Back to Journals » Journal of Blood Medicine » Volume 14
The Correlation Between sP-Selectin and Platelet Count in COVID-19 Patients in Referral Hospital, West Java Indonesia
Authors Prihatni D, Budianto FC, Andriyoko B , Trisa S
Received 13 June 2023
Accepted for publication 16 October 2023
Published 27 October 2023 Volume 2023:14 Pages 555—561
DOI https://doi.org/10.2147/JBM.S425667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin H Bluth
Delita Prihatni,1 Frany Charisma Budianto,1 Basti Andriyoko,1 Suryarini Trisa2
1Clinical Pathology Department, Medical Faculty of Padjadjaran University, Hasan Sadikin Hospital, Bandung, West Java, Indonesia; 2Depati Hamzah Hospital, Bangka Belitung, Indonesia
Correspondence: Delita Prihatni, Hasan Sadikin Hospital, Jln. Pasteur No. 38, Bandung, West Java, Indonesia, Tel +628122314089, Email [email protected]
Introduction: sP-selectin is a glycoprotein located in α granules of platelet and endothelial’s Weibel Palade body, as expression to platelet activation and endothelial cell stimulation by SARS-CoV-2 binding with ACE2 receptor. Consumptive thrombocytopenia is also related to platelet activation. Elevation of sP-selectin and thrombocytopenia are related to COVID-19 complication and often correlated with severity of COVID-19.
Purpose: Assess the correlation between sP-selectin and platelet in COVID-19 patients at intensive care and non-intensive care.
Patients and Methods: The study population was hospitalized COVID-19 patients confirmed by Real-Time PCR that underwent platelet examination within 48 hours upon admission, divided into intensive care and non-intensive care group. sP-selectin examination using ELISA methods. Platelet cell count and sP-selectin divided based on normal reference range.
Results: The subjects consist of 24 were in intensive care, 25 were in non-intensive care group. A 66.7% of subject in intensive care group has an elevation in sP-selection (> 44.0 ng/mL). Thrombocytopenia was significantly correlated with intensive group (r =− 0.32, p< 0.05). The combination of platelet count < 150.000/mm3 and sP-selectin > 44.0ng/mL was not correlated with the intensive and non-intensive group. Platelet and sP-selectin were not correlated with each other.
Conclusion: Thrombocytopenia is able to induce the expression of sP-selectin.
Keywords: COVID-19, sP-selectin, platelet, thrombocytopenia
Introduction
Coronavirus Disease 2019 (COVID-19) is a new infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) which was first identified in the city of Wuhan in December 2019. SARS-CoV-2 belongs to the Coronaviridae family and the genus Betacoronavirus. This virus is a positive single-stranded RNA virus that is encapsulated, non-segmented, and spherical in shape with a diameter of 60–140 nanometers. Transmission of this virus can be transmitted from human to human through respiratory droplets and aerosols. In addition, the RNA of this virus has also been found in the feces of people with COVID-19.1,2
SARS-Cov-2 consists of four main structures, namely spike glycoprotein (S), matrix (M), nucleocapsid (N), and capsule (E). Protein S will bind to Angiotensin-converting enzyme 2 (ACE2) as a receptor to enter the host cell after being activated by transmembrane protease serine 2 (TMPRSS2).2
Angiotensin-converting enzyme 2 (ACE2) is a type 1 transmembrane protein with 805 amino acids attached to the outer surface of cells.1 ACE2 is found mostly on the surface of alveolar epithelial cells and enterocyte cells in the small intestine. The expression of the ACE2 receptor on the surface of the alveoli that causes the clinical symptoms of COVID-19 is generally related to damage to the lung parenchyma.3 ACE2 receptor is also present in the endothelial cells of arteries and veins, as well as in arterial smooth muscle cells, therefore patients with COVID-19 may also experience complications of coagulopathy.4
