Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Successful Treatment of Cutaneous Protothecosis Due to Prototheca wickerhamii with Terbinafine
Authors Chen Y , Gao A, Ke Y, Zhou X, Lin L, Lu S, Liu Y
Received 10 December 2023
Accepted for publication 31 January 2024
Published 24 April 2024 Volume 2024:17 Pages 913—919
DOI https://doi.org/10.2147/CCID.S453620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Yue Chen,1,* Aili Gao,1,* Yanan Ke,1 Xin Zhou,1 Li Lin,2 Sha Lu,2 Yumei Liu1
1Department of Dermatology, Guangzhou Dermatology Hospital, Guangzhou, 510095, People’s Republic of China; 2Department of Dermatology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, 510120, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yumei Liu, Director of Department of Dermatology, Guangzhou Dermatology Hospital, Guangzhou, People’s Republic of China, Tel/Fax +86- 20-83593472, Email [email protected]
Abstract: Protothecosis, an infrequent human infection, is caused by achlorophyllic algae belonging to the genus Prototheca, particularly Prototheca wickerhamii. The skin stands as the most commonly affected organ. This report documents a case involving an 82-year-old male with Protothecosis. Histopathological analysis revealed granulomatous inflammation in the dermis, exhibiting necrotic features and hosting numerous non-budding spherical organisms. These organisms were positively stained using methenamine silver and periodic acid–Schiff stains, confirming identification as P. wickerhamii after validation through tissue culture and sequencing procedures. Initially, the patient received oral itraconazole at a dosage of 200 mg daily, accompanied by topical 1% naftifine-0.25% ketoconazole cream for a duration of 4 weeks, resulting in significant improvement. Subsequently, due to gastrointestinal discomfort presumably linked to itraconazole, terbinafine was administered. Over a span of 3 months, the patient received oral terbinafine at a dosage of 250 mg/day alongside the application of topical 1% naftifine-0.25% ketoconazole cream, leading to complete healing of the skin lesion, leaving behind a fibrotic scar.
Keywords: protothecosis, Prototheca wickerhamii, terbinafine
Introduction
Protothecosis represents a rare opportunistic human infection caused by the genus Prototheca, consisting of achlorophyllic algae widely present in nature.1,2 Prototheca species, including P. ciferrii (previously known as P. zopfii genotype 1), P. bovis (previously known as P. zopfii genotype 2), P. wickerhamii, P. blaschkeae, P. cutis, and P. miyajii3, can cause infections in both humans and animals. While Prototheca species are commonly isolated from domestic animals, such as cattle, dogs, and cats, cases have also been reported in goats, horses, and other nondomestic animals, with P. wickerhamii being the primary cause of human infections.3–5 Prototheca algae have been found to be isolated from terrestrial sources, including soil, mud, and stream sediments, in regions characterized by a high organic matter content and moisture level.6 The prevailing belief is that Prototheca species infect humans either through direct contact with exposed areas or via traumatic inoculation of materials contaminated with the algae.1,7–10 This infection typically manifests in three primary forms: intra-cutaneous to subcutaneous nodules and plaques, olecranon bursitis, and systemic (disseminated) disease.11 Notably, the skin emerges as the organ most frequently affected. Cutaneous infections commonly arise in immunocompetent patients, while disseminated infections predominantly impact immunocompromised individuals.1,11,12 Clinical management lacks a standardized protocol due to varying treatment efficacies.13,14 In this context, we present a case of cutaneous protothecosis effectively cured through a three-month course of terbinafine treatment.
