Back to Journals » International Journal of Nanomedicine » Volume 19
The Application of Drugs and Nano-Therapies Targeting Immune Cells in Hypoxic Inflammation
Authors Luo J, Wang H, Chen J, Wei X, Feng J, Zhang Y, Zhou Y
Received 9 January 2024
Accepted for publication 29 March 2024
Published 9 April 2024 Volume 2024:19 Pages 3441—3459
DOI https://doi.org/10.2147/IJN.S456533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Jiaxin Luo,1,2,* Hanchi Wang,1,2,* Jingxia Chen,1,2 Xuyan Wei,1,2 Jian Feng,1,2 Yidi Zhang,1,2 Yanmin Zhou1,2
1Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China; 2Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yidi Zhang; Yanmin Zhou, Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Immune cells are pivotal in the dynamic interplay between hypoxia and inflammation. During hypoxic conditions, HIF-1α, a crucial transcription factor, facilitates the adaptation of immune cells to the hypoxic micro-environment. This adaptation includes regulating immune cell metabolism, significantly impacting inflammation development. Strategies for anti-inflammatory and hypoxic relief have been proposed, aiming to disrupt the hypoxia-inflammation nexus. Research extensively focuses on anti-inflammatory agents and materials that target immune cells. These primarily mitigate hypoxic inflammation by encouraging M2-macrophage polarization, restraining neutrophil proliferation and infiltration, and maintaining Treg/TH17 balance. Additionally, oxygen-releasing nano-materials play a significant role. By alleviating hypoxia and clearing reactive oxygen species (ROS), these nano-materials indirectly influence immune cell functions. This paper delves into the response of immune cells under hypoxic conditions and the resultant effects on inflammation. It provides a comprehensive overview of various therapies targeting specific immune cells for anti-inflammatory purposes and explores nano-materials that either carry or generate oxygen to alleviate anoxic micro-environments.
Keywords: hypoxia, inflammation, immunometabolism, immunotherapy, nanotherapy
Introduction
The recognition that hypoxia can precipitate inflammation is now widely acknowledged. A key feature of acute respiratory distress syndrome (ARDS) is hypoxemia. In ARDS patients, a notable decrease in monocytes accompanies this hypoxemia. Thus, by establishing a mouse model of acute lung injury, Ananda found that impaired monopoiesis led to reduced accumulation of monocyte-derived macrophages and enhanced neutrophil-mediated inflammation in the lungs.1 Moreover, cycling hypoxia may occur in the tumor area due to intermittent flow of red blood cells into tumor vessels. Scholars have demonstrated that cycling hypoxia can affect the polarization of tumor-associated macrophages (TAMs) and activate endothelial cell activation through nuclear factor-kappa B (NF-kB) pathway, thus promoting tumor inflammation.2 Furthermore, hypoxia can promote anaerobic glycolysis, resulting in increased production and release of lactic acid. Prior studies have indicated that lactate escalates the differentiation of T helper 1 cells and the generation of interferon-γ (IFN γ). Additionally, the augmentation of lactic acid encourages M2-like polarization and vascular endothelial growth factor (VEGF) expression in tumor-associated macrophages (TAMs). This is partially mediated by the activation of hypoxia-inducible factor 1α (HIF1α).3,4 Under normoxic conditions, HIF-1α is rendered inactive through hydroxylation by prolyl hydroxylases (PHDs) and factor-inhibiting HIF (FIH). In hypoxic states, these hydroxylases are inactivated due to the scarcity of available non-mitochondrial oxygen, leading to the stabilization and transcription of HIF.5 HIF expression occurs in innate immune cells, including macrophages, neutrophils, and TH17 cells. As a principal transcriptional regulator of immune cell function, HIF aids these cells in adapting to hypoxic environments and enhances the expression of inflammatory genes.6 The relationship between hypoxia and inflammation is synergistic, not merely cause-and-effect. Hypoxia in inflammatory conditions arises from the recruitment of immune cells, the increased metabolic demands of various cells, and the depletion of metabolic substrates due to trauma and compression.7,8 In inflamed tissues, infiltrating immune cells alter the local tissue micro-environment by depleting molecular oxygen. Studies have revealed that activated neutrophils can promote HIF-1α transcription by consuming local oxygen, particularly when epithelial cells detect proximate hypoxia.9 Moreover, the proliferation of intracellular pathogens can exacerbate oxygen deprivation in infected tissues.7
To address the interplay between hypoxia and inflammation, strategies focusing on anti-inflammatory actions and hypoxic mitigation have been developed. Bio-active drugs and materials that target immune cells primarily curb hypoxic inflammation by influencing the functionalities of macrophages, neutrophils, T cells, and other immune constituents.10,11 Additionally, recent advancements have brought forth various nano-therapies designed to supply or generate oxygen at inflammatory sites. These include hemoglobin-based oxygen carriers, perfluorocarbon-based oxygen carriers, catalase-mediated oxygen generation, nano-enzyme-mediated oxygen generation, and metal peroxide decomposition.12 However, comprehensive reviews detailing how hypoxia influences inflammation’s onset and progression, and the application of drugs or bio-active nano-materials for treating inflammation under hypoxic conditions, particularly from an immune cell perspective, are scarce. Based on immune cells, this paper examines the alterations in immune cells under hypoxic conditions and their implications for inflammation. It methodically reviews different therapies that target various immune cells to exert anti-inflammatory effects. In addition to bio-active drugs and materials targeting immune cells, the paper also thoroughly evaluates nano-materials capable of transporting or generating oxygen to alleviate anoxic micro-environments. This exploration aims to uncover more bio-active materials for the prevention and treatment of hypoxic inflammation, with a focus on underlying mechanisms.
The Mechanism of Inflammation Under Hypoxia
The host immune response is typically categorized into innate and adaptive immune responses. The innate response is rapid and non-specific against pathogens, whereas the adaptive response, characterized by antigen receptor gene rearrangement, develops slowly but with specificity, leading to classical immune memory.13
Hypoxia and Innate Immunity
This section focuses on two primary innate immune cells: macrophages and neutrophils. Macrophages exhibit diverse activation states influenced by their local environment, broadly categorized into classically activated M1 macrophages and alternatively activated M2 phenotypes. While M1 macrophages are generally pro-inflammatory, M2 macrophages are regarded as “immunomodulatory”, playing roles in wound healing and anti-inflammation.14 Neutrophils, significant in inflammation, exacerbate the inflammatory state by aiding macrophage recruitment and interacting with antigen-presenting cells.15 In addition, other innate immune cells, like dendritic cells, natural killer cells, and mast cells, also variably impact inflammation under hypoxic conditions.
Hypoxia and Macrophage
Hypoxia triggers increased hypoxia signaling, lactic acid buildup, and metabolic acidosis (Figure 1). Studies have shown that hypoxia elevates HIF-α secretion.16,17 Hypoxia initially inhibits PHD activity, reducing PHD’s suppressive effect on HIF-α. Additionally, IL-1, induced by cancer necrotic debris under severe and prolonged hypoxia, augments HIF-α synthesis.18 Elevated HIF-α is crucial for macrophage differentiation and inflammatory chemokine release. HIF-1α stabilizes the long noncoding RNA (HISLA), a myeloid-specific lnc-RNA, influencing miR-210 and miR-383 levels in lesion-associated macrophages, affecting their aerobic glycolysis and anti-apoptotic capabilities.19,20 Furthermore, the mTOR/HIF-1α/glycolysis pathway activation is a novel mechanism for NLRP3 inflammasome activation in macrophages.21 The NLRP3 inflammasome, a multi-protein complex, facilitates pro-inflammatory cytokine IL-1β secretion in a caspase-1-dependent manner, regulating inflammation.22 Along with the NLRP3 inflammasome, HIF-1α promotes the polarization of M1 macrophages,23 which leads to the release of pro-inflammatory cytokines such as TNF-α, IL-1, nitric oxide, and other chemokines. The increase in IFN-γ levels, mediated by HIF-1α, significantly influences macrophage cytokine production, antigen-presenting activity, and phagocytic function in hypoxic conditions.24
In conditions of mild hypoxia (4–8% Oxygen), the metabolic alterations mediated by HIF-1α-PDK1 significantly contribute to macrophage migration and activation, critical processes in inflammation.25 During the inflammatory activation of macrophages, both HIF-1α and glycolytic pathway metabolites can intensify inflammation. Hypoxia and inflammation collectively enhance anaerobic and aerobic glycolysis, leading to increased lactic acid release and metabolic acidosis.3 Lactic acid plays a crucial role in signaling, notably by inducing vascular endothelial growth factor expression and M2 macrophage polarization.26 In anaerobic tumor environments, lactic acid activates mTORC1, which in turn inhibits TFEB-mediated expression of the macrophage-specific vacuolar ATPase subunit ATP6V0d2, targeting HIF-2α. The absence of ATP6V0d2 results in increased macrophage polarization, elevated VEGF production, and enhanced HIF-2α stability.27 HIF-2α elevates Arg-1 expression in macrophages, promoting M2 macrophage polarization.28 M2 macrophages aid in resolving inflammation, tissue repair, and cell proliferation by releasing anti-inflammatory cytokines like IL-10 and clearing necrotic cell debris.29
Hypoxia and Neutrophil
