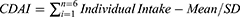Back to Journals » Clinical Interventions in Aging » Volume 19
The Association Between the Composite Dietary Antioxidant Index and Frailty Symptoms: Mediating Effects of Oxidative Stress
Authors Wu Y, Cheng S, Lei S, Li D, Li Z, Guo Y
Received 6 November 2023
Accepted for publication 19 January 2024
Published 3 February 2024 Volume 2024:19 Pages 163—173
DOI https://doi.org/10.2147/CIA.S448354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Yue Wu,1 Siqi Cheng,1 Shaoyuan Lei,2 Dongxiao Li,3 Zhongzhong Li,4 Yansu Guo1,2,5
1Beijing Geriatric Healthcare Center, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Evidence-Based Medicine, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 3Department of Neurology, Hebei Provincial Hospital of Traditional Chinese Medicine, Shijiazhuang, People’s Republic of China; 4Department of Neurology, The Second Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 5Beijing Municipal Geriatric Medical Research Center, Beijing, People’s Republic of China
Correspondence: Yansu Guo, Beijing Geriatric Healthcare Center, Xuanwu Hospital, Capital Medical University, Changchun Street 45, Beijing, People’s Republic of China, Email [email protected]
Background: There is growing evidence that an antioxidant diet is a protective factor against frailty. However, few studies have examined the effect of comprehensive dietary antioxidants on frailty symptoms. The aim of this study was to examine the relationships between the composite dietary antioxidant index (CDAI) and frailty and the underlying mechanisms involved.
Methods: Based on the National Health and Nutrition Survey (NHANES) 2003– 2018, this study included 11,277 older persons aged ≥ 60 years. In this study, frailty was defined as having a total score > 0.21 on the 49-item frailty index. Six dietary antioxidants were selected for use in calculating the CDAI. A weighted multiple logistic regression model with subgroup analysis and restricted cubic splines (RCSs) were used to examine the association between the CDAI and frailty. To examine the role of oxidative stress, mediation analyses were also conducted.
Results: The association between the CDAI score and frailty risk was significant according to the multivariate model. Compared with participants in tertile 1, participants in both tertile 2 and tertile 3 had lower odds of developing frailty symptoms (OR=0.86; 95% CI=0.75– 0.97; P=0.02; and OR=0.81; 95% CI=0.70– 0.93; P=0.003). According to the subgroup analyses, the differences in interactions were not statistically significant. There was also a potential nonlinear relationship between the CDAI score and frailty risk. The serum albumin concentration and uric acid concentration had significant mediating effects on the association between the CDAI score and frailty index, with 19.25% (P=0.002) and 21.26% (P < 0.001) of the total, respectively.
Conclusion: Frailty is negatively associated with the CDAI score, which may be partially mediated by oxidative stress.
Keywords: frailty, CDAI, NHANES, elderly adults
Introduction
Frailty is a complex biological syndrome characterized by a decrease in the reserve and function of multiple physiological systems.1 The prevalence of frailty is high in the aging population.2 According to a systematic review, the overall weighted prevalence of frailty in community-dwelling adults aged >60 years ranged from 4.0 to 59.1%.2 Frailty increases the risk of falls, disability, and mortality and is becoming a major global public health problem in older people.3 However, frailty is a dynamic process, and early detection of frailty stages may provide a window of opportunity for timely preventive or therapeutic interventions, which may delay or even reverse frailty.4
Aging, nutritional status, diet, physical activity, and inflammation are known risk factors for frailty.5,6 Oxidative stress is another important risk factor for frailty.7 Oxidative stress is caused by an imbalance between the oxidation and reduction systems, resulting in excessive reactive oxygen species (ROS). ROS-induced changes at the cellular level can lead to organ dysfunction and loss of muscle mass, eventually resulting in frailty in older persons.8
As a result, preventing oxidative stress has also become a priority. According to the Kraków study, daily intake of antioxidants can increase antioxidant defense and reduce oxidative stress by increasing plasma antioxidant levels.9 Studies have shown that antioxidants such as vitamin E and vitamin C can effectively control oxidative stress and improve frailty status.10 A population-based longitudinal study conducted by Millar et al suggested that carotenoid intake is associated with frailty prevention over time.11 At present, the effects of single antioxidants on frailty have been reported. However, few observational studies have examined the relationship between comprehensive dietary antioxidant intake and frailty.