The mechanism of coagulopathy in COVID-19 is not fully understood, but it is thought to be related to the binding of SARS-Cov-2 to ACE2 receptors on endothelial cells. SARS-Cov-2 can directly cause endothelial disease, leading to endothelial dysfunction, endothelial cell lysis and death. Furthermore, activation of neutrophils, formation of pro-inflammatory cytokines and vasoactive molecules due to inflammation can also increase vascular permeability, leading to endothelial cell damage. Endothelial cell damage will expose a thrombogenic basement membrane that activates the coagulation cascade and activates platelets. Pro-inflammatory cytokines (IL-1β and TNF) exposure in endothelial cell will result in expression of P-selectin, von Willebrand factor and fibrinogen and will lead to further thrombosis and coagulopathy. Furthermore, platelet activation can also increase the expression of P-selectin on the surface of platelets.5–12 Therefore, elevated plasma levels of P-selectin in patients with COVID-19 can be used as a marker of endothelial dysfunction and platelet activation and is associated with coagulopathy and disease severity.13
Platelet activation in COVID-19 coagulopathy may lead to thrombocytopenia in COVID-19 patients as the severity of COVID-19 patients increases. Thrombocytopenia in patients with COVID-19 was found in several studies, one of them was the study by Liu et al, which found a correlation between platelets and mortality in patients with COVID-19.14
According to that theory, coagulopathy leads to worsening of COVID-19 may be manifested by increased sP-selectin and thrombocytopenia. SP-selectin was considered as a new and limited parameter in Indonesia, so there are not many studies about the correlation between sP-selectin and platelet counts in COVID-19 patients in Indonesia. Therefore, investigators are interested to study about a correlation between these two parameters in patients with COVID-19 treated in ICU and non-ICU isolation wards.
Materials and Methods
This analytical cross-sectional study that included COVID-19 patients admitted into the in-patient ward (intensive and non-intensive care) was conducted at RSUP Dr. Hasan Sadikin Bandung from August to September 2021. This study used purposive sampling with minimal sample was determined using minimal sample equation for correlation study. Subjects were taken from a population of COVID-19 patients who met the inclusion criteria: (1) adult patients (≥18 years) diagnosed with COVID-19 based on real-time reverse transcription PCR (RT-PCR) in nasopharyngeal swabs, (2) platelet and D-Dimer examinations are carried out at the initial admission to the isolation inpatient room, or at least within 48 hours after admission to the COVID isolation inpatient care. The exclusion criteria of this study were the patient died before entering the inpatient room, incomplete and inaccessible medical record data during the study period, subjects with incomplete laboratory data.
The subjects were grouped based on the ward requirements, that is intensive and non-intensive wards. Data regarding the treatment room for COVID-19 patients was obtained by tracing the patient’s medical record data. D-dimer level was taken for characteristic data. The D-Dimer parameter was divided into two mortality risk groups based on Zhou’s research, which were >1.0 µg/mL.15
Data about platelet counts were taken from the laboratory information system based on the results of the examination using the Sysmex XN-1000 hematology analyzer. Platelet results in the study subjects were then divided into two groups, the first group as abnormal group with thrombocytopenia (platelet count <150.000/mm3) and the second group if the platelet count was normal or above the reference value (≥150.000/mm3).
Blood samples of COVID-19 patients were collected at the time of hospital admission. Citrate peripheral blood was centrifuged at 1200 rpm for 10 minutes at room temperature to obtain plasma samples. After that, 500 ul of plasma samples were stored at −80ᵒC before analyzed to obtain the quantification of soluble P-selectin performed ELISA assay (eLabscience; detection range: 1.56–100 ng/mL). The reference value for sP-selectin is taken based on the reference value on the insert kit, which is 26–44 ng/mL. The results of the sP-selectin were divided into two groups: group one is considered abnormal if the results of the p-selectin examination were >44 ng/mL and group two as normal results if sP-selectin was ≤44 ng/mL.