Neutrophils are the most important component of innate immunity which play an important role in inflammation. When inflammation occurs, neutrophils migrate across the vascular barrier along the concentration of cytokines to the site of inflammation. In hypoxia, HIF is activated which is controlled by the oxygen sensors of neutrophils such as prolyl and asparaginyl hydroxylase enzymes.30 In colitis-associated colon cancer model mice, over-expression of HIF-2α in the intestinal epithelium promotes the recruitment of neutrophiles to the colon and regulates the infiltration to tumor-associated neutrophils to regulate the colon tumor micro-environment.31 As the neutrophils arrive the area of inflammation, the main functions of neutrophils are phagocytosis, production of reactive oxygen species (ROS), release of inflammatory proteins, and apoptosis.32 Neutrophils maintain polarized mitochondria, produce ROS and regulate HIF-1α stability by using the glycerol 3-phosphate pathway as a way of directly regulating mitochondrial function through glycolysis.33 Enhanced HIF1-α expression up-regulates critical glycolytic enzymes, including G3PDH and triosephosphate isomerase-1, thus increasing energy production and cell viability. Additionally, HIF-1α serves as an upstream regulator of NF-κβ, augmenting pro-inflammatory cytokine production and thereby extending inflammation.34,35 Conversely, hypoxia may induce neutrophil degranulation, potentially due to an initial HIF-independent phase involving PI3Kγ signaling pathway activation, or a subsequent HIF-dependent gene transcription response.30 This heightened degranulation in hypoxic conditions aids in pathogen clearance and enhances interactions with other immune cell types in neutrophils.36 However, research indicates that increased neutrophil degranulation under hypoxic conditions may lead to the release of harmful substances, like active nitrogen and toxic proteases, causing local tissue toxicity.37,38 Moreover, a dual host defense mechanism, neutrophil extracellular traps (NETs), is released via autophagy-associated signaling pathways, controlled by HIF-1α through the neutrophil stress-response protein REDD1.39 NETs combat bacteria but can also damage tissue and blood vessels during inflammation.40 Dysregulated NETs formation during pathological stimulation can result in sepsis and systemic inflammatory response syndrome.41 Furthermore, hypoxia may delay apoptosis and regulate neutrophil retention at inflammation sites through HIF-1α stabilization and NF-κ B activation.35
Hypoxia and Other Innate Immune Cells
Dendritic cells (DCs) form a vital link between innate and adaptive immunity, with the key function of recognizing pathogens and presenting antigenic peptides to T cells.42 Initially, human-derived monocytes differentiate into DCs and migrate to peripheral tissues, where immature DCs assess the environment and gather antigenic materials.43 However, some studies suggest that hypoxia down-regulates the uptake of antigens and phagocytosis in DCs, in a process seemingly independent of HIF-1α.42 Following antigen phagocytosis, DCs travel to draining lymph nodes via afferent lymphatic vessels to present the antigens to T cells.43 Research indicates that hypoxia inhibits the migration of human monocyte-derived DCs in vitro by reducing MMP-9 expression and increasing protease inhibitor TIMP1 levels.44,45 Conversely, other studies have shown that hypoxia enhances the migratory capability of DCs via the HIF-1α and PI3K/Akt pathways.43 DCs present antigens to T cells by expressing surface costimulatory molecules, prompting T cells to release specific cytokines.46 Yet, the impact of hypoxia on the expression of co-stimulatory molecules (eg, CD80, CD83) and the release of pro-inflammatory cytokines remains a topic of debate. Reports have shown that hypoxia can lead to a decrease,47,48 an increase,49,50 or no change51 in the expression of surface co-stimulatory molecules. Additionally, hypoxia has been reported to have both stimulatory42,46,49,51 and inhibitory42,48 effects on cytokine release (eg, interleukin (IL)-6, IL-10, IL-12, and TNF-α). The variability in these effects can be attributed to: (1) different environments in which DCs are induced and matured in vitro; (2) variations in the types of co-stimulatory molecules on human and animal DCs.48 (3) The interaction between the TLR signaling pathway and HIF-1α, leading to varied immune responses in different diseases.46 Consequently, further in vivo research is essential to better understand the delicate balance between necessary immune induction to combat diseases and the excessive activation of immune cells that can harm the host.
Natural killer (NK) cells, a type of cytotoxic innate lymphoid cells, induce apoptosis in target cells by up-regulating death-inducing ligands such as Fas-L and TRAIL and producing various pro-inflammatory cytokines and chemokines.52 Hypoxia sustains NK cell glycolysis by enhancing HIF-1α expression and inhibiting NK cell apoptosis via the HIF-1α/NAD axis.53,54 Moreover, hypoxia alone can diminish NK cell cytotoxicity by reducing the phosphorylation levels of ERK and STAT3 in a SHP-1-dependent manner,52 but it does not increase adhesion molecules or NK cell adhesion to human endothelial cells.55 Contrarily, Maurus demonstrated that the combination of TNF-α, a pro-inflammatory cytokine, with hypoxia augments the expression of adhesion molecules like ICAM-1 and enhances NK cell adhesion.55 Anti-inflammatory treatment strategies have been shown to mitigate hypoxia-induced damage. Mast cells, pivotal in coordinating inflammatory processes, produce and release abundant inflammatory cytokines.56 Hypoxia preserves the degranulation ability of mast cells and boosts their proliferation and secretion of pro-inflammatory cytokines, such as TNF-α and IL-6.57 Additionally, mast cells express various adhesion molecules, including integrin receptors, crucial for their localization and penetration into inflammatory tissue sites.56 Hypoxia down-regulates mast cell adhesion to hyaluronic acid by decreasing hyaluronic acid receptor affinity,58 but up-regulates mast cell adhesion to fibronectin via the PI3K/AKT signaling pathway.56 Both hyaluronic acid and fibronectin are key components of the extracellular matrix.58 These contrasting findings indicate that hypoxia significantly influences mast cell interactions with the extracellular matrix, potentially playing a role in mast cell accumulation at disease sites.
Hypoxia in Adaptive Immunity
T cells and B cells constitute the primary components of adaptive immunity. T cells differentiate into CD4+ and CD8+ subsets, marked by specific surface proteins. CD8T cells exhibit cytotoxicity, capable of directly eliminating damaged and cancerous cells. In contrast, CD4+ helper T cells indirectly induce cellular damage. Upon activation, CD4 T cells diverge into subpopulations influenced by local antigens and cytokines. These subpopulations of helper T cells are instrumental in eradicating specific microbial pathogens and stimulating pro-inflammatory responses in other immune cells. T follicular helper (Tfh) cells enhance the survival and proliferation of germinal center B cells, while regulatory T cells (Tregs) are crucial in maintaining immune homeostasis and self-tolerance, mitigating inflammation, and preventing autoimmune diseases.59,60 Activated T cells propel B cell development, whereas soluble antigens stimulate B cells through B cell receptors, leading to antibody responses and the generation of memory B cells alongside CD8 memory T cells. These memory cells are primed for rapid proliferation upon subsequent pathogen encounters.59
Hypoxia and T Cells
In hypoxic conditions, T cells are activated and drawn to inflammation sites. HIF-1α associates with a transcriptionally active hypoxia-response element (HRE) in the PD-L1 proximal promoter, inhibiting PD-L1 activation in hypoxia. This enhances T cell activation while downregulating IL-6 and IL-10.61 Hypoxia impacts CD8 T cell glycolytic metabolism, their ability to eliminate target cells, and infiltration, all in an HIF-1α-dependent manner.62 HIF-1α is crucial for CD8 T cells’ effector state. Hypoxia fosters CD8 T cell differentiation via the IL-4-HIF-1α-IL-13 axis, enhancing CD8 T cell-dependent airway hyperreactivity and inflammation, potentially contributing to steroid-refractory asthma development.63 Additionally, IL-2, a vital cytokine for preventing chronic inflammation, upholds high levels of glucose metabolism and glycolysis in CD8 T cells by maintaining mTORC1 activity and HIF1α protein expression.64,65 HIF also regulates CD4 T cell populations by promoting CD154 expression on Tfh cells, vital for stimulating CD40 on germinal center B (GCB) cells. This affects the quantity of switch cytokines secreted by differentiated helper cells and alters the metabolic programming of activated T cells. Hypoxia diminishes cytokine secretion upon re-stimulation of differentiated CD4 T cells and modifies their metabolic programming. Conversely, HIF enhances the capacity of activated CD4 T cells to produce switch cytokines.66
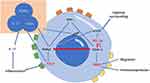
Regulatory T cells (Tregs), a specialized subpopulation of CD4 T cells, are essential in suppressing T cell activation, maintaining peripheral immune tolerance, and curbing inflammatory responses.67 The impact of hypoxia on Tregs is multifaceted and somewhat contradictory. Clambey demonstrated that Treg-intrinsic HIF-1α significantly restrains inflammation. He observed that hypoxia in mucosal inflammation stimulates Foxp3 transcription via HIF-1α, thereby enhancing the abundance and functionality of Tregs.68 Foxp3 counters the activity of the retinoid-related orphan receptor-γ t (ROR γ T). It competes with ROR γ T protein binding, thereby inhibiting Treg binding to DNA and promoting differentiation.67 Contrarily, some studies indicate that HIF-1α impairs the function and stability of Tregs.69 HIF-1α is known to facilitate FOXP3 proteasomal degradation and hinder the development of Tregs by altering T cell metabolism.70,71 Addressing this complexity, Miska established that hypoxia, rather than suppressing, actually promotes Treg migration through a Treg-specific ablation of HIF-1α in an animal model. In hypoxic conditions, HIF-1α diverts glucose away from mitochondria, making Tregs reliant on fatty acids for mitochondrial metabolism. This positions HIF-1α as a metabolic switch in Tregs, toggling between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression.71 In contrast to Tregs, TH17 cells predominantly represent an anti-inflammatory subset.72 As illustrated in Figure 2, HIF-1α acts as a crucial metabolic sensor, balancing Treg and TH17 cell dynamics. It modulates TH17 signature genes and amplifies TH17 development through the transcriptional activation of ROR γ T. ROR γ T and p300 congregate at the IL-17 promoter, forming a complex.67,73 Upon reaching a threshold expression level of ROR γ T, the cell transitions to a TH17 phenotype, potentially inciting inflammation.74
Hypoxia and B Cells