The composite dietary antioxidant index (CDAI) is the comprehensive score of a variety of dietary antioxidants, including vitamin A, C, and E; selenium; zinc; and carotenoid.12 The CDAI can represent an individual’s overall dietary antioxidant intake. Epidemiological studies have shown that the CDAI is significantly associated with reduced risks of several cancers13–15 (cervical cancer, colorectal cancer and lung cancer), cardiovascular disease16 and osteoporosis.17 Notably, frailty and chronic diseases frequently occur together. Some studies believe that the presence of chronic diseases is a major determinant of frailty.18 According to previous studies, the CDAI may be associated with frailty.13–18 There is growing interest in understanding the pathways involved in the effects of the CDAI on frailty. A study by Luu et al showed that the CDAI is associated with oxidative stress and inflammation biomarkers, which provides some support for the validity of the CDAI as an indicator of oxidative/antioxidant balance.19 The serum albumin concentration and uric acid concentration can reflect the level of oxidative stress and can be used as markers of oxidative stress.20,21 Another study revealed that the oxidative balance score (OBS), which combines antioxidant and pro-oxidant components of dietary and lifestyle factors, is positively correlated with cognitive function in older adults, and both albumin and uric acid could mediate this relationship.22 Based on these studies, we hypothesize that the CDAI may be associated with oxidative stress biomarkers for reducing the risk of frailty. Nevertheless, few studies have examined whether the effects of the CDAI on frailty are related to oxidative stress markers.
To address this critical gap in knowledge, we first used data from the National Health and Nutrition Examination Survey (NHANES) to investigate the association between the CDAI and frailty after adjustment for several potential confounders. In addition, we further explored whether oxidative stress biomarkers mediate the association between the CDAI and frailty.
Methods
Study Population
Eight cycles of NHANES data (2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018) were analyzed. The NHANES is a national survey of health conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS) to analyze the health and nutritional status of a nationally representative sample of noninstitutionalized US citizens. In this analysis, only those individuals who were 60 years and older, had complete CDAI scores, or had no missing data were considered. During 2003–2018, NHANES collected data from 80,312 participants, among whom 15,381 were 60 years and older. A total of 1914 participants lacked information on CDAI components and were excluded (13,467 included). Then, we collected frailty data from the NHANES. The frailty index was calculated using the standard 49-item procedure.23 To include more participants, participants who completed 80% of the questionnaire were included, and 471 respondents with serious deficiencies in the frailty scale were excluded (12,996 included). A total of 11,276 participants were ultimately included in the analysis after excluding participants with incomplete oxidative stress biomarker data or missing data for any other covariate. The NCHS Research Ethics Review Board approved the survey protocol, while all participants provided written informed consent.
Composite Dietary Antioxidant Indices
Through a 24-hour dietary recall interview, the NHANES collected participants’ food intake over two days. The first dietary recall was conducted in person, and the second was conducted by telephone within three to ten days. Dietary intake data from two days were more accurate than data from a single day.24 Participants were asked to recall what foods and drinks they had consumed in the 24 hours preceding the interview. In this study, six dietary antioxidants were studied: vitamin A, vitamin C, vitamin E, zinc, selenium, and carotenoids. Based on Wright et al’s Composite Dietary Antioxidant Index (CDAI), joint exposure from dietary antioxidant intake was assessed.12 The CDAI was calculated using the following formula.
Frailty Index
According to Searle et al,23 the frailty index was constructed based on a standard procedure. The frailty index covered 49 deficits, including cognition, dependence, depression, comorbidities, hospital utilization, general health, physical performance and anthropometry, and laboratory values. The frailty index was calculated as the number of acquired deficits divided by the total number of potential deficits. Table S1 shows the frailty indices and their scoring systems. The frailty index cutoff point was 0.21.25 Frailty was diagnosed when the frailty index was >0.21, and nonfrailty was diagnosed when the frailty index was ≤0.21.