Case Report
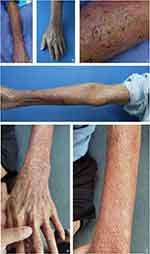
An 82-year-old man presented at the hospital with a 4-month history of skin lesions. Preceding these lesions, he experienced a stab wound on the dorsal side of his left forearm from firewood, promptly cleaned and dressed with an antiseptic iodine solution. Subsequently, he noticed moderate itching and redness in the same area a few days later. Initially diagnosed with eczema, he received symptomatic treatment involving a potent corticosteroid cream containing clobetasol and antihistamine, which failed to control the rash. Instead, the affected area rapidly expanded to the flexor aspect of the left arm and the back of the left hand, accompanied by itching and pain. Concurrently, he received oral cefdinir, compound glycyrrhizin, and loratadine. The patient denied any prior medical history or conditions except hypertension. Dermatological examination revealed diffuse infiltrated erythematous and indurated plaques covering the dorsal side of the left forearm, the back of the left hand, and the flexor aspect of the left arm. Within the affected area, numerous insect bite-like papules, ulcers, papillae, pustules, atrophic scars, and scabs were observed (Figure 1a–d). There was no tenderness upon pressure, and axillary lymph nodes were impalpable. Laboratory tests indicated an elevated white blood cell count of 12.89 x 10^9/L (normal, 3.5–9.5 x 10^9/L), decreased albumin at 34.7g/L (normal, 40–50 g/L), elevated urea nitrogen at 19.28 mmol/L (normal, 1.7–8.3 mmol/L), and elevated total cholesterol levels at 7.57 mmol/L (normal, 2.9–5.68 mmol/L) and 4.31 mmol/L (normal, <3.36 mmol/L). Blood sugar, urinalysis, ANA, and HIV tests showed no abnormalities.
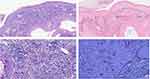
Histopathological analysis depicted granulomatous inflammation with necrosis in the dermis, revealing abundant non-budding spherical structures observed under 100x magnification (Figure 2a). The application of Periodic acid-Schiff staining demonstrated thick spore cell walls with characteristic internal septations. Highlighted in Figure 2b were multiple sporangia containing endospores, displaying a morula appearance. Conversely, acid-fast staining yielded negative results (Figure 2c), while hexamine silver staining showcased positivity (Figure 2d).
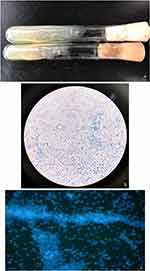
Culturing the biopsy specimen on Sabouraud dextrose agar (SDA) at 30°C resulted in smooth, creamy white, yeast-like colonies after 48 hours (Figure 3a). Further microscopic examination stained with lactophenol cotton blue and fluorochrome revealed characteristic endospores scattered or enclosed in a sporangium (Figure 3b and c). All of the reagents were purchased from Sigma-Aldrich. Molecular biology identification was performed using PCR with fungal-specific universal primers. The TIANquick Yeast Genomic DNA Extraction Kit (W9317) was utilized to extract DNA from the saved strain. PCR was conducted using fungal-specific universal primers, namely NS3: 5’-GCAA GTCTGGCCAGCAGCC-3’; and NS8: 5’- TCCGC AGGTTCACCTACGGA-3’. The amplification parameters were as follows: an initial denaturation at 94°C for 4 minutes, followed by 34 cycles of denaturation at 94 °C for 1 minute, annealing at 49°C for 45 seconds, and extension at 72°C for 30 seconds, with a final extension at 72°C for 5 minutes. The amplified DNA sequence showed a 92.63% sequence homology with Prototheca wickerhamii (GenBank Accession No. MG832940.1), as confirmed by MALDI-TOF analysis. In vitro susceptibility testing yielded minimum inhibitory concentration (MIC) values for various antifungals: amphotericin B (AMB) 0.5 mg/L, itraconazole (ITR) 1.0 mg/L, fluconazole (FCA) >128 mg/L, voriconazole (VOR) 0.25 mg/L, 5-flucytosine (5-FC) >64 mg/L, caspofungin (CAS) >8 mg/L, anidulafungin (ANI) >8 mg/L, and posaconazole (POS) 1.0 mg/L. Confirmation of antifungal susceptibility was achieved using the microdilution method according to Clinical and Laboratory Standards Institute (CLSI) standards, indicating MIC values of ITR 0.5 mg/L, AMB 0.125 mg/L, and terbinafine >16 mg/L. Based on prior experiences, the patient received oral itraconazole at 200 mg/day combined with 1% naftifine-0.25% ketoconazole cream for external application. After 4 weeks of treatment, the lesions significantly improved, with the erythematous lesions and patches halting their extension. While papillomatous hyperplasia, atrophic scars, and scabs remained, the pustules, insect-bite-like erosions, and ulcers healed. However, due to gastrointestinal discomfort from itraconazole, the treatment shifted to terbinafine at 250 mg/day along with topical 1% naftifine-0.25% ketoconazole cream, considering the patient’s age and compromised kidney function. No further side effects were reported thereafter. Over 3 months of treatment, the skin lesions continued to improve, resulting in wound healing and leaving behind only fibrotic scars (Figure 1e and f). Subsequent follow-ups of one and a half years showed no evidence of relapse.