B cells, integral to adaptive immunity, exhibit varying responses to hypoxic environments, which remain incompletely understood. Research on mouse models has revealed that the germinal center (GC) micro-environment is hypoxic.75,76 Hypoxia has been observed to enhance B cell proliferation and promote class switch recombination (CSR) and plasmacyte differentiation.76 HIF-1α, a pivotal transcription factor, is crucial for the production of IL-6 and IL-10 in B cells. It binds to their promoters, augmenting their transcription.77,78 In patients with Rheumatoid Arthritis (RA), B cells are the primary source of IL-6 in peripheral blood and are closely associated with RA disease activity.77 The production of IL-10, which modulates the differentiation of innate-like B cells and B10 cells, leads to reduced IgM secretion.76 IL-10 is known to mitigate inflammation; conversely, IL-10-knockout mice have displayed chronic inflammation.79 Furthermore, the expression of hCXCR4 is vital in regulating B cell viability under hypoxia, influenced by hypoxia-induced ROS, HIF-1α, and Nrf2. CXCR4 also facilitates the migration of B-1a cells to the bone marrow, where they produce IgM antibodies.80 However, HIF-1α plays a role in the regeneration process post-pancreatitis by limiting B cell accumulation in the pancreas. Lee’s experiments showed that mice lacking pancreas-specific HIF-1α expression exhibited significantly impaired pancreatic regeneration, coupled with abnormal B cell accumulation in the pancreas following cerulein-induced pancreatitis.81
Influence Factors
Degree of Hypoxia
Hypoxia can be categorized based on its duration as either acute or chronic. Acute hypoxia typically arises during ARDS, acute cerebrovascular diseases, and cardiac arrest.82 In contrast, chronic hypoxia is more common in conditions such as chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), and anemia. Recovery is often achievable following acute hypoxia, but chronic hypoxia tends to show a diminished recovery amplitude, attributed to specific molecular mechanisms that evolve with prolonged hypoxia.82 Hypoxia can also be classified according to its frequency into continuous or intermittent forms. Intermittent hypoxia is associated with a heightened inflammatory response compared to continuous hypoxia.83,84 Specifically, intermittent hypoxia notably escalates the activation of c-Jun and NF-κβ in M1-type macrophages, thereby fostering a pro-inflammatory phenotype.85 Moreover, the severity of hypoxia determines the extent of HIF-α activation in immune cells. Severe hypoxia can precipitate a bioenergetic crisis in cells, leading to necrosis. This cellular necrosis further exacerbates the inflammatory process by releasing cellular contents into the extracellular space.5
Body State
Various bodily conditions, such as age, obesity, and diabetes, significantly influence the degree of inflammation experienced under hypoxic conditions.86,87 Hypoxia activates related signaling pathways and interacts with aging pathways, potentially accelerating the aging process. In an aging state, two key developments occur. Firstly, aging-related inflammation leads to the accumulation of local inflammatory factors, potentially causing severe inflammation and even cytokine storms. Secondly, aging impairs various cellular functions, notably in epithelial cells and vascular smooth muscle cells, leading to frequent oxidative stress damage.86 Obesity induces a mild yet chronic inflammatory state within adipose tissue,87 driven by several pathways, including oxidative stress, endoplasmic reticulum stress, and adipose tissue hypoxia.87,88 Hypoxia acts as a principal trigger for adipokine dysregulation in obesity, promoting the expression of macrophage genes regulated by HIF-1α.89,90 Elevated expression and protein levels of HIF-1α have been observed in the adipose tissue of obese mice.91 Moreover, high glucose levels foster chronic inflammation and oxidative stress, elevating the expression of various pro-inflammatory cytokines.92,93 Zhao showed that the HIF-1α/JMJD1A signaling pathway is involved in oxidative stress and inflammation in human umbilical vein endothelial cells (HUVECs) that are caused by high glucose and low oxygen levels.92
The Anti-Inflammatory Therapies Under Hypoxia
In the above study, we explored how hypoxia promotes the development of inflammation by influencing immune cells. Therefore, one of the effective strategies for the treatment of hypoxic inflammation is to regulate the activity of immune cells under hypoxic environment by targeting immune cells, so as to achieve the purpose of anti-inflammation and tissue regeneration. More information on immune cell targeting therapy strategies is summarized in Table 1.
 |
Table 1 Summary of Different Drugs Targeting Immune Cells |
Therapy in Macrophage
Numerous studies have shown that inhibiting HIF-1α steers macrophages towards an anti-inflammatory phenotype.95,100 Glucose metabolism significantly influences HIF activity. D-mannose elevates intracellular mannose-6-phosphate, hindering succinate-mediated HIF-1α activation.95 Similarly, Tan-IIA obstructs HIF-1α induction by reducing succinate levels.97 Drugs like Norisoboldine, Cynaroside, and Iminostilbene target the PKM2/HIF-1α axis. They decrease PKM2-HIF-1α binding and inhibit glycolysis-related proteins such as PFKFB3, HK2, and HIF-1α.96,98,100 Suppressing HIF-1α activation primarily influences macrophage polarization by modifying the release of anti-inflammatory cytokines, the glycolytic pathway balance, and NLRP3 inflammasome inhibition. Notably, IL-6 and TNF-α levels, M1 macrophage markers, decline with treatment. Conversely, IL-4 and IL-10 levels, which induce M2 macrophage polarization, increase significantly.10,114 Deng’s experiments revealed that BMSCs-derived exosomes regulate macrophage polarization by inhibiting glycolysis, with HIF-1α playing a crucial role in this anti-glycolysis effect.99 Tiliroside prevents classical M1 macrophage polarization by blocking glycolytic enzyme expression and reducing 2-NBDG uptake, a fluorescent deoxy-glucose analog.94 Therapies such as the co-assembly of hypoxia-sensitive macrocyclic amphiphiles with extracellular vesicles and ferulic acid induce the M1-to-M2 macrophage transition by inhibiting HIF-1α expression and the downstream NF-κβ signaling pathway.102,115 Beyond HIF-1α, the PPAR γ/STAT3 and GSK-3β/Nrf-2 pathways also regulate macrophage polarization.116–118 Curcumin, via the ERK signaling pathway, reduces HIF-1α levels, thereby lowering total cholesterol and lipid levels in macrophages and decreasing apoptosis.101 As highlighted, the NLRP3 inflammasome, crucial in inflammation development, is activated through the mTOR/HIF-1α/glycolysis pathway,119,120 Hydroxychloroquine impedes NLRP3 inflammasome activation via the NF-κβ signaling pathway.103
Therapy in Neutrophil
Anti-inflammatory drugs effectively suppress inflammation by inhibiting the release of inflammatory cytokines and reducing neutrophil infiltration, migration, and adhesion. HIF-1α, crucial for oxygen homeostasis under hypoxic conditions, is a significant target in this process. FG-4592 is a special small-molecule PHD inhibitor that stabilizes HIF-1α. This stops neutrophils from entering the cell, prevents cell damage, and reduces inflammation caused by low oxygen.105 Lu’s research indicated that Cyclosporine enhances neutrophil HIF-1α expression via the SIRT6–HIF‐1α–glycolysis axis. This elevation aids in fueling neutrophil glycolysis and the TCA cycle, reducing neutrophil migration, and alleviating severe ulcerative colitis.104 Moreover, targeting neutrophil adhesion is a vital therapeutic strategy. Sevoflurane diminishes neutrophil adhesion molecule expression and inhibits migration by stabilizing hypoxia-inducible factor 1α and the adenosine A2B receptor.106 In addition to the stabilizing effects of HIF-1α, research indicates that inhibitors of HIF-1α also play a crucial role in modulating neutrophil functions to mitigate inflammation. Itaconic acid, an endogenous product of macrophages, stimulates the transcription of the anti-inflammatory nuclear factor erythroid 2-related factor 2 (Nrf2). This activation leads to an upregulation of the cytoprotective enzyme heme oxygenase (HO-1) and concurrently reduces the release of pro-inflammatory cytokines.121 Gabriela discovered a derivative of itaconic acid, specifically 4-octyl itaconate (4-OI), which was introduced exogenously. This compound has been shown to inhibit the formation of NETs in mice. It achieves this by suppressing HIF-1α and activating HO-1.107 While the formation of neutrophil NETs is advantageous during the initial stages of infection, it becomes detrimental in subsequent inflammation stages caused by the infection.122 Additionally, YC-1, functioning as a HIF-1 inhibitor, effectively impedes the HMGB1/TLR4/NF-κβ signaling pathway. This inhibition subsequently reduces HIF-1α expression, curtailing the release of pro-inflammatory cytokines and the infiltration of neutrophils.108
Therapy in Adaptive Immunity
In the context of hypoxia, a key factor contributing to heightened inflammation is the imbalance between Treg and TH17 cells. Addressing this imbalance involves interventions at three levels: cytokines, receptors, and signaling pathways. TH17 cells, known for their pro-inflammatory actions, facilitate the release of cytokines such as TNF, IL-6, and IL-17. TNF inhibitors, including infliximab, etanercept, and adalimumab, are extensively utilized in treating rheumatoid arthritis.109 IL-17 inhibitors have demonstrated anti-inflammatory properties in both human and rat models of inflammatory diseases.67,113 Additionally, the TH17 transcription factor ROR γ t presents another target. Laura introduced SR1001, a high-affinity synthetic ligand, which inhibits TH17 cell differentiation and function by targeting ROR γ t.110 Hif-1α plays a crucial role in modulating the interplay between inflammation and T cells. Melatonin, an endogenous hormone, mitigates the expression of IL-17A and IFN-γ, thereby suppressing TH17 cells in experimental autoimmune uveitis mice through the oxidative stress/TXNIP/HIF-1α axis.112 Yao suggested that the HIF-1α inhibitor echinomycin improves Treg development by blocking the interaction between HIF-1α and Foxp3, and it weakens TH17 responses by blocking HIF-1α’s connection to the ROR γ t gene.111 STAT3 is crucial in triggering ROR γ t, a transcription factor specific to TH17 cells, which is essential for inducing the expression of IL17.123 Thus, Consequently, therapeutic strategies targeting the JAK-STAT signaling pathway are also frequently used in immune diseases and inflammatory diseases.123,124
Therapy Alleviating Hypoxic Micro-Environment Function
The previously discussed therapies focus on modulating immune cell responses to suppress inflammation induced by hypoxia. However, these do not directly address the hypoxic micro-environment itself. To counteract this, materials that either deliver or generate oxygen in hypoxic regions can mitigate hypoxic inflammation. This is achieved by alleviating anoxia, inhibiting anaerobic bacteria, and reducing ROS. Table 2 lists representative nanomaterials used to alleviate hypoxia.