Albumin and Uric Acid
Detailed information about the laboratory methods can be found on the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). Albumin and uric acid were detected using a Beckman Coulter UniCel DxC800 (Beckman Coulter, Fullerton, CA, USA). The serum albumin concentration was measured using the bichromatic digital endpoints method, and the serum uric acid concentration was determined via timed-endpoint analysis.
Variables of Interest
The sociodemographic factors were age (60–69, 70–79, and ≥ 80), sex (female or male), and race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American, and other races/ethnicities). Education was divided into three categories (college or above, high school, and less than high school). For each subject, metabolic equivalents (METs) were calculated. Participants without any physical activity (PA) and performing <600 METs min/week were classified as inactive.26 Those performing ≥600 METs min/week were active. It has been consistently demonstrated that PA levels of 600 METs min/week are associated with substantial health benefits.27 The poverty-income ratio was used to measure income inequality, and it was classified into four categories (<1, 1–1.99, 2–3.99, and 4). Finally, the smoking status of the participants was divided into three categories: never (reported smoking fewer than 100 cigarettes in their lifetime), former (reported smoking more than 100 cigarettes in their lifetime and smoking not at all now), and current (reported smoking more than 100 cigarettes in life and smoking some days or every day). Other variables of interest included hypertension, diabetes, and chronic obstructive pulmonary disease (COPD).
Statistical Analysis
Due to the complex sampling design of the NHANES, sample weights were included in the analyses. The baseline characteristics of the study participants are summarized as the means and standard errors (SEs) for continuous variables and cases and percentages for categorical variables. The CDAI was modeled as a tertile, with the lowest tertile used as a reference group. Multivariate logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) associated with the CDAI score and risk of frailty. We developed three models: Model 1 was unadjusted; Model 2 was adjusted for age, sex, and race/ethnicity; and Model 3 was modified for all covariates. Stratified and interaction analyses were conducted using age, sex, and race/ethnicity. Restricted cubic splines were used to determine the shape of the dose–response correlation between the CDAI score and risk of frailty. Furthermore, we evaluated the association between the CDAI and frailty indices using multivariate linear regression models. Using similar methods, we also examined the CDAI and its associations with the serum albumin concentration and uric acid concentration and between the serum albumin concentration and the serum uric acid concentration and the frailty index.
Two mediation models were used to estimate the potential mediating effects of serum albumin and uric acid concentrations on the association between the CDAI and the frailty index. We estimated the individual mediating effects of the serum albumin concentration and uric acid concentration using the R package. To make a mutual adjustment, we entered all the covariates into the mediation analyses. We present the indirect effect size (βindirect), the direct effect size (βdirect), the total effect size (βtotal), the proportion mediated (PM), and the P values in our results. All the statistical analyses were conducted using R 4.2.1. Two-sided P values <0.05 were considered to indicate statistical significance.
Results
Patient General Characteristics
The baseline characteristics of the research participants are shown in Table 1. This study involved a total of 11,276 participants (Figure 1). Table 1 shows the characteristics of the target population based on the CDAI tertiles. Sex, age, race/ethnicity, educational levels, smoking status, physical activity, the poverty income ratio, frailty status, hypertension, diabetes, COPD, albumin, and uric acid were significantly different among the three CDAI tertiles. The CDAI scores were significantly different between the frail and non-frail groups (Table S2).