Discussion
Protothecosis, an infrequently reported infection, stems from the Prototheca genus, achlorophyllous algae derived from common green algae like chlorella. The pathogenesis remains largely elusive.1,2 These algae thrive in diverse environmental reservoirs such as water, grass, trees, animals, food items, and even household waste. Human protothecosis primarily results from Prototheca wickerhamii, causing localized infections in immunocompetent individuals, yet dissemination is more prevalent in immunocompromised cases.15 This report details a case of cutaneous protothecosis induced by Prototheca wickerhamii in a non-immunocompromised male, notably lacking exposure to stagnant water or animal waste, with no significant medical history except for hypertension. His otherwise healthy status preceding lesion onset was disrupted by a stab injury on his left forearm, a route also posing risk factors for deep fungal diseases like sporotrichosis and chromoblastomycosis. Clinical symptom aggravation may be linked to a potent corticosteroid cream containing clobetasol.
Protothecosis presents with a heterogeneous, nonspecific array of skin lesions, documented as papules, pustules, plaques, nodules, ulcers, crusts, erosions, and various eczematous, herpetiform, granulomatous, and verrucous lesions.16,17 Our patient displayed insect bite-like erosions, ulcers, papillae, atrophic scars, and scabs diffusely covering an indurated erythematous area. Given the nonspecific clinical morphology, histopathological examination, aided by Periodic Acid Schiff and Grocott’s modification of Gomori methenamine silver staining, remains crucial for diagnosis. Characteristic morulae structure and endosporulation, better highlighted by these stains, were evident in our case. Skin biopsies typically reveal pan-dermal granulomatous inflammatory infiltrates, occasionally accompanied by necrosis and pseudocarcinomatous epidermal hyperplasia.18 Organism cultivation and identification further support diagnosis.
Establishing a standardized treatment regimen for protothecosis remains elusive, with treatment varying on a case-by-case basis.14 Amphotericin B appears most effective against Prototheca spp., while azole antifungals, particularly itraconazole, are preferred for localized infections.19–21 Previous studies have indicated that AMB and most azoles exhibit activity against clinical isolates of Prototheca and primarily exert an algicidal effect. However, the activities of individual drugs vary significantly between different Prototheca species and among strains of the same species.22 Moreover, several studies have demonstrated the beneficial effects of blue light on pathogenic species of Prototheca.23 Additionally, the study by Jagielski et al demonstrated that silver nanoparticles (AgNPs) and 3-Bromopyruvate also exhibited positive effects on pathogenic species of the Prototheca genus.24,25 In this case, our patient initially received oral itraconazole (200 mg/day) based on in vitro MIC results but had to discontinue due to side effects, despite significant lesion improvement. Subsequently, a 3-month terbinafine treatment successfully eradicated the infection. While in vitro susceptibility tests suggested Prototheca wickerhamii’s resistance to terbinafine, contradicting clinical efficacy, our patient exhibited gradual wound healing and resolved itching and pain with terbinafine (250 mg/day). The discordance between in vitro susceptibility and clinical outcomes challenges current understanding, with no established guidelines for testing this genus.26 Antibacterial agents like amikacin, tetracycline, and gentamicin have shown some efficacy in vitro.1,26 However, diverse susceptibility profiles in Prototheca species limit establishing direct correlations between in vitro activity and clinical responses.27 As of March 2021, a total of 269 human Prototheca infections have been reported since the first case in a barefoot Sierra Leone rice farmer’s foot in 1964.28–32 Routine in vitro antifungal susceptibility testing for protothecosis is not recommended unless empirical therapy fails.