 |
Table 2 Summary of Oxygenated Materials |
Oxygen Delivery in Hypoxic Regions
Currently, materials like perfluorocarbons (PFCs) and biomimetic nano-materials based on red blood cells are predominant in oxygen transport. To make these man-made oxygen carriers (AOCs), scientists use a variety of techniques, including electrospinning/electro-spraying, mechanical emulsification, SPG membrane emulsification, flow focusing, and the layer-by-layer technique with template particles.137
Perfluorocarbons
PFCs, with their unique ability to bind oxygen due to fluorine atoms replacing hydrogen, serve as efficient artificial oxygen carriers.138 Their capacity to dissolve significant amounts of oxygen stems from weak van der Waals forces, facilitating passive diffusion into oxygen-deprived zones.139 The dense electron clouds, elevated ionization potentials, and increased electron affinities in PFCs confer pronounced hydrophobicity and fluorophilicity. These properties, while characteristic of PFCs, present challenges in biological aqueous environments due to their inherent incompatibility with hydrophilic structures, unless appropriately conjugated with hydrophilic entities.125 Their oxygen release, which depends only on diffusion across concentration gradients, is noticeably inefficient.140 Additionally, PFCs can release metal ions, potentially leading to acute toxicity and oxidative damage in vital organs.141,142 Therefore, Patil introduced meth-acrylamide chitosan-modified perfluorocarbon chains (MACF) to create hydrogel dressings for dermal wounds. This hydrogel, as illustrated in Figure 3C, facilitates oxygen transport and enhances wound healing by modulating arginine and proline metabolism. Notably, it regulates M1 and M2 macrophage activation without macrophage phenotype polarization in wound tissues, with untreated wounds showing fewer macrophages.125 Additionally, oxygenating microgels were developed using PFC-modified chitosan through a water-in-oil mini-emulsion method and incorporated into 3D spheroid cultures to boost oxygen transport (Figure 3).126 Another good way to address the shortcomings of PFCs is to wrap them in nanoparticles. Yang synthesized perfluoro-decalin-encapsulated albumin nanoparticles within a hyaluronate gel, forming a nano-oxygenated gel. This gel effectively delivered oxygen to overcome local hypoxia and foster tissue regeneration, reducing macrophage density and inflammation.127 Moreover, Lee incorporated perfluorocarbon (PFC) nano-emulsions into a hydrogel dressing, enhancing its composition with epidermal growth factor (EGF)-loaded nanoparticles and poly-hexamethylene biguanide. This innovative blend was designed to simultaneously deliver anti-inflammatory and antimicrobial effects, as well as supplement oxygen. The experimental results demonstrated the hydrogel’s anti-inflammatory capabilities, attributed primarily to the release of chitosan molecules.128 This leads to a compelling proposition: combining the previously mentioned anti-inflammatory agents with PFCs to develop novel nanoparticles or hydrogels that play a multi-functional role.
 |
Figure 3 Perfluorocarbons deliver oxygen to hypoxia areas. (A) Representative PO2 vs time data for MACF initially saturated with oxygen (+O2) and MAC + O2 at RT. (B) Oxygen transport rate measurements. Superscript symbols “*” indicate p < 0.0001. (C) Arginine and proline metabolism pathway supplies fibrillary collagen synthesis. Reprinted from Acta Biomater, volume: 36, Patil PS, Fountas-Davis N, Huang H, et al. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. 164–174, Copyright 2016, with permission from Elsevier.125 (D) Illustration of radial oxygen measurements taken at various depths in control versus microgel loaded spheroids after 4 days (96 h) in culture. (E) Partial pressure of oxygen at various depths inside the spheroids. Reprinted from Patil PS, Mansouri M, Leipzig ND. Fluorinated Chitosan Microgels to Overcome Internal Oxygen Transport Deficiencies in Microtissue Culture Systems. Adv Biosyst. 2020; 4 (8): e1900250. © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.126 |
Hemoglobin-Based Oxygen Carriers
Artificial materials often pose biocompatibility challenges and can elicit immune responses.143 In response, bionic materials, such as those derived from white or red blood cell membranes, are increasingly utilized.144 Red blood cells, notable for their double-concave structure and ability to deform mechanically, efficiently transport oxygen by forming oxy-hemoglobin.129,145 Guo capitalized on this by employing silica bio-replication to reconstruct red blood cells, embedding them with functional cargo such as hemoglobin (Hb) drugs and magnetic nanoparticles for enhanced oxygen transport.129 Hemoglobin consists of four peptide subunits, each containing a heme group. This group, featuring a porphyrin ring with centrally bound iron ions (Fe2+), is crucial for oxygen carriage. Oxygenated hemoglobin (HbO2) forms through reversible binding with oxygen. At mildly elevated temperatures, Hb acts as an efficient oxygen carrier, modulating its oxygen-binding ability for controlled release.130,146 Yang developed an injectable hydrogel combining hyaluronic acid-graft-dopamine (HA-DA) and polydopamine (PDA)-coated Ti3C2 Mxene nano-sheets. This formulation undergoes catalytic cross-linking via an oxyhemoglobin/hydrogen peroxide (HbO2/H2O2) system, activated by mild photothermal stimulation. HbO2 in this system releases oxygen in response to mild heat and captures atmospheric oxygen when near-infrared irradiation (NIR) is deactivated. HA-DA molecules in the hydrogel also help macrophages change from M1 to M2, which adds to its anti-inflammatory properties.130 However, hemoglobin molecules, when devoid of protective red blood cell membranes or biological environments, degrade quickly and lose oxygen transport efficiency. It’s been observed that modifying hemoglobin through polymerization, cross-linking, conjugation, encapsulation, or genetic recombination can enhance its stability and functionality.147 These modifications help reduce hemoglobin dissociation, prevent exosmosis of Hb, and maintain effective life cycles and transport capacities.148
Oxygen Production in situ in Hypoxic Areas
Catalase-Based Nanoparticles
Catalase, a vital endogenous antioxidant enzyme, plays a dual role. It not only acts as an antioxidant to eliminate ROS but also functions as an oxygen generator. This dual functionality is essential for oxygenating wound tissue, stimulating epithelial regeneration, fibroblast proliferation, migration, and angiogenesis, thereby facilitating tissue regeneration and wound healing.149 Zhou synthesized encapsulated S-methylisothiourea hemi-sulfate salt (SMT) and catalase (CAT) within ZIF-8 nanoparticles (NPs). These NPs, upon decomposition in the acidic endosomal environment, release CAT and SMT (Figure 4). SMT inhibits the release of inducible nitric oxide synthase (iNOS), thereby controlling mitochondrial respiration disruption caused by nitric oxide (NO). This regulation assists in moderating the release of H2O2 and ROS. Concurrently, CAT catalyzes the decomposition of excess H2O2 in the body into oxygen, further regulating mitochondrial respiration and suppressing HIF-1α expression.131 Building on this, researchers have proposed novel therapeutic strategies that integrate immune-targeted therapy with catalase. Han pioneered a combination of dendritic cell (DC)-targeted nanoplatforms with retinoic acid (RA) therapy, creating ZnO2/Catalase @liposome-Mannose nanoparticles (ZnCM NPs). These NPs modulate zinc and oxygen homeostasis; catalase within these NPs transforms H2O2 into oxygen, alleviating intracellular hypoxia. This assists Zn2+ in promoting the transition from DC to tolerogenic dendritic cells (tDCs), reducing immune damage in rheumatoid arthritis (RA).132 However, the concentration of H2O2 in wounds is limited, and its exogenous delivery is challenging to control, potentially exacerbating oxidative stress and causing toxicity. Scholars suggest a multi-enzyme cascade reaction approach, combining catalase with superoxide dismutase (SOD), to enhance ROS removal and oxygen production.150 SOD, a primary antioxidant enzyme, converts superoxide radicals to H2O2 and then to O2, acting as an antioxidant and anti-inflammatory agent.151 Yet the enzyme’s stability limits its application, which restricts its stable delivery in the body.152 Furthermore, catalase concentrations exceeding 500 U/mL may disrupt key cellular signaling molecules, posing potential cytotoxic risks.153 Therefore, optimizing catalase concentration to balance its oxygen-producing role in wound healing without inducing cytotoxicity is an area of ongoing research.