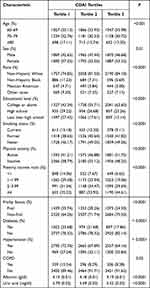 |
Table 1 Survey-Weighted Characteristics of the NHANES Sample According to CDAI Tertiles |
 |
Figure 1 Participants are selected according to the flowchart. |
Association Between the CDAI Score and Risk of Frailty
Table 2 illustrates the relationship between the CDAI and the risk of frailty based on logistic regression. We found that participants in the highest CDAI tertile had lower odds of frailty (OR=0.58; 95% CI=0.51–0.67; P < 0.001). The relationship persisted even after potential confounders were included in Model 3. Participants in tertile 3 of the CDAI had the lowest risk of frailty, with an OR of 0.81 (95% CI=0.70–0.93).
 |
Table 2 Multiple Logistic Regression Analysis on the Association Between CDAI and the Risk of Frailty |
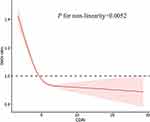
Subgroup analyses of the CDAI score and risk of frailty stratified by age, sex, and race/ethnicity are presented in Figure 2. After adjusting for the covariates, the CDAI was associated with a lower risk of frailty among people aged 70–79 years, females, and non-Hispanic white individuals. Interactions were not found to be significant. Restricted cubic splines showed a nonlinear relationship between the CDAI and risk of frailty after adjusting for all covariates (p for nonlinearity = 0.0052; Figure 3).
Association Between the CDAI and Frailty Indices
Table S3 shows the association between the CDAI and the frailty index according to linear regression. Compared with participants in tertile 1, participants in both tertile 2 and tertile 3 had a lower frailty index (β:-0.01; 95% CI:-0.01–0.00; P =0.02; and β:-0.01; 95% CI:-0.02–0.00; P < 0.001, respectively).
Associations Between the CDAI and Biomarkers and Between Biomarkers and the Frailty Index
Tables S4 and S5 show the associations between the CDAI and serum albumin concentration and between the CDAI and uric acid concentration, respectively. Table S6 shows the relationships of the serum albumin concentration and uric acid concentration with the frailty index. We found that higher CDAIs were associated with lower uric acid levels and that lower uric acid levels were associated with a lower frailty index. Similarly, higher CDAIs were related to higher levels of albumin, and higher albumin levels were related to a lower frailty index.
Mediating Role of Albumin and Uric Acid
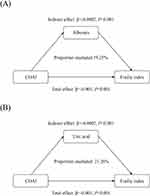
Based on the mediation analyses, both the serum albumin concentration and the serum uric acid concentration had significant mediating effects on the association between the CDAI score and the frailty index, with 19.25% and 21.26%, respectively (Table S7; Figure 4).
Discussion
In this study, we examined the association between the CDAI score and frailty in a sample of elderly individuals in the United States. After we adjusted for all the covariates, participants in CDAI tertile 3 had lower odds of developing frailty than did those in tertile 1. A restricted cubic spline curve showed a decreasing relationship between the CDAI and the risk of frailty, which seemed more like L-shaped associations. The CDAI was negatively associated with the frailty index according to the multivariate linear model. According to our mediation analyses, the serum albumin concentration and serum uric acid concentration were found to mediate the association between the CDAI and the frailty index.
To our knowledge, this is the first study to report the relationship between the CDAI and frailty. According to our findings, older adults in the US with a higher CDAI were at lower risk of frailty. The CDAI is a composite score that considers the biological interaction between dietary antioxidants to reflect an individual’s antioxidant status. Our results are in agreement with those of a prospective study reporting that a low intake of antioxidants may be a factor in the development of frailty in older men.28 Another study based on NHANES data showed that antioxidant-rich dietary patterns (Mediterranean diet) were associated with lower levels of frailty symptoms among community-dwelling US adults.29 According to a cross-sectional study of Japanese older persons, participants in the highest quintile of dietary total antioxidant capacity (DTAC) were less likely to suffer from frailty than were those in the lowest quintile.30 The same results revealed an inverse association between total antioxidant capacity (TAC) and the occurrence of frailty in older Australian men.31 These results indicated that an antioxidant diet is an important factor in the pathogenesis and progression of frailty. It is necessary to conduct further prospective studies to examine the contribution of the CDAI to frailty.
Although the mechanism by which the CDAI affects the risk of frailty is not fully understood, oxidative stress may play an important role. Oxidative stress increases intracellular calcium levels, promoting proteasomal activity and enhancing muscle decomposition, which may lead to a decline in muscle function and strength.32 Physical activity levels can be directly affected by a loss of muscle mass and strength, and frailty is characterized by low physical activity.33 In addition, oxidative stress can promote immune activation and generate inflammatory cytokines. Inflammatory cytokines may affect frailty either directly by promoting protein degradation or indirectly by affecting important metabolic pathways.34 This evidence may explain the link between the CDAI score and frailty symptoms.
