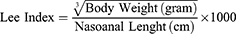Back to Journals » Research and Reports in Urology » Volume 16
The Effects of High Fat Diet on the Incidence of Obesity and Monocyte Chemoattractant Protein-1 Levels on Histological Changes in Prostate Wistar Rats
Authors Syarif , Rasyid H, Aman M, Lawrence GS, Bukhari A, Patellongi I, Cangara H , Putra MZDA
Received 27 August 2023
Accepted for publication 22 February 2024
Published 5 March 2024 Volume 2024:16 Pages 57—63
DOI https://doi.org/10.2147/RRU.S437322
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Syarif,1 Haerani Rasyid,2 Makbul Aman,2 Gatot Susilo Lawrence,3 Agussalim Bukhari,4 Ilhamjaya Patellongi,5 Husni Cangara,3 Muhammad Zulharyahya Dandy Asmara Putra6
1Division of Urology, Department of Surgery, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 2Department of Internal Medicine, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 3Department of Pathology Anatomy, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 4Department of Clinical Nutrition, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 5Department of Physiology, Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 6Faculty of Medicine, Hasanuddin University, Makassar, South Sulawesi, Indonesia
Correspondence: Syarif, Division of Urology, Department of Surgery, Faculty of Medicine, Hasanuddin University, Jalan Perintis Kemerdekaan KM 11, Makassar, South Sulawesi, 90245, Indonesia, Fax +62411587571, Email [email protected]
Introduction: Benign prostatic hyperplasia (BPH) is a histopathological diagnosis characterized by the increase in stromal cells and epithelial cells of the prostate gland in the transitional zone surrounding the urethra. Obesity is the risk factor of BPH. The most frequent cause of obesity is a high-fat diet (HFD). Obesity and HFD lead to pro-inflammatory conditions. One of the pathomechanisms for the occurrence of BPH is a low-degree inflammatory factor, one of which is the level of monocyte chemoattractant protein-1/MCP-1. This study aims to determine the influence of HFD on the incidence of obesity and inflammatory factors (monocyte chemoattractant protein-1/MCP-1 levels) on the histopathological picture of the prostate.
Methods: Experimental research was performed on male Wistar rats with each of the 6 rats given normal fat (ND) and HFD intake and terminated at 8 weeks and 6 rats given each ND and HFD were terminated at 16 weeks. The determination of obesity was determined based on Lee’s criteria which were categorized as obese if the Lee index > 0.3 and non-obese if ≤ 0.3. Examination of circulating MCP-1 was carried out by the ELISA method and determination of prostatic hyperplasia was done by calculating the percentage of prostate glands that had a large per-field cystic dilatation on light microscopy examination. All data are analyzed statistically with the Fisher Exact Test and Spearman Correlation Test.
Results: Of the 12 rats that were given ND, none of them became obese according to Lee’s criteria, on the other hand, of the 12 rats that were given HFD 8 became obese (66.7%, p = 0.001). Serum MCP-1 levels and the percentage of prostate glands that had cystic dilatation were significantly higher in mice receiving HFD than ND; both at week-8 (MCP-1; 18.87 vs 15.66) and (prostate gland experiencing cystic dilatation; 63.46% vs 47.24%) and week-16 (MCP-1; 21.27 vs 21.27) and (prostate gland experiencing cystic dilatation; 67.79% vs 56.39%). Spearman correlation analysis showed that only circulating MCP-1 levels were significantly correlated (p < 0.05) to the percentage of the prostate gland that had cystic dilatation; especially in week 16 (r = 0.713 and p < 0.001). At 8 weeks, it was not statistically significant (r = 0.406 and p = 0.095).
Conclusion: High fat intake has been shown to increase the risk of obesity, but obesity does not increase inflammatory status and the incidence of prostate glands with cystic dilatation. On the other hand, high-fat intake increases inflammatory status which in turn causes prostate glands to develop cystic dilatation.