 |
Figure 4 Catalase-based nanoparticles produce oxygen in situ in hypoxia areas. (A) Schematic illustration of the synthesis processes for CAT&SMT@ZIF-8-Ab NPs. (B) Modified ZIF-8 NPs target synovial macrophages to regulate intracellular gases and reprogram the metabolic pathway, thus attenuating OA. (C) The percentages of M1 (CD206-negative and CD16/32-positive cells) and M2 (CD206-positive and CD16/32-negative cells cells) macrophages were evaluated by flow cytometry. (D) Expression levels of genes related to M1-type macrophage including IL-1β, IL-12b, IL-6, TNF-α, and Inos/Arg-1 were measured by Qrt-PCR. Superscript symbol “*” indicates comparisons with the first group, and superscript symbol “#” indicates comparisons with the second group. Comparisons between ZIF-8 and CAT&SMT@ZIF-8 NP-treated groups and CAT&SMT@ZIF-8 and CAT&SMT@ZIF-8-Ab NP-treated groups were calculated and marked with the superscript symbol “&”. Superscript symbols “*”, “#”, and “&” indicate p < 0.05; superscript symbols “**”, “##”, and “&&” indicate p < 0.01; NS means not significant. Reprinted from Zhou F, Mei J, Yang S, et al. Modified ZIF-8 Nanoparticles Attenuate Osteoarthritis by Reprogramming the Metabolic Pathway of Synovial Macrophages. ACS Appl Mater Interfaces. 2020; 12 (2): 2009–2022. Copyright 2022 American Chemical Society.131 |
Nano-Enzymes
Nano-particles exhibiting enzyme-like catalytic activity can alleviate hypoxia by catalyzing hydrogen peroxide (H2O2) into oxygen. Prominent nano-materials with such activity include Manganese dioxide (MnO2), Prussian blue (PB), Platinum (Pt), Fe3O4, etc.133,134,151,154 Platinum nanoparticles, in particular, can break the oxygen-oxygen bond of H2O2 to generate O2,155 demonstrating various oxidoreductase-like activities (type 2 NZ), including oxidase (OX), peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD).156 These nanoparticles also regulate ROS levels, impacting inflammatory signal transduction and pathways. Both in vitro and in vivo experiments have confirmed Pt nanoparticles’ potential anti-inflammatory effects, which mitigate phagocytic activation by clearing ROS-producing chambers and disrupting pathways involved in intracellular aggregation and pro-inflammatory gene transcription.156,157 However, the detailed mechanisms of their action in inflammation remain largely unexplored. Prussian blue nanoparticles stand out as promising catalysts. Tong utilized chlorin e6 (Ce6)-loaded Prussian blue nanoparticles for combating drug-resistant bacteria. These nanoparticles catalyze H2O2 into O2, alleviating the anaerobic bacterial environment. Concurrently, photosensitizers (PS) in the nanoparticles enhance the antibacterial effect by utilizing oxygen to generate significant amounts of ROS. Additionally, PB nanoparticles promote wound healing and anti-inflammatory effects by regulating macrophage polarization.152 MnO2 nano-sheets, a novel nano-enzyme, catalyze the abundant endogenous ROS (H2O2) into O2, presenting a promising solution for oxidative stress and hypoxia.134,158 Wang developed an injectable multifunctional hydrogel composed of ε-poly-lysine (EPL)-coated MnO2 nano-sheets (EM) and insulin-loaded, self-assembled aldehyde Pluronic F127 (FCHO) micelles. The hydrogel responds to elevated glucose levels in diabetes, which are associated with increased ROS production, impeding wound healing. MnO2 nano-sheets serve a dual function: clearing ROS and replenishing oxygen. When H2O2 breaks down EM nano-sheets, it starts a redox reaction that helps break down the hydrogel and speed up insulin release. This process not only scavenges ROS but also reduces glucose levels, thereby decreasing inflammation. Moreover, MnO2 nano-sheets have demonstrated excellent antibacterial properties.134
Metal Peroxides Decomposition Strategy
Metal peroxides, comprising metal ions and peroxide groups, interact with water to produce hydrogen peroxide (H2O2) and release metal ions. Current peroxide nano-systems primarily include copper peroxide (CuO2), calcium peroxide (CaO2), magnesium peroxide (MgO2), zinc peroxide (ZnO2), barium peroxide (BaO2), and titanium peroxide (TiOx).159 Among these, calcium peroxide is the most widely used in wound healing, owing to its optimal oxygen-producing potential and environmentally friendly by-products.12 Ma developed a pH-responsive O2 and H2O2 self-supplying zeolitic imidazolate framework-67 (ZIF-67) nano-system for photodynamic (PDT) and chemo-dynamic therapy (CDT) of wound infections. This system uses CaO2 to make oxygen and H2O2. The oxygen lowers hypoxia, which improves PDT caused by light from the outside, and H2O2, which is catalyzed by endogenous Co2+, makes hydroxyl radicals for CDT that is set off by Co2+.135 A major challenge with calcium peroxide is its rapid initial release of oxygen upon direct contact with water. Addressing this, Lim designed an oxygen-generating biomaterial using calcium peroxide, coated with catalase (which quickly decomposes H2O2 into water and oxygen), and hydrophobic beeswax (BW) for prolonged oxygen release. Experiments demonstrated that the beeswax addition extended the oxygen burst duration from 2 to 12 hours.136 However, the decomposition by-products of calcium peroxide may cause olfactory disorders, and its direct exposure to the lower respiratory tract can lead to pulmonary edema. Therefore, the bio-safety of calcium peroxide warrants further enhancement.136
Conclusion
Immune cells play a crucial role in the interplay between hypoxia and inflammation. While targeting hypoxic signaling pathways, there is a potential trade-off between the regulatory and pro-inflammatory functions of immune cells. As shown in Figure 5, understanding how hypoxia exacerbates inflammation via immune cells is essential for effectively treating hypoxia-induced inflammatory diseases. This paper has reviewed existing drugs and materials that target immune cells for the treatment and mitigation of hypoxia-induced inflammation, paving the way for new therapeutic targets. The paper also highlights the significance of bio-active nano-materials capable of delivering or producing oxygen in situ at infected sites. These materials, known for reducing hypoxia and clearing ROS, also influence immune cell functionality. Therefore, the development of bio-active materials must be informed by a thorough understanding of their underlying mechanisms. Strategically selecting and combining these bio-active materials from a mechanistic viewpoint can lead to the design of novel, multifunctional materials. Such an approach promises more effective prevention and treatment of hypoxic inflammation.
Data Sharing Statement
No data was used for the research described in the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mirchandani AS, Jenkins SJ, Bain CC, et al. Hypoxia shapes the immune landscape in lung injury and promotes the persistence of inflammation. Nat Immunol. 2022;23(6):927–939. doi:10.1038/s41590-022-01216-z
2. Delprat V, Huart C, Feron O, Soncin F, Michiels C. The impact of macrophages on endothelial cells is potentiated by cycling hypoxia: enhanced tumor inflammation and metastasis. Front Oncol. 2022;12:961753. doi:10.3389/fonc.2022.961753
3. Ivashkiv LB. The hypoxia-lactate axis tempers inflammation. Nat Rev Immunol. 2020;20(2):85–86. doi:10.1038/s41577-019-0259-8
4. Peng M, Yin N, Chhangawala S, et al. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354(6311):481–484. doi:10.1126/science.aaf6284
5. Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17(12):774–785. doi:10.1038/nri.2017.103
6. Corcoran SE, O’Neill LA. HIF1α and metabolic reprogramming in inflammation. J Clin Invest. 2016;126(10):3699–3707. doi:10.1172/JCI84431
7. Eltzschig HK, Carmeliet P, Schwartz RS. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi:10.1056/NEJMra0910283
8. Colgan SP, Campbell EL, Kominsky DJ. Hypoxia and Mucosal Inflammation. Annu Rev Pathol. 2016;11(1):77–100. doi:10.1146/annurev-pathol-012615-044231
9. Campbell EL, Bruyninckx WJ, Kelly CJ, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40(1):66–77. doi:10.1016/j.immuni.2013.11.020
10. Tu C, Lu H, Zhou T, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597. doi:10.1016/j.biomaterials.2022.121597
11. Lin Y, Xue K, Li Q, et al. Cyclin-dependent kinase 7 promotes Th17/Th1 cell differentiation in psoriasis by modulating glycolytic metabolism. J Invest Dermatol. 2021;141(11):2656–2667.e2611. doi:10.1016/j.jid.2021.04.018
12. Han X, Ju LS, Irudayaraj J. Oxygenated wound dressings for hypoxia mitigation and enhanced wound healing. Mol Pharm. 2023;20(7):3338–3355. doi:10.1021/acs.molpharmaceut.3c00352
13. Netea MG, Joosten LA, Latz E, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. doi:10.1126/science.aaf1098
14. Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60(5):1090–1096. doi:10.1016/j.jhep.2013.12.025
15. Arrese M, Cabrera D, Kalergis AM, et al. Innate immunity and inflammation in NAFLD/NASH. Dig Dis Sci. 2016;61(5):1294–1303. doi:10.1007/s10620-016-4049-x
16. Li Y, Liang Q, Zhou L, et al. An ROS-responsive artesunate prodrug nanosystem co-delivers dexamethasone for rheumatoid arthritis treatment through the HIF-1α/NF-κB cascade regulation of ROS scavenging and macrophage repolarization. Acta Biomater. 2022;152:406–424. doi:10.1016/j.actbio.2022.08.054
17. Doi K, Murata K, Ito S, et al. Role of lysine-specific demethylase 1 in Metabolically integrating osteoclast differentiation and inflammatory bone resorption through hypoxia-inducible factor 1α and E2F1. Arthritis Rheumatol. 2022;74(6):948–960. doi:10.1002/art.42074
18. Zhang J, Zhang Q, Lou Y, et al. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67(5):1872–1889. doi:10.1002/hep.29681
19. Chen F, Chen J, Yang L, et al. Extracellular vesicle-packaged HIF-1α-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol. 2019;21(4):498–510. doi:10.1038/s41556-019-0299-0
20. Karshovska E, Wei Y, Subramanian P, et al. HIF-1α (Hypoxia-Inducible Factor-1α) promotes macrophage necroptosis by regulating miR-210 and miR-383. Arterioscler Thromb Vasc Biol. 2020;40(3):583–596. doi:10.1161/ATVBAHA.119.313290
21. Zhong WJ, Liu T, Yang HH, et al. TREM-1 governs NLRP3 inflammasome activation of macrophages by firing up glycolysis in acute lung injury. Int J Biol Sci. 2023;19(1):242–257. doi:10.7150/ijbs.77304
22. Li W, Cao T, Luo C, et al. Crosstalk between ER stress, NLRP3 inflammasome, and inflammation. Appl Microbiol Biotechnol. 2020;104(14):6129–6140. doi:10.1007/s00253-020-10614-y
23. Chen MH, Wang YH, Sun BJ, et al. HIF-1α activator DMOG inhibits alveolar bone resorption in murine periodontitis by regulating macrophage polarization. Int Immunopharmacol. 2021;99:107901. doi:10.1016/j.intimp.2021.107901
24. Acosta-Iborra B, Elorza A, Olazabal IM, et al. Macrophage oxygen sensing modulates antigen presentation and phagocytic functions involving IFN-gamma production through the HIF-1 alpha transcription factor. J Immunol. 2009;182(5):3155–3164. doi:10.4049/jimmunol.0801710
25. Semba H, Takeda N, Isagawa T, et al. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun. 2016;7(1):11635. doi:10.1038/ncomms11635
26. Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi:10.1038/nature13490
27. Liu N, Luo J, Kuang D, et al. Lactate inhibits ATP6V0d2 expression in tumor-associated macrophages to promote HIF-2α-mediated tumor progression. J Clin Invest. 2019;129(2):631–646. doi:10.1172/JCI123027
28. Choe SS, Shin KC, Ka S, et al. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes. 2014;63(10):3359–3371. doi:10.2337/db13-1965
29. Li M, Sun X, Zhao J, et al. CCL5 deficiency promotes liver repair by improving inflammation resolution and liver regeneration through M2 macrophage polarization. Cell Mol Immunol. 2020;17(7):753–764. doi:10.1038/s41423-019-0279-0
30. Lodge KM, Cowburn AS, Li W, Condliffe AM. The impact of hypoxia on neutrophil degranulation and consequences for the host. Int J Mol Sci. 2020;21(4):1183. doi:10.3390/ijms21041183
31. Triner D, Xue X, Schwartz AJ, et al. Epithelial hypoxia-inducible factor 2α facilitates the progression of colon tumors through recruiting neutrophils. Mol Cell Biol. 2017;37(5). doi:10.1128/MCB.00481-16
32. Curi R, Levada-Pires AC, Silva EBD, et al. The critical role of cell metabolism for essential neutrophil functions. Cell Physiol Biochem. 2020;54(4):629–647.