Statistical Anaysis
All research data were processed using SPSS version 25.0 and tabulated against the subject’s treatment room (ICU/non-ICU). Correlation between sP-selectin, platelet counts, and patient’s ward were calculated using Mann–Whitney U method. Correlation between sP-selectin level and Platelet Counts was performed using Goodman and Kruskal’s gamma coefficient, if the results were near to 1 then the correlation is stronger. All analyses were two-sided and a p value smaller than 0.05 was considered statistically significant. The results in this study will then be presented in the form of tables and charts.
Results
There were 49 subjects those met the inclusion criteria. Of the 49 subjects, 24 subjects were treated in the ICU and 25 subjects in the non-ICU isolation room. Characteristics data of the subjects of this study can be seen in Table 1.
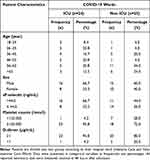 |
Table 1 Characteristics of the Research Subjects |
Based on Table 1, it was found that the age of subjects who were treated in the ICU was almost evenly distributed, while in non-ICU isolation wards were mostly in the age group of 56–65 years (44.0%). The gender distribution of research subjects was dominated by male (66.7% and 60.0%) in the ICU and non-ICU, respectively. Based on laboratory results, it was found that most of the sP-selectin levels in patients who were treated in the ICU had increased levels of sP-selectin (66.7%), while for the group of subjects treated in non-ICU isolation rooms, most of them had a normal levels of sP-selectin (56.0%). The mean platelet counts in the ICU and non-ICU groups were normal (95.8% and 72.0%) in both subjects group. Meanwhile, D-Dimer levels were elevated for both of subjects group (95.8% for ICU subjects and 80.0% for subjects in non-ICU isolation).
From Table 2, we found that only platelet parameters had a significant relationship between ICU and non-ICU with a weak effect (r =−0.32, p < 0.05). Meanwhile, for the parameters of sP-selectin and the combination of platelet and sP-selectin parameters did not have a significant correlation.
 |
Table 2 The Correlation of sP-Selectin and Platelets with COVID-19 ICU Patients and Non-ICU Ward |
Based on the analysis using Goodman and Kruskal’s Gamma coefficient, it shows that the sP-selectin and platelet counts has no correlation. To be clearer, the result of this correlation’s analysis is shown in Figure 1.
Discussion
SARS-CoV-2 attacks by binding to ACE2 receptors on the surface of endothelial cells, not only in the lungs but also in the blood vessels.4 There is one theory that underlies COVID-19 can cause a spectrum of diseases involving the hemostatic system, ie abnormal coagulopathy.16,17 Coagulopathy that occurs in COVID-19 patients consists of increased D-dimer, followed by mild thrombocytopenia and finally disseminated intravascular coagulation (DIC).18
In this study, thrombocytopenia occurred in both subjects treated in the ICU and non-ICU isolation inpatients. Thrombocytopenia in this study had a weak but significant correlation between COVID-19 patients treated in the ICU and non-ICU. These results are consistent with the study of Yang et al who found that thrombocytopenia was common in COVID-19 patients with an increased risk of mortality in patients with lower platelets.19 The results of a meta-analysis by Lippi et al also stated that there was a correlation between thrombocytopenia and the severity of COVID-19.20 The incidence of thrombocytopenia in COVID-19 patients is associated with an increase in platelet consumption due to thrombus formation; however, it usually tends to be mild because it is usually followed by an increase in platelet production. Decreased platelets may indicate worsening of thrombosis and are associated with increased mortality.18
Based on this study, we found that the levels of sP selectin in patients treated in the ICU mostly increased (66.67%), meanwhile in the non-ICU group mostly had normal levels of sP selectin (56%). These results are following the study by Manne et al which showed an increase in P-selectin levels on the surface of the platelet membrane in COVID-19 patients.21 Agrati et al also mentioned a higher plasma P-selectin level in COVID-19 patients compared to healthy controls [median 65.2 (IQRs: 45.1–81.1) vs 40.3 (IQRs: 24.3–48.7), respectively, p=0.0023].3