Conclusions
The correlation between in vitro susceptibility and clinical outcomes, as well as established in vitro antimicrobial breakpoints, remains undefined. While itraconazole and AMB typically serve as the primary treatment options for cutaneous protothecosis, our evidence suggests that terbinafine could serve as a viable alternative in specific situations for treating this condition.
Data Sharing Statement
All data used in this work are publicly available.
Ethical Approval
The ethical committee of the hospital gave the agreement to report this case.
Consent Statement
A formal written consent was obtained for publication the case details and associated images from the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Science and Technology Program of Guangzhou (Grant No. 2023A03J0473).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20(2):230–242. doi:10.1128/CMR.00032-06
2. Phair JP, Williams JE, Bassaris HP, Zeiss CR, Morlock BA. Phagocytosis and algicidal activity of human polymorphonuclear neutrophils against Prototheca wickerhamii. J Infect Dis. 1981;144(1):72–77. doi:10.1093/infdis/144.1.72
3. Jagielski T, Bakuła Z, Gawor J, et al. The genus Prototheca (Trebouxiophyceae, Chlorophyta) revisited: implications from molecular taxonomic studies. Algal Res. 2019;43:101639. doi:10.1016/j.algal.2019.101639
4. Leimann BC, Monteiro PC, Lazéra M, Candanoza ER, Wanke B. Protothecosis. Med Mycol. 2004;42(2):95–106. doi:10.1080/13695780310001653653
5. Wang X, Ran Y, Jia S, et al. Human disseminated protothecosis: the skin is the “Window”? Front Immunol. 2022;13:880196. doi:10.3389/fimmu.2022.880196
6. Jagielski T, Iskra M, Bakuła Z, et al. Occurrence of prototheca microalgae in aquatic ecosystems with a description of three new species, Prototheca fontanea, Prototheca lentecrescens, and Prototheca vistulensis. Appl Environ Microbiol. 2022;88(22):e0109222. doi:10.1128/aem.01092-22
7. Silva PC, Costa ESS, Lima RB, D’Acri AM, Lupi O, Martins CJ. Cutaneous protothecosis--case report. An Bras Dermatol. 2013;88(6 Suppl 1):183–185. doi:10.1590/abd1806-4841.20132232
8. Hillesheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med. 2011;135(7):941–944. doi:10.5858/2010-0017-RSR.1
9. Mithari HS, Gole PV, Darkase B, Kalantri MS, Khopkar U. Cutaneous protothecosis. Indian J Dermatol. 2022;67(4):464–465. doi:10.4103/ijd.ijd_127_21
10. Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, Muñoz-Estrada VF, Welsh O. Protothecosis. Clin Dermatol. 2012;30(4):432–436. doi:10.1016/j.clindermatol.2011.09.016
11. Okazaki C, Wakusawa C, Chikama R, et al. A case of cutaneous protothecosis in a polyarteritis nodosa patient and review of cases reported in Japan. Dermatol Online J. 2011;17(9):2.