33. Willson JA, Arienti S, Sadiku P, et al. Neutrophil HIF-1α stabilization is augmented by mitochondrial ROS produced via the glycerol 3-phosphate shuttle. Blood. 2022;139(2):281–286. doi:10.1182/blood.2021011010
34. Harris AJ, Mirchandani AS, Lynch RW, et al. IL4Rα signaling abrogates hypoxic neutrophil survival and limits acute lung injury responses in vivo. Am J Respir Crit Care Med. 2019;200(2):235–246. doi:10.1164/rccm.201808-1599OC
35. Fresneda Alarcon M, McLaren Z, Wright HL. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: same foe different M.O. Front Immunol. 2021;12:649693. doi:10.3389/fimmu.2021.649693
36. Tan BH, Meinken C, Bastian M, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol. 2006;177(3):1864–1871. doi:10.4049/jimmunol.177.3.1864
37. Hajdamowicz NH, Hull RC, Foster SJ, Condliffe AM. The impact of hypoxia on the host-pathogen interaction between neutrophils and Staphylococcus aureus. Int J Mol Sci. 2019;20(22):5561. doi:10.3390/ijms20225561
38. Alfaidi M, Wilson H, Daigneault M, et al. Neutrophil elastase promotes interleukin-1β secretion from human coronary endothelium. J Biol Chem. 2015;290(40):24067–24078. doi:10.1074/jbc.M115.659029
39. Jing H, Chen X, Zhang S, et al. Neutrophil extracellular traps (NETs): the role of inflammation and coagulation in COVID-19. Am J Transl Res. 2021;13(8):8575–8588.
40. Tang YY, Wang DC, Wang YQ, Huang AF, Xu WD. Emerging role of hypoxia-inducible factor-1α in inflammatory autoimmune diseases: a comprehensive review. Front Immunol. 2022;13:1073971. doi:10.3389/fimmu.2022.1073971
41. McInturff AM, Cody MJ, Elliott EA, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 α. Blood. 2012;120(15):3118–3125. doi:10.1182/blood-2012-01-405993
42. Flück K, Breves G, Fandrey J, Winning S. Hypoxia-inducible factor 1 in dendritic cells is crucial for the activation of protective regulatory T cells in murine colitis. Mucosal Immunol. 2016;9(2):379–390. doi:10.1038/mi.2015.67
43. Filippi I, Morena E, Aldinucci C, et al. Short-term hypoxia enhances the migratory capability of dendritic cell through HIF-1α and PI3K/Akt pathway. J Cell Physiol. 2014;229(12):2067–2076. doi:10.1002/jcp.24666
44. Qu X, Yang MX, Kong BH, et al. Hypoxia inhibits the migratory capacity of human monocyte-derived dendritic cells. Immunol Cell Biol. 2005;83(6):668–673. doi:10.1111/j.1440-1711.2005.01383.x
45. Liu J, Zhang X, Chen K, et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity. 2019;50(3):600–615.e615. doi:10.1016/j.immuni.2019.01.021
46. Paardekooper LM, Bendix MB, Ottria A, et al. Hypoxia potentiates monocyte-derived dendritic cells for release of tumor necrosis factor α via MAP3K8. Biosci Rep. 2018;38(6). doi:10.1042/BSR20182019
47. Fliesser M, Wallstein M, Kurzai O, Einsele H, Löffler J. Hypoxia attenuates anti-Aspergillus fumigatus immune responses initiated by human dendritic cells. Mycoses. 2016;59(8):503–508. doi:10.1111/myc.12498
48. Wang Q, Liu C, Zhu F, et al. Reoxygenation of hypoxia-differentiated dentritic cells induces Th1 and Th17 cell differentiation. Mol Immunol. 2010;47(4):922–931. doi:10.1016/j.molimm.2009.09.038
49. Pierobon D, Bosco MC, Blengio F, et al. Chronic hypoxia reprograms human immature dendritic cells by inducing a proinflammatory phenotype and TREM-1 expression. Eur J Immunol. 2013;43(4):949–966. doi:10.1002/eji.201242709
50. Winning S, Fandrey J. Dendritic cells under hypoxia: how oxygen shortage affects the linkage between innate and adaptive immunity. J Immunol Res. 2016;2016:5134329. doi:10.1155/2016/5134329
51. Elia AR, Cappello P, Puppo M, et al. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84(6):1472–1482. doi:10.1189/jlb.0208082
52. Teng R, Wang Y, Lv N, et al. Hypoxia impairs NK cell cytotoxicity through SHP-1-mediated attenuation of STAT3 and ERK signaling pathways. J Immunol Res. 2020;2020:4598476. doi:10.1155/2020/4598476
53. Pelletier A, Nelius E, Fan Z, et al. Resting natural killer cell homeostasis relies on tryptophan/NAD(+) metabolism and HIF-1α. EMBO Rep. 2023;24(6):e56156. doi:10.15252/embr.202256156
54. Maurus CF, Schmidt D, Schneider MK, et al. Hypoxia and reoxygenation do not upregulate adhesion molecules and natural killer cell adhesion on human endothelial cells in vitro. Eur J Cardiothorac Surg. 2003;23(6):976–983; discussion 983. doi:10.1016/S1010-7940(03)00146-5
55. Maurus CF, Schneider MK, Schmidt D, Zünd G, Seebach JD. Activation of human microvascular endothelial cells with TNF-alpha and hypoxia/reoxygenation enhances NK-cell adhesion, but not NK-Cytotoxicity. Transplantation. 2006;81(8):1204–1211. doi:10.1097/01.tp.0000205175.53938.bd
56. Pastwińska J, Walczak-Drzewiecka A, Łukasiak M, Ratajewski M, Dastych J. Hypoxia regulates human mast cell adhesion to fibronectin via the PI3K/AKT signaling pathway. Cell Adh Migr. 2020;14(1):106–117. doi:10.1080/19336918.2020.1764690
57. Wang X, Lin L, Chai X, et al. Hypoxic mast cells accelerate the proliferation, collagen accumulation and phenotypic alteration of human lung fibroblasts. Int J Mol Med. 2020;45(1):175–185. doi:10.3892/ijmm.2019.4400
58. Pastwińska J, Walczak-Drzewiecka A, Kozłowska E, et al. Hypoxia modulates human mast cell adhesion to hyaluronic acid. Immunol Res. 2022;70(2):152–160. doi:10.1007/s12026-021-09228-x
59. Gray KJ, Gibbs JE. Adaptive immunity, chronic inflammation and the clock. Semin Immunopathol. 2022;44(2):209–224. doi:10.1007/s00281-022-00919-7
60. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
61. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi:10.1084/jem.20131916
62. Palazon A, Tyrakis PA, Macias D, et al. An HIF-1α/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32(5):669–683.e665. doi:10.1016/j.ccell.2017.10.003
63. Ning F, Takeda K, Schedel M, et al. Hypoxia enhances CD8(+) T(C)2 cell-dependent airway hyperresponsiveness and inflammation through hypoxia-inducible factor 1α. J Allergy Clin Immunol. 2019;143(6):2026–2037.e2027. doi:10.1016/j.jaci.2018.11.049
64. Finlay DK. mTORC1 regulates CD8+ T-cell glucose metabolism and function independently of PI3K and PKB. Biochem Soc Trans. 2013;41(2):681–686. doi:10.1042/BST20120359
65. Zhou L, Chu C, Teng F, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568(7752):405–409. doi:10.1038/s41586-019-1082-x
66. Cho SH, Raybuck AL, Blagih J, et al. Hypoxia-inducible factors in CD4(+) T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc Natl Acad Sci U S A. 2019;116(18):8975–8984. doi:10.1073/pnas.1811702116
67. Arias C, Sepúlveda P, Castillo RL, Salazar LA. Relationship between hypoxic and immune pathways activation in the progression of neuroinflammation: role of HIF-1α and Th17 cells. Int J Mol Sci. 2023;24(4):3073. doi:10.3390/ijms24043073
68. Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109(41):E2784–E2793. doi:10.1073/pnas.1202366109
69. Hsu TS, Lai MZ. Hypoxia-inducible factor 1α plays a predominantly negative role in regulatory T cell functions. J Leukoc Biol. 2018;104(5):911–918. doi:10.1002/JLB.MR1217-481R
70. Lee JH, Elly C, Park Y, Liu YC. E3 ubiquitin ligase VHL regulates hypoxia-inducible factor-1α to maintain regulatory T cell stability and suppressive capacity. Immunity. 2015;42(6):1062–1074. doi:10.1016/j.immuni.2015.05.016
71. Miska J, Lee-Chang C, Rashidi A, et al. HIF-1α is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of tregs in glioblastoma. Cell Rep. 2019;27(1):226–237.e224. doi:10.1016/j.celrep.2019.03.029
72. Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13(6):668–677. doi:10.1016/j.autrev.2013.12.004
73. Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–784. doi:10.1016/j.cell.2011.07.033
74. Harnanik T, Soeroso J, Suryokusumo MG, Juliandhy T. Effects of hyperbaric oxygen on T helper 17/regulatory T polarization in antigen and collagen-induced arthritis: hypoxia-inducible factor-1α as a target. Oman Med J. 2020;35(1):e90. doi:10.5001/omj.2020.08
75. Jellusova J, Cato MH, Apgar JR, et al. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol. 2017;18(3):303–312. doi:10.1038/ni.3664
76. Abbott RK, Thayer M, Labuda J, et al. Germinal center hypoxia potentiates immunoglobulin class switch recombination. J Immunol. 2016;197(10):4014–4020. doi:10.4049/jimmunol.1601401
77. Fan C, Li J, Li Y, et al. Hypoxia-inducible factor-1α regulates the interleukin-6 production by B cells in rheumatoid arthritis. Clin Transl Immunology. 2023;12(5):e1447. doi:10.1002/cti2.1447
78. Meng X, Grötsch B, Luo Y, et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat Commun. 2018;9(1):251. doi:10.1038/s41467-017-02683-x
79. Zhu Y, Zhang X, Xie S, et al. Oxidative phosphorylation regulates interleukin-10 production in regulatory B cells via the extracellular signal-related kinase pathway. Immunology. 2022;167(4):576–589. doi:10.1111/imm.13554
80. Jang JW, Thuy PX, Lee JW, Moon EY. CXCR4 promotes B cell viability by the cooperation of nuclear factor (erythroid-derived 2)-like 2 and hypoxia-inducible factor-1α under hypoxic conditions. Cell Death Dis. 2021;12(4):330. doi:10.1038/s41419-021-03615-w
81. Lee KE, Spata M, Maduka R, Vonderheide RH, Simon MC. Hif1α deletion limits tissue regeneration via aberrant B cell accumulation in experimental pancreatitis. Cell Rep. 2018;23(12):3457–3464. doi:10.1016/j.celrep.2018.05.071
82. Wang X, Cui L, Ji X. Cognitive impairment caused by hypoxia: from clinical evidences to molecular mechanisms. Metab Brain Dis. 2022;37(1):51–66. doi:10.1007/s11011-021-00796-3
83. Taylor CT, Kent BD, Crinion SJ, McNicholas WT, Ryan S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun. 2014;447(4):660–665. doi:10.1016/j.bbrc.2014.04.062
84. Korbecki J, Simińska D, Gąssowska-Dobrowolska M, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. 2021;22(19):10701. doi:10.3390/ijms221910701
85. Delprat V, Tellier C, Demazy C, et al. Cycling hypoxia promotes a pro-inflammatory phenotype in macrophages via JNK/p65 signaling pathway. Sci Rep. 2020;10(1):882. doi:10.1038/s41598-020-57677-5
86. Wei Y, Giunta S, Xia S. Hypoxia in aging and aging-related diseases: mechanism and therapeutic strategies. Int J Mol Sci. 2022;23(15):8165.