This result was supported by the theory that the expression of P-selectin in COVID-19 is related to damage of endothelial cells leading to coagulation cascade and platelet activation. At normal condition, P selectin is located on the α granules of platelets as well as on the endothelium. Megakaryocytes and endothelial cells produce P-selectin and store it in platelet α granules and Weibel Palade bodies from endothelial cells. In COVID-19, binding of SARS-CoV-2 to ACE2 receptors on the endothelium causes endothelial damage and activates the coagulation cascade and platelet activation.5,6 The expression of P-selectin consists of two processes. The first process is when platelet and endothelial cells receive a stimulus in the form of inflammatory mediators such as histamine, thrombin, or a state of hypoxia, they will translocate P-selectin to the surface in the first two minutes and will reach a peak within 10 minutes. This expression will reach normal after three hours. The second process happens when there is stimulation by cytokines that makes P-selectin rise again within 12 hours.3,14,22 Therefore, this study shows a higher sP-selectin levels in COVID-19 patients in ICU wards compared to the normal level in non-ICU patients so the sP-selectin level can be considered as the screening of the worsening COVID-19 condition.
When we performed an analytical correlation, this study found no correlation between sP-selectin in ICU-treated COVID-19 patients and non-ICU. This is consistent with the study by Agrati et al, who find no differences in P-selectin levels between ICU-treated COVID-19 patients and in the ICU setting.3
Based on the results of this study, sP-selectin does not correlate with platelet count. According to previous theory, the expression and elevation of sP-selectin levels are associated with platelet activation. Although thrombocytopenia occurs due to platelet activation in coagulation in COVID-19, it is also being balanced by increasing platelet production.18 In addition, sP-selectin levels are also associated with endothelial stimulation. Therefore, changes in sP-selectin levels are not directly related to changes in platelet count in this study.
This study has several limitations. This study had a small research sample because it was conducted at only one site and was conducted at the end of the COVID Delta period, so the number of incoming patients had decreased. We used no healthy control in this study and using the previous research as the validation of sP-selectin.3 Lopez et al also found that P selectin is less specific for thrombosis than other biomarkers such as von Willebrand Factor, lupus anticoagulant, D-dimer.11 Because of the data limitations in our hospital, we cannot explore more about the platelet activity, either from platelet index or platelet aggregation test and also the comorbidity characteristic is not well mentioned. We suggest to include the platelet index data and platelet aggregation test to see the platelet activation process for further research.
Conclusion
In this study, an increase in sP-selectin levels was found in the group of subjects treated in the COVID-19 ICU, while in the group treated in non-ICU isolation rooms, most of them had normal sP-selectin levels. There is no correlation between sP-selectin levels in COVID-19 patients treated in ICU and non-ICU. Meanwhile, for thrombocytopenia, a significant correlation was found with a weak effect between COVID-19 patients treated in the ICU and non-ICU rooms. While progress has been made in elucidating the molecular mechanisms leading to platelet adhesion, aggregation, shape change and secretion, clinically useful tests of platelet function have been developed. A number of dedicated platelet function instruments have now become available. Some instruments have been incorporated into routine clinical use and can be utilized not only as general screening tests of platelet function but as monitors of antiplatelet therapy and to potentially assess both risk of bleeding and/or thrombosis.23 The results of this study are expected to provide information regarding sP-selectin and platelet counts in the development of COVID-19 disease especially in Indonesia as one of the limited resource and developing country.
Ethics Approval and Consent to Participate
The institutional review board of Dr. Hasan Sadikin General Hospital Bandung, Indonesia approved the protocol no LB.02.01/X.6.5/337/2022 and all procedures were in compliance with the Helsinki Declaration. All patients gave their informed consent to participate in the study, which was approved by our local Ethics Committee of Dr. Hasan Sadikin General Hospital Bandung, Indonesia.
