higher AIH incidence rate among smokers (incidence rate of 2.24, 95% CI 1.94–2.55 in smokers vs 1.96, 95% CI 1.79–2.12 per 100,000 population per year in non-smokers), but the difference was not statistically significant.12 Another study with a main aim of assessing the incidence of autoimmune liver diseases in relation to latitude examined the association between smoking and AIH in the United Kingdom.12 That study showed a similar prevalence of smokers (current and ex-smokers) among AIH patients as our study (45% vs 44%), but it was unspecified how the smoking categories were defined. In line with our study, their results suggested a higher incidence of AIH among smokers compared with non-smokers.
In our study, the stratified analyses might indicate a slightly stronger association between smoking and the risk of AIH in women and younger persons, though we were unable to demonstrate statistical significance for this. We did not have the data to evaluate any differences in the intensity of smoking between women and men and between younger and older persons, and we cannot rule out the possibility that any such differences contributed to our results. In general, the risk of autoimmunity is higher in women compared with men, and this may relate to hormonal, immunological, and genetic differences that may influence the susceptibility to environmental factors, such as smoking.18 Changing environmental factor exposures and interactions are also the likely explanation for the increasing incidence of AIH over time. The prevalence of smoking in the UK for both sexes and all ages has been declining during the last two decades (https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2021), but exposure to other unknown environmental risk factors may have increased or exposure to protective factors may have dropped. We assume generalisability of our results, but with the complexity of AIH aetiology, our results are not necessarily reproducible in other countries where genetic pre-dispositions and exposure to other environmental factors may be different.
In conclusion, we found that tobacco smoking was associated with an increased risk of AIH. Our study, like many others, suggests the need for sustained prevention of tobacco smoking.
Abbreviations
AIH, Autoimmune hepatitis; CPRD, clinical practice research datalink; CI, confidence interval; GWAS, Genome-wide association studies; HES, hospital episode statistics; HLA, human leucocyte antigen; ICD-10, international classification of diseases 10th edition; IMD, index of multiple deprivation; OR, odds ratio; PBC, primary biliary cholangitis; PSC, primary sclerosing cholangitis.
Permission to Reproduce Material from Other Sources
This study is based in part on data from the Clinical Practice Research Datalink (CPRD) obtained under the University of Nottingham licence from the United Kingdom Medicines and Healthcare Products Regulatory Agency. All data were reused with permission, and we extracted the dataset under this license. The anonymised data is provided by patients and collected by the NHS as part of their care and support. The interpretation and conclusion contained in this study are those of the authors alone. Hospital Episode Statistics Data copyright® (2015) was reused with the permission of the Health & Social Care Information Centre, all rights reserved.
Data Sharing Statement
The data that support the findings of this study can be available by applying it to the Independent Scientific Advisory Committee for MHRA database Research, United Kingdom.
Ethics Approval Statement
The study was approved by the Independent Scientific Advisory Committee for MHRA Database Research, United Kingdom (Protocol 18_022R). Only approved researchers can gain access to the data, and research applications are subject to a rigorous research data governance approvals process (https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/clinical-practice-research-datalink-cprd-research-database/).
Acknowledgment
The abstract of this paper was presented at the seventh annual meeting for the Danish society for Gastroenterology and Hepatology, 2019, as a poster presentation with interim findings. The poster’s abstract with the title “Is smoking a risk factor for autoimmune hepatitis? An English registry-based matched case–control study” was published in the corresponding abstract book (https://dsgh.dk/wp-content/uploads/2022/06/abstract2019-1.pdf).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
LG received funding from the Danish Foundation of 17.12.1981 and the A.P. Møller Foundation for the Advancement of Medical Science. The funding sources were not involved in the conduct of the research or preparation of the article.
Disclosure
LB and HO are currently employees of Evidera, a business unit of PPD, a ThermoFisher Scientific company. Evidera was not involved in the conduct of the research or preparation of the article. LG, CJC, TRC, PJ, and JW declare that they have nothing to disclose with respect to this manuscript.
References
1. Grønbæk L, Vilstrup H, Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi:10.1016/j.jhep.2013.10.020
2. Grønbæk L, Otete H, Ban L, et al. Incidence, prevalence and mortality of autoimmune hepatitis in England 1997–2015. A population-based cohort study. Liver Int. 2020;40:1634–1644. doi:10.1111/liv.14480
3. Manns MP, Lohse AW, Vergani D. Autoimmune hepatitis - Update 2015. J Hepatol. 2015;62:S100–S111.
4. Grønbæk L, Vilstrup H, Pedersen L, et al. Family occurrence of autoimmune hepatitis: a Danish nationwide registry-based cohort study. J Hepatol. 2018;69:873–877. doi:10.1016/j.jhep.2018.05.035
5. Karlsen TH, Chung BK. Genetic risk and the development of autoimmune liver disease. Dig Dis. 2015;33(2):13–24. doi:10.1159/000440706
6. Mahid SS, Minor KS, Soto RE, et al. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006;81:1462–1471. doi:10.4065/81.11.1462
7. Costenbader KH, Karlson EW. Cigarette smoking and autoimmune disease: what can we learn from epidemiology? Lupus. 2006;15:737–745. doi:10.1177/0961203306069344
8. Vazquez H, Smecuol E, Flores D, et al. Relation between cigarette smoking and celiac disease: evidence from a case-control study. Am J Gastroenterol. 2001;96:798–802. doi:10.1111/j.1572-0241.2001.03625.x
9. Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–57. doi:10.1152/ajplung.00241.2005
10. Bose T. Bitter correlationship between autoimmune hepatitis and smoking. Med Hypotheses. 2015;84:118–121. doi:10.1016/j.mehy.2014.12.006
11. Lammert C, Chalasani SN, Atkinson EJ, et al. Environmental risk factors are associated with autoimmune hepatitis. Liver Int. 2021;41:2396–2403. doi:10.1111/liv.14944
12. Webb GJ, Ryan RP, Marshall TP, et al. The epidemiology of UK autoimmune liver disease varies with geographic latitude. Clin Gastroenterol Hepatol. 2021;19:2587–2596. doi:10.1016/j.cgh.2021.01.029
13. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: clinical practice research datalink (CPRD). Int J Epidemiol. 2015;44:827–836. doi:10.1093/ije/dyv098
14. Booth HP, Prevost AT, Gulliford MC. Validity of smoking prevalence estimates from primary care electronic health records compared with national population survey data for England, 2007 to 2011. Pharmacoepidemiol Drug Saf. 2013;22:1357–1361. doi:10.1002/pds.3537
15. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi:10.1002/sim.4067
16. Hedstrom AK, Ronnelid J, Klareskog L, et al. Complex relationships of smoking, HLA-DRB1 genes, and serologic profiles in patients with early rheumatoid arthritis: update from a Swedish population-based case-control study. Arthritis Rheumatol. 2019;71:1504–1511. doi:10.1002/art.40852
17. Hedstrom AK, Sundqvist E, Baarnhielm M, et al. Smoking and two human leukocyte antigen genes interact to increase the risk for multiple sclerosis. Brain. 2011;134:653–664. doi:10.1093/brain/awq371
18. Ngo ST, Steyn FJ, McCombe PA. Gender differences in autoimmune disease. Front Neuroendocrinol. 2014;35:347–369. doi:10.1016/j.yfrne.2014.04.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