87. González-Muniesa P, Garcia-Gerique L, Quintero P, et al. Effects of hyperoxia on oxygen-related inflammation with a focus on obesity. Oxid Med Cell Longev. 2015;2015:8957827. doi:10.1155/2016/8957827
88. Le Lay S, Simard G, Martinez MC, Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev. 2014;2014:908539. doi:10.1155/2014/908539
89. Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93(1):1–21. doi:10.1152/physrev.00017.2012
90. Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68(4):370–377. doi:10.1017/S0029665109990206
91. He Q, Gao Z, Yin J, et al. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab. 2011;300(5):E877–E885. doi:10.1152/ajpendo.00626.2010
92. Zhao M, Wang S, Zuo A, et al. HIF-1α/JMJD1A signaling regulates inflammation and oxidative stress following hyperglycemia and hypoxia-induced vascular cell injury. Cell Mol Biol Lett. 2021;26(1):40. doi:10.1186/s11658-021-00283-8
93. Escobar-Morreale HF, Martínez-García M, Montes-Nieto R, et al. Effects of glucose ingestion on circulating inflammatory mediators: influence of sex and weight excess. Clin Nutr. 2017;36(2):522–529. doi:10.1016/j.clnu.2016.01.015
94. Zhuang H, Lv Q, Zhong C, et al. Tiliroside ameliorates ulcerative colitis by restoring the M1/M2 macrophage balance via the HIF-1α/glycolysis pathway. Front Immunol. 2021;12:649463. doi:10.3389/fimmu.2021.649463
95. Torretta S, Scagliola A, Ricci L, et al. D-mannose suppresses macrophage IL-1β production. Nat Commun. 2020;11(1):6343. doi:10.1038/s41467-020-20164-6
96. Lu S, Tian Y, Luo Y, et al. Iminostilbene, a novel small-molecule modulator of PKM2, suppresses macrophage inflammation in myocardial ischemia–reperfusion injury. J Adv Res. 2021;29:83–94. doi:10.1016/j.jare.2020.09.001
97. Liu QY, Zhuang Y, Song XR, et al. Tanshinone IIA prevents LPS-induced inflammatory responses in mice via inactivation of succinate dehydrogenase in macrophages. Acta Pharmacol Sin. 2021;42(6):987–997. doi:10.1038/s41401-020-00535-x
98. Chen Q, Shao X, He Y, et al. Norisoboldine attenuates sepsis-induced acute lung injury by modulating macrophage polarization via PKM2/HIF-1α/PGC-1α pathway. Biol Pharm Bull. 2021;44(10):1536–1547. doi:10.1248/bpb.b21-00457
99. Deng H, Wu L, Liu M, et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate LPS-induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock. 2020;54(6):828–843. doi:10.1097/SHK.0000000000001549
100. Pei L, Le Y, Chen H, et al. Cynaroside prevents macrophage polarization into pro-inflammatory phenotype and alleviates cecal ligation and puncture-induced liver injury by targeting PKM2/HIF-1α axis. Fitoterapia. 2021;152:104922. doi:10.1016/j.fitote.2021.104922
101. Ouyang S, Yao YH, Zhang ZM, Liu JS, Xiang H. Curcumin inhibits hypoxia inducible factor-1α-induced inflammation and apoptosis in macrophages through an ERK dependent pathway. Eur Rev Med Pharmacol Sci. 2019;23(4):1816–1825. doi:10.26355/eurrev_201902_17145
102. Sun X, Ma L, Li X, et al. Ferulic acid alleviates retinal neovascularization by modulating microglia/macrophage polarization through the ROS/NF-κB axis. Front Immunol. 2022;13:976729. doi:10.3389/fimmu.2022.976729
103. Tang TT, Lv LL, Pan MM, et al. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9(3):351. doi:10.1038/s41419-018-0378-3
104. Lu H, Lin J, Xu C, et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin Transl Med. 2021;11(2):e334. doi:10.1002/ctm2.334
105. Miao AF, Liang JX, Yao L, Han JL, Zhou LJ. Hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) protects against renal ischemia/reperfusion injury by inhibiting inflammation. Ren Fail. 2021;43(1):803–810. doi:10.1080/0886022X.2021.1915801
106. Ngamsri KC, Fabian F, Fuhr A, et al. Sevoflurane exerts protective effects in murine peritonitis-induced sepsis via hypoxia-inducible factor 1α/adenosine A2B receptor signaling. Anesthesiology. 2021;135(1):136–150. doi:10.1097/ALN.0000000000003788
107. Burczyk G, Cichon I, Kolaczkowska E. Itaconate suppresses formation of Neutrophil Extracellular Traps (NETs): involvement of Hypoxia-Inducible Factor 1α (Hif-1α) and Heme Oxygenase (HO-1). Front Immunol. 2022;13:864638. doi:10.3389/fimmu.2022.864638
108. Kong L, Ma Y, Wang Z, et al. Inhibition of hypoxia inducible factor 1 by YC-1 attenuates tissue plasminogen activator induced hemorrhagic transformation by suppressing HMGB1/TLR4/NF-κB mediated neutrophil infiltration in thromboembolic stroke rats. Int Immunopharmacol. 2021;94:107507. doi:10.1016/j.intimp.2021.107507
109. Atzeni F, Benucci M, Sallì S, et al. Different effects of biological drugs in rheumatoid arthritis. Autoimmun Rev. 2013;12(5):575–579. doi:10.1016/j.autrev.2012.10.020
110. Solt LA, Kumar N, Nuhant P, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–494. doi:10.1038/nature10075
111. Yao Y, Wang L, Zhou J, Zhang X. HIF-1α inhibitor echinomycin reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. J Transl Med. 2017;15(1):28. doi:10.1186/s12967-017-1132-9
112. Huang J, Li Z, Hu Y, et al. Melatonin, an endogenous hormone, modulates Th17 cells via the reactive-oxygen species/TXNIP/HIF-1α axis to alleviate autoimmune uveitis. J Neuroinflammation. 2022;19(1):124. doi:10.1186/s12974-022-02477-z
113. Miossec P. IL-17 and Th17 cells in human inflammatory diseases. Microbes Infect. 2009;11(5):625–630. doi:10.1016/j.micinf.2009.04.003
114. Kim J, Kim HY, Song SY, et al. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano. 2019;13(3):3206–3217. doi:10.1021/acsnano.8b08785
115. Cheng YQ, Yue YX, Cao HM, et al. Coassembly of hypoxia-sensitive macrocyclic amphiphiles and extracellular vesicles for targeted kidney injury imaging and therapy. J Nanobiotechnology. 2021;19(1):451. doi:10.1186/s12951-021-01192-w
116. Liu ZJ, Ran YY, Qie SY, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019;25(12):1353–1362. doi:10.1111/cns.13261
117. Cai M, Sun S, Wang J, et al. Sevoflurane preconditioning protects experimental ischemic stroke by enhancing anti-inflammatory microglia/macrophages phenotype polarization through GSK-3β/Nrf2 pathway. CNS Neurosci Ther. 2021;27(11):1348–1365. doi:10.1111/cns.13715
118. Liu Z, Meng Y, Miao Y, et al. Propofol ameliorates renal ischemia/reperfusion injury by enhancing macrophage M2 polarization through PPARγ/STAT3 signaling. Aging. 2021;13(11):15511–15522. doi:10.18632/aging.203107
119. Lorenz G, Darisipudi MN, Anders HJ. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol Dial Transplant. 2014;29(1):41–48. doi:10.1093/ndt/gft332
120. Anders HJ. Of inflammasomes and alarmins: IL-1β and IL-1α in kidney disease. J Am Soc Nephrol. 2016;27(9):2564–2575. doi:10.1681/ASN.2016020177
121. Ryan DG, O’Neill LAJ. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38(1):289–313. doi:10.1146/annurev-immunol-081619-104850
122. Santocki M, Kolaczkowska E. On Neutrophil Extracellular Trap (NET) removal: what we know thus far and why so little. Cells. 2020;9(9):2079. doi:10.3390/cells9092079
123. Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15(1):23. doi:10.1186/s12964-017-0177-y
124. Tsiogka A, Kyriazopoulou M, Kontochristopoulos G, et al. The JAK/STAT pathway and its selective inhibition in the treatment of atopic dermatitis: a systematic review. J Clin Med. 2022;11(15):4431. doi:10.3390/jcm11154431