Acknowledgment
We thank Mrs. Hijrianti for helping us in preanalytic and analytic sample process of this research, Mulya Nurmansyah for statistical analysis, and Verina Logito for helping us in submitting this research.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Tahmineh Mokhtari FH, Ghaffari N, Ghaffari N, et al. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–628. doi:10.1007/s10735-020-09915-3
2. Mohammad Madjid PS-N, Solomon SD, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020;5(7):831–840. doi:10.1001/jamacardio.2020.1286
3. Agrati C, Bordoni V, Sacchi A, et al. Elevated P-selectin in severe Covid-19: considerations for therapeutic options. Mediterr J Hematol Infect Dis. 2021;13(1):e2021016. doi:10.4084/mjhid.2021.016
4. Hamming I, Timens W, Bulthuis M, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS Coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi:10.1002/path.1570
5. Freedman JE, Lascalzo J. Platelets and fibrinolysis/thrombolysis. Fibrinolysis in Disease Molecular and Hemovascular Aspects of Fibrinolysis. CRC; 2019:65–69.
6. Varga Z, Flammer AJ, Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi:10.1016/S0140-6736(20)30937-5
7. Lopez-Castaneda S, García-Larragoiti N, Cano-Mendez A, et al. Inflammatory and prothrombotic biomarkers associated with the severity of COVID-19 infection. Clin Appl Thromb Hemost. 2021;27:1–9.
8. Tommaso Neri DN, Celi A, Celi A. P-selectin blockade in COVID-19 related ARDS. Am J Physiol Lung Cell Mol Physiol. 2020;318(6):L1237–L1238. doi:10.1152/ajplung.00202.2020
9. Alicja Polek WS, Matowicka-Karna J. P-Selectin and Its Role in Some Diseases. Postepy Hig Med Dosw. 2009;63:465–470.
10. Corlia Grobler SC, Maphumulo SC, Grobbelaar LM, et al. COVID-19: the Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int J Mol Sci. 2020;21(14):5168. doi:10.3390/ijms21145168
11. López Reboiro ML, Suárez Fuentetaja R, Gutiérrez López R, et al. Role of lupus anticoagulant and von Willebrand factor in chronic reactive endotheliitis in COVID-19. J Infect. 2021;82(6):e27–e28. doi:10.1016/j.jinf.2021.03.006
12. Ming Chen M, Geng J-G. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis, and cancer growth and metastasis. Arch Immunol Ther Exp. 2006;54(2):75–84. doi:10.1007/s00005-006-0010-6
13. Nader Yatim JB, Chocron R, Chocron R. Platelet Activation in Critically Ill COVID-19 Patients. Ann Intensive Care. 2021;11(1):113. doi:10.1186/s13613-021-00899-1
14. Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: retrospective cohort study. Platelets. 2020;31(4):490–496. doi:10.1080/09537104.2020.1754383
15. Litao Zhang XY, Fan Q, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1324–1329. doi:10.1111/jth.14859
16. Bikdeli B, Madhavan MV, Jimenez D, et al.; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi:10.1016/j.jacc.2020.04.031
17. Helms J, Tacquard C, Severac F, et al.; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi:10.1007/s00134-020-06062-x
18. Wool G, Miller D, J L. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88(1):15–27. doi:10.1159/000512007
19. Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469–1472. PMID: 32302435. doi:10.1111/jth.14848
20. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. PMID: 32178975; PMCID: PMC7102663. doi:10.1016/j.cca.2020.03.022
21. Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317–1329. doi:10.1182/blood.2020007214
22. Blann AD, Nadar SK, Lip GYH. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24(24):2166–2179. doi:10.1016/j.ehj.2003.08.021
23. Pakala R, Waksman R. Currently available methods for platelet function analysis: advantages and disadvantages. Cardiovasc Revascular Med. 2011;12(5):312–322. doi:10.1016/j.carrev.2010.09.005
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