As part of our investigation, we further explored potential pathways that link the CDAI to frailty. We found that the serum albumin concentration and uric acid concentration mediated the relationship between the CDAI score and frailty in older persons. In the present study, the CDAI was significantly correlated with oxidative stress indicators, positively correlated with the serum albumin concentration, and negatively correlated with the serum uric acid concentration. Moreover, our results indicated that the serum albumin concentration was negatively associated with the frailty index and that the serum uric acid concentration was positively associated with the frailty index. The biochemical structure of albumin has made it a good indicator of oxidative stress in the circulatory system.35 As an extracellular antioxidant and major transporter, albumin may protect aging cells from excessive oxidative stress induced by inflammation and could be an important indicator of frailty. A protective relationship between the serum albumin concentration and frailty status was found in our study, which was in agreement with the findings of an observational study from Japan.36 Some research indicates that uric acid promotes oxidative stress in human cells.37 Oxidative stress induced by elevated uric acid may play a role in aging and apoptosis of endothelial cells.38 García-Esquinas et al found that uric acid is a risk factor for frailty symptoms in older persons.39 Our findings further support this view. It has also been shown that uric acid performs important antioxidant functions, particularly in the central nervous system. However, further research is needed to determine whether the antioxidant protective effect of uric acid occurs within a certain range.
There were several strengths in our study. First, to assess an individual’s antioxidant status, we used the CDAI, a composite index that combines the dietary intake of six antioxidant factors. Second, our study examined the association between the CDAI and frailty indices in a large, nationally representative sample. Third, mediation analyses were used to further explore the mechanisms underlying the relationship between the CDAI and frailty indices.
This study has several limitations worth mentioning. First, the reliability of the results may have been affected by self-reported frailty symptoms. Second, individual diet estimations were based on the average of two 24-hour recalls, which may be biased. Third, we conducted mediation studies using a cross-sectional study design, making causal association inference difficult. Fourth, frailty is a geriatric syndrome whose underlying mechanisms remain elusive. It may be influenced by genetic, physical, biological, social, environmental, and a variety of risk factors.3,40 Therefore, we cannot eliminate residual and unmeasured confounding variables, despite adjusting for several possible confounders. Considering the limitations of the current study, our findings should be interpreted with caution, and further research is required to confirm them.
Conclusions
In conclusion, a higher CDAI was associated with lower odds of frailty. We also found that the serum albumin concentration and uric acid concentration may mediate the association between the CDAI and frailty indices.
Abbreviations
NHANES, National Health and Nutrition Examination Survey; CDAI, Composite Dietary Antioxidant Index; PA, physical activity; DTAC, dietary total antioxidant capacity; TAC, total antioxidant capacity; NCHS, National Center for Health Statistics; CDC, Centers for Disease Control and Prevention; ORs, odds ratios; CIs, confidence intervals.
Data Sharing Statement
The Data are publicly available on the internet throughout the world (www.cdc.gov/nchs/nhanes/).
Ethics Approval and Informed Consent
This study is exempted from ethics review based on national legislation guidelines, “Notice on the Issuance of Ethical Review Measures for Life Science and Medical Research Involving Humans” National Health Commission’s Department of Health Science, Technology and Education No. 4 (2023).
Acknowledgments
Thanks to the US Centers for Disease Control and Prevention for providing free National Health and Nutrition Examination Survey data for analysis.