125. Patil PS, Fountas-Davis N, Huang H, et al. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. Acta Biomater. 2016;36:164–174. doi:10.1016/j.actbio.2016.03.022
126. Patil PS, Mansouri M, Leipzig ND. Fluorinated chitosan microgels to overcome internal oxygen transport deficiencies in microtissue culture systems. Adv Biosyst. 2020;4(8):e1900250. doi:10.1002/adbi.201900250
127. Yang Z, Chen H, Yang P, et al. Nano-oxygenated hydrogels for locally and permeably hypoxia relieving to heal chronic wounds. Biomaterials. 2022;282:121401. doi:10.1016/j.biomaterials.2022.121401
128. Lee YH, Lin SJ. Chitosan/PVA hetero-composite hydrogel containing antimicrobials, perfluorocarbon nanoemulsions, and growth factor-loaded nanoparticles as a multifunctional dressing for diabetic wound healing: synthesis, characterization, and in vitro/in vivo evaluation. Pharmaceutics. 2022;14(3). doi:10.3390/pharmaceutics14030537
129. Guo J, Agola JO, Serda R, et al. Biomimetic rebuilding of multifunctional red blood cells: modular design using functional components. ACS Nano. 2020;14(7):7847–7859. doi:10.1021/acsnano.9b08714
130. Li Y, Fu R, Duan Z, Zhu C, Fan D. Artificial nonenzymatic antioxidant mxene nanosheet-anchored injectable hydrogel as a mild photothermal-controlled oxygen release platform for diabetic wound healing. ACS Nano. 2022;16(5):7486–7502. doi:10.1021/acsnano.1c10575
131. Zhou F, Mei J, Yang S, et al. Modified ZIF-8 nanoparticles attenuate osteoarthritis by reprogramming the metabolic pathway of synovial macrophages. ACS Appl Mater Interfaces. 2020;12(2):2009–2022. doi:10.1021/acsami.9b16327
132. Qiao H, Mei J, Yuan K, et al. Immune-regulating strategy against rheumatoid arthritis by inducing tolerogenic dendritic cells with modified zinc peroxide nanoparticles. J Nanobiotechnology. 2022;20(1):323. doi:10.1186/s12951-022-01536-0
133. Tong A, Tong C, Fan J, et al. Prussian blue nano-enzyme-assisted photodynamic therapy effectively eradicates MRSA infection in diabetic mouse skin wounds. Biomater Sci. 2023;11(18):6342–6356. doi:10.1039/D3BM01039B
134. Wang S, Zheng H, Zhou L, et al. Nanoenzyme-reinforced injectable hydrogel for healing diabetic wounds infected with multidrug resistant bacteria. Nano Lett. 2020;20(7):5149–5158. doi:10.1021/acs.nanolett.0c01371
135. Ma Y, Xu H, Sun B, et al. pH-responsive oxygen and hydrogen peroxide self-supplying nanosystem for photodynamic and chemodynamic therapy of wound infection. ACS Appl Mater Interfaces. 2021;13(50):59720–59730. doi:10.1021/acsami.1c19681
136. Lim DJ, Skinner D, West JM, et al. In vitro evaluation of a novel oxygen-generating biomaterial for chronic rhinosinusitis therapy. Int Forum Allergy Rhinol. 2022;12(2):181–190. doi:10.1002/alr.22875
137. Zhang Q, Inagaki NF, Ito T. Recent advances in micro-sized oxygen carriers inspired by red blood cells. Sci Technol Adv Mater. 2023;24(1):2223050. doi:10.1080/14686996.2023.2223050
138. Wu M, Chen C, Liu Z, Tian J, Zhang W. Regulating the bacterial oxygen microenvironment via a perfluorocarbon-conjugated bacteriochlorin for enhanced photodynamic antibacterial efficacy. Acta Biomater. 2022;142:242–252. doi:10.1016/j.actbio.2022.02.013
139. Krafft MP. Alleviating tumor hypoxia with perfluorocarbon-based oxygen carriers. Curr Opin Pharmacol. 2020;53:117–125. doi:10.1016/j.coph.2020.08.010
140. Zhou Z, Zhang B, Wang H, et al. Two-stage oxygen delivery for enhanced radiotherapy by perfluorocarbon nanoparticles. Theranostics. 2018;8(18):4898–4911. doi:10.7150/thno.27598
141. Zou J, Zhu J, Yang Z, et al. A phototheranostic strategy to continuously deliver singlet oxygen in the dark and hypoxic tumor microenvironment. Angew Chem Int Ed Engl. 2020;59(23):8833–8838. doi:10.1002/anie.201914384
142. Wang W, Wang X, Tao F, et al. Fluorinated hyaluronic acid encapsulated perfluorocarbon nanoparticles as tumor-targeted oxygen carriers to enhance radiotherapy. Mol Pharm. 2022;19(11):3948–3958. doi:10.1021/acs.molpharmaceut.2c00432
143. Xu CH, Ye PJ, Zhou YC, et al. Cell membrane-camouflaged nanoparticles as drug carriers for cancer therapy. Acta Biomater. 2020;105:1–14. doi:10.1016/j.actbio.2020.01.036
144. Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220(Pt B):600–607. doi:10.1016/j.jconrel.2015.07.019
145. Wu P, Jiang X, Yin S, et al. Biomimetic recombinant of red blood cell membranes for improved photothermal therapy. J Nanobiotechnology. 2021;19(1):213. doi:10.1186/s12951-021-00949-7
146. Zhang X, Chen G, Liu Y, et al. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing. ACS Nano. 2020;14(5):5901–5908. doi:10.1021/acsnano.0c01059
147. Chen L, Yang Z, Liu H. Hemoglobin-based oxygen carriers: where are we now in 2023? Medicina. 2023;59(2):396. doi:10.3390/medicina59020396
148. Sen Gupta A. Hemoglobin-based oxygen carriers: current state-of-the-art and novel molecules. Shock. 2019;52(1S Suppl 1):70–83. doi:10.1097/SHK.0000000000001009
149. Bryan N, Ahswin H, Smart N, et al. Reactive oxygen species (ROS)--a family of fate deciding molecules pivotal in constructive inflammation and wound healing. Eur Cell Mater. 2012;24:249–265. doi:10.22203/eCM.v024a18
150. Gil D, Rodriguez J, Ward B, et al. Antioxidant activity of SOD and catalase conjugated with nanocrystalline ceria. Bioengineering. 2017;4(1):18. doi:10.3390/bioengineering4010018
151. Ling Y, Nie D, Huang Y, et al. Antioxidant cascade nanoenzyme antagonize inflammatory pain by modulating MAPK/p-65 signaling pathway. Adv Sci. 2023;10(12):e2206934. doi:10.1002/advs.202206934
152. Singh N, NaveenKumar SK, Geethika M, Mugesh G. A cerium vanadate nanozyme with specific superoxide dismutase activity regulates mitochondrial function and ATP synthesis in neuronal cells. Angew Chem Int Ed Engl. 2021;60(6):3121–3130. doi:10.1002/anie.202011711
153. Kang JI, Park KM. Oxygen-supplying syringe to create hyperoxia-inducible hydrogels for in situ tissue regeneration. Biomaterials. 2023;293:121943. doi:10.1016/j.biomaterials.2022.121943
154. Yan K, Mu C, Zhang C, et al. Pt nanoenzyme decorated yolk-shell nanoplatform as an oxygen generator for enhanced multi-modality imaging-guided phototherapy. J Colloid Interface Sci. 2022;616:759–768. doi:10.1016/j.jcis.2022.02.042
155. Zhang DY, Liu H, Rizwan Younis M, et al. Corrigendum to “Ultrasmall platinum nanozymes as broad-spectrum antioxidants for theranostic application in acute kidney injury” [Chem. Eng. J. 409 (2020) 127371]. Chem Eng J. 2021;421(421):129963. doi:10.1016/j.cej.2021.129963
156. Bardi G, Boselli L, Pompa PP. Anti-inflammatory potential of platinum nanozymes: mechanisms and perspectives. Nanoscale. 2023;15(35):14284–14300. doi:10.1039/D3NR03016D
157. Tsuji G, Hashimoto-Hachiya A, Takemura M, et al. Palladium and platinum nanoparticles activate AHR and NRF2 in human keratinocytes-implications in vitiligo therapy. J Invest Dermatol. 2017;137(7):1582–1586. doi:10.1016/j.jid.2017.02.981
158. Zhang W, Li S, Liu X, et al. Oxygen-generating MnO2 nanodots-anchored versatile nanoplatform for combined chemo-photodynamic therapy in hypoxic cancer. Adv Funct Mater. 2018;28(13):1706375.
159. Mbugua SN, Keramidas A. Targeting tumor microenvironment by metal peroxide nanoparticles in cancer therapy. Bioinorg Chem Appl. 2022;2022:5041399. doi:10.1155/2022/5041399
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.