12. Carey WP, Kaykova Y, Bandres JC, Sidhu GS, Bräu N. Cutaneous protothecosis in a patient with AIDS and a severe functional neutrophil defect: successful therapy with amphotericin B. Clin Infect Dis. 1997;25(5):1265–1266. doi:10.1086/516974
13. Krcméry VJ. Systemic chlorellosis, an emerging infection in humans caused by algae. Int J Antimicrob Agents. 2000;15(3):235–237. doi:10.1016/s0924-8579(00)00150-3
14. Wang F, Feng P, Lin Y, et al. Human cutaneous protothecosis: a case report and review of cases from Mainland China, Hong Kong, and Taiwan. Mycopathologia. 2018;183(5):821–828. doi:10.1007/s11046-018-0292-3
15. Khan ID, Sahni AK, Sen S, Gupta RM, Basu A. Outbreak of Prototheca wickerhamii algaemia and sepsis in a tertiary care chemotherapy oncology unit. Med J Armed Forces India. 2018;74(4):358–364. doi:10.1016/j.mjafi.2017.07.012
16. Woolrich A, Koestenblatt E, Don P, Szaniawski W. Cutaneous protothecosis and AIDS. J Am Acad Dermatol. 1994;31(5 Pt 2):920–924. doi:10.1016/s0190-9622(94)70260-8
17. Zhang Q, Li L, Zhu L, et al. Cutaneous protothecosis in patient with diabetes mellitus and review of published case reports. Mycopathologia. 2012;173(163):163–171. doi:10.1007/s11046-011-9480-0
18. Seok JY, Lee Y, Lee H, Yi SY, Oh HE, Song JS. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47(6):575–578. doi:10.4132/KoreanJPathol.2013.47.6.575
19. El HK, Abdel-Halim M, El-Nabarawy E, Shalaby S. Cutaneous protothecosis as an unusual complication following dermal filler injection: a case report. J Clin Aesthet Dermatol. 2019;12(12):13–16.
20. Chao SC, Hsu MM, Lee JY. Cutaneous protothecosis: report of five cases. Br J Dermatol. 2002;146(4):688–693. doi:10.1046/j.1365-2133.2002.04609.x
21. Boyd AS, Langley M, King LJ. Cutaneous manifestations of Prototheca infections. J Am Acad Dermatol. 1995;32(5 Pt 1):758–764. doi:10.1016/0190-9622(95)91456-0
22. Proskurnicka A, Żupnik K, Bakuła Z, Iskra M, Rösler U, Jagielski T. Drug susceptibility profiling of prototheca species isolated from cases of human protothecosis. Antimicrob Agents Chemother. 2023;67(4):e0162722. doi:10.1128/aac.01627-22
23. Dos Anjos C, Sellera FP, Gargano RG, et al. Algicidal effect of blue light on pathogenic Prototheca species. Photodiagn Photodyn. 2019;26:210–213. doi:10.1016/j.pdpdt.2019.04.009
24. Jagielski T, Niedźwiecka K, Roeske K, Dyląg M. 3-Bromopyruvate as an Alternative Option for the Treatment of Protothecosis. Front Pharmacol. 2018;9:375. doi:10.3389/fphar.2018.00375
25. Jagielski T, Bakuła Z, Pleń M, et al. The activity of silver nanoparticles against microalgae of the Prototheca genus. Nanomedicine. 2018;13(9):1025–1036. doi:10.2217/nnm-2017-0370
26. Shahan TA, Pore RS. In vitro susceptibility of Prototheca spp. to gentamicin. Antimicrob Agents Chemother. 1991;35(11):2434–2435. doi:10.1128/AAC.35.11.2434
27. Wayne PA. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. In: CLSI Document M27-A2. Clinical and Laboratory Standards Institute; 2002.
28. Lee JS, Moon GH, Lee NY, Peck KR. Case report: protothecal tenosynovitis. Clin Orthop Relat Res. 2008;466(12):3143–3146. doi:10.1007/s11999-008-0487-x
29. Davies RR, Spencer H, Wakelin PO. A CASE OF HUMAN PROTOTHECOSIS. Trans R Soc Trop Med Hyg. 1964;58(5):448–451. doi:10.1016/0035-9203(64)90094-x
30. Zhao F, Chen M, Fu Y. Multiple cutaneous infections caused by Prototheca wickerhamii. J Clin Lab Anal. 2020;34(11):e23492. doi:10.1002/jcla.23492
31. Minato K, Yoshikawa M, Nakanishi H, Hasegawa K. Long term follow-up of prototheca keratitis: a case report. Int Med Case Rep J. 2020;13:503–506. doi:10.2147/IMCRJ.S268696
32. Herold S, Klodt T, Toelle D, et al. Lethal systemic and brain infection caused by Prototheca zopfii algae in a patient with acute myeloid leukemia. Med Mycol Case Rep. 2021;32:17–20. doi:10.1016/j.mmcr.2021.01.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.