Funding
This work was supported by the Beijing Municipal Natural Science Foundation, China (grant no. 7222082) and the Beijing Municipal Health Commission, China (grant no. 11000023T000002036320). The funding source had no role in the design, methods, subject recruitment, data collections, analysis, and preparation of this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi:10.1016/S0140-6736(12)62167-9
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487–1492. doi:10.1111/j.1532-5415.2012.04054.x
3. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interventions Aging. 2014;9:433–441. doi:10.2147/CIA.S45300
4. Lee JS, Auyeung TW, Leung J, Kwok T, Woo J. Transitions in frailty states among community-living older adults and their associated factors. J Am Med Directors Assoc. 2014;15(4):281–286. doi:10.1016/j.jamda.2013.12.002
5. Angulo J, El Assar M, Álvarez-bustos A, Rodríguez-Mañas L. Physical activity and exercise: strategies to manage frailty. Redox Biol. 2020;35:101513.
6. Ni Lochlainn M, Cox NJ, Wilson T, et al. Nutrition and frailty: opportunities for prevention and treatment. Nutrients. 2021;13(7):2349. doi:10.3390/nu13072349
7. Chen S-Y, Wang T-Y, Zhao C, Wang H-J. Oxidative stress bridges the gut microbiota and the occurrence of frailty syndrome. World J Gastroenterol. 2022;28(38):5547–5556. doi:10.3748/wjg.v28.i38.5547
8. Mulero J, Zafrilla P, Martinez-Cacha A. Oxidative stress, frailty and cognitive decline. J Nutr Health Aging. 2011;15(9):756–760. doi:10.1007/s12603-011-0130-5
9. Daneshzad E, Keshavarz SA, Qorbani M, Larijani B, Azadbakht L. Dietary total antioxidant capacity and its association with sleep, stress, anxiety, and depression score: a cross-sectional study among diabetic women. Clin Nutr ESPEN. 2020;37:187–194. doi:10.1016/j.clnesp.2020.03.002
10. Bartali B, Frongillo EA, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61(6):589–593. doi:10.1093/gerona/61.6.589
11. Millar CL, Costa E, Jacques PF, et al. Adherence to the Mediterranean-style diet and high intake of total carotenoids reduces the odds of frailty over 11 years in older adults: results from the Framingham offspring study. Am J Clin Nutr. 2022;116(3):630–639. doi:10.1093/ajcn/nqac130
12. Wright ME, Mayne ST, Stolzenberg-Solomon RZ, et al. Development of a comprehensive dietary antioxidant index and application to lung cancer risk in a cohort of male smokers. Am J Epidemiol. 2004;160(1):68–76. doi:10.1093/aje/kwh173
13. Yang J, Qian S, Na X, Zhao A. Association between dietary and supplemental antioxidants intake and lung cancer risk: evidence from a cancer screening trial. Antioxidants. 2023;12(2). doi:10.3390/antiox12020338
14. Maugeri A, Barchitta M, Magnano San Lio R, Scalisi A, Agodi A. Antioxidant and inflammatory potential of diet among women at risk of cervical cancer: findings from a cross-sectional study in Italy. Public Health Nutrition. 2022;25(6):1577–1585. doi:10.1017/S1368980021001944
15. Yu YC, Paragomi P, Wang R, et al. Composite dietary antioxidant index and the risk of colorectal cancer: findings from the Singapore Chinese health study. Int J Cancer. 2022;150(10):1599–1608. doi:10.1002/ijc.33925
16. Wang L, Yi Z. Association of the composite dietary antioxidant index with all-cause and cardiovascular mortality: a prospective cohort study. Front Cardiovasc Med. 2022;9:993930. doi:10.3389/fcvm.2022.993930
17. Chen Y, Tang W, Li H, Lv J, Chang L, Chen S. Composite dietary antioxidant index negatively correlates with osteoporosis among middle-aged and older US populations. Am J Transl Res. 2023;15(2):1300–1308.
18. Onder G, Vetrano DL, Marengoni A, Bell JS, Johnell K, Palmer K. Accounting for frailty when treating chronic diseases. Eur J Internal Med. 2018;56:49–52. doi:10.1016/j.ejim.2018.02.021
19. Luu HN, Wen W, Li H, et al. Are dietary antioxidant intake indices correlated to oxidative stress and inflammatory marker levels? Antioxid Redox Signaling. 2015;22(11):951–959. doi:10.1089/ars.2014.6212
20. Bengesser SA, Lackner N, Birner A, et al. Peripheral markers of oxidative stress and antioxidative defense in euthymia of bipolar disorder--Gender and obesity effects. J Affective Disorders. 2015;172:367–374. doi:10.1016/j.jad.2014.10.014
21. Gersch C, Palii SP, Kim KM, Angerhofer A, Johnson RJ, Henderson GN. Inactivation of nitric oxide by uric acid. Nucleosides Nucleotides Nucleic Acids. 2008;27(8):967–978. doi:10.1080/15257770802257952
22. Song L, Li H, Fu X, Cen M, Wu J. Association of the oxidative balance score and cognitive function and the mediating role of oxidative stress: evidence from the National Health and Nutrition Examination Survey (NHANES) 2011–2014. J Nutr. 2023;153(7):1974–1983. doi:10.1016/j.tjnut.2023.05.014
23. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi:10.1186/1471-2318-8-24
24. Yang T, Yi J, He Y, et al. Associations of dietary fats with all-cause mortality and cardiovascular disease mortality among patients with cardiometabolic disease. Nutrients. 2022;14(17). doi:10.3390/nu14173608
25. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatrics. 2015;60(3):464–470. doi:10.1016/j.archger.2015.01.016
26. Cao C, Friedenreich CM, Yang L. Association of daily sitting time and leisure-time physical activity with survival among US cancer survivors. JAMA Oncol. 2022;8(3):395–403. doi:10.1001/jamaoncol.2021.6590
27. Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. doi:10.1001/jama.2018.14854
28. Das A, Cumming RG, Naganathan V, et al. Prospective associations between dietary antioxidant intake and frailty in older Australian men: the concord health and ageing in men project. J Gerontol A Biol Sci Med Sci. 2020;75(2):348–356. doi:10.1093/gerona/glz054
29. Jayanama K, Theou O, Godin J, et al. Relationship between diet quality scores and the risk of frailty and mortality in adults across a wide age spectrum. BMC Med. 2021;19(1):64. doi:10.1186/s12916-021-01918-5
30. Kobayashi S, Suga H, Sasaki S. Diet with a combination of high protein and high total antioxidant capacity is strongly associated with low prevalence of frailty among old Japanese women: a multicenter cross-sectional study. Nutr J. 2017;16(1):29. doi:10.1186/s12937-017-0250-9
31. Tembo MC, Holloway-Kew KL, Bortolasci CC, et al. Total antioxidant capacity and frailty in older men. Am J Mens Health. 2020;14(5):1557988320946592. doi:10.1177/1557988320946592
32. Boittin F-X, Petermann O, Hirn C, et al. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J Cell Sci. 2006;119(18):3733–3742. doi:10.1242/jcs.03184
33. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146
34. Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology. 2009;55(5):539–549. doi:10.1159/000211949
35. Anraku M, Chuang VT, Maruyama T, Otagiri M. Redox properties of serum albumin. BBA. 2013;1830(12):5465–5472.
36. Yamamoto M, Adachi H, Enomoto M, et al. Lower albumin levels are associated with frailty measures, trace elements, and an inflammation marker in a cross-sectional study in Tanushimaru. Environ Health Preventative Med. 2021;26(1):25. doi:10.1186/s12199-021-00946-0
37. Sautin YY, Johnson RJ. Uric acid: the oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):608–619. doi:10.1080/15257770802138558
38. Roumeliotis S, Roumeliotis A, Dounousi E, Eleftheriadis T, Liakopoulos V. Dietary antioxidant supplements and uric acid in chronic kidney disease: a review. Nutrients. 2019;11(8):1.
39. García-Esquinas E, Guallar-Castillón P, Carnicero JA, et al. Serum uric acid concentrations and risk of frailty in older adults. Exp Gerontol. 2016;82:160–165. doi:10.1016/j.exger.2016.07.002
40. Semmarath W, Seesen M, Yodkeeree S, et al. The association between frailty indicators and blood-based biomarkers in early-old community dwellers of Thailand. Int J Environ Res Public Health. 2019;16(18):3457. doi:10.3390/ijerph16183457
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.