Keywords: prostate, monocyte chemoattractant proteins, inflammatory status, obesity, high-fat diet, Wistar rat, animal model
Introduction
Obesity is among the most common and costly chronic disorders worldwide1 and is defined as a disease of excess body fat that had negative repercussions on health.2 Obesity is associated with consumption of a high-fat diet (HFD), a worldwide epidemic prevalent in occidental populations, is a precursor of insulin resistance and type 2 diabetes,3 and is associated with numerous types of cancer, including colon, breast, and prostate cancer.4 HFD with consequent obesity causes adipose tissue inflammation and cytokine secretion. Accordingly, Liu et al find that ten mice receiving a high-calorie diet exhibited neoplastic progression with stromal infiltration of inflammatory cells, such as macrophages, T cells, and inflammatory monocytes, into the prostates.5 Increase in inflammatory response to a high-calorie diet was supported by the elevation in the expression of CD3, CD45, FoxP3, MCP-1, IL-6, and TNF alpha. Microarray analysis using TRAMP mice models showed that HFD feeding increased serum levels of MCP-1, MCP-5, TIMP-1, IL-16, CCL12, CXCL1, CXCL10, and CXCL13. Metabolic disorders induced by HFD may induce prostate damage.6
Benign prostatic hyperplasia (BPH) is defined by the American Urological Association (AUA) as a histologic diagnosis referring to the proliferation of smooth muscle and epithelial cells within the prostatic transition zone.7 Prostate primary functions are to contribute approximately 20% of the secretions from the seminal fluid, together with those produced by the seminal vesicles and the bulbourethral glands.8 Male obesity has been linked to increased risk of BPH and an increased severity of LUTS in the men affected by BPH.7
MCP-1 is a chemotactic factor for lymphocytes and macrophages, immune cells that are often observed in BPH specimens. Elevated MCP-1 levels in the prostate may also further attract inflammatory cells and contribute to a positive-feedback loop to stimulate BPH.9
Previous studies have found that a regular high-fat diet (HFD) for a duration of 8 and 16 weeks may induce obesity and increase the level of circulating MCP-1 in Wistar rats. The longer the HFD feeding, the higher the MCP-1 level.10 In this study, we examined the effects of high fat diet on the incidence of obesity and monocyte chemoattractant protein-1 levels on Histological Changes in Prostate Wistar Rats.
Methods
This was an experimental research study using rats with a post-test control group design comprised of an experimental group and control group. All experiment has been approved by the animal ethics committee of Universitas Hasanuddin and procedures conformed to the guidelines for animal experimentation of Universitas Hasanuddin.
Animal Model and Housing Conditions
Twenty-four male Wistar (Rattus norvegicus) albino aged 8–12 weeks with a weight of 180–250 grams obtained from the Veterinary Laboratory of the Faculty of Medicine, Universitas Brawijaya Malang. Animals were maintained for fourteen days in a cage with standard shape, Humidity 50%±10%, given lighting (12 h:12 h dark/light), cleaned regularly, an average temperature of 28 ± 2 °C, and had ad libitum access to piped water and standard food chow composed of 16.1% protein, carbohydrates 25%, 3.1% lipids, 0.9–1.2% calcium, 0.7–0.9% phosphorus.11 The Wistar rats were divided into 2 groups randomly with the same number (n = 12) in each group. Group I was a group of experimental mice given ND (n=6) and HFD (n=6) that were terminated in week 8 and group II was a group of experimental mice that were given ND (n=6) and HFD (n=6) that were released at week 16.
Supplementation with the HFD started at 60 days of age. Animals in the HFD groups were supplemented during the 10 weeks of the protocol and maintained on an HFD composed of 17.5% protein, 42% lipids, and 21.4% carbohydrates. As mentioned above, animals in the ND group were kept on a standard diet (16.1% protein, carbohydrates 25%, 3.1% lipids, 0.9–1.2% calcium, 0.7–0.9% phosphorus).
Euthanasia Protocol
All animals were anesthetized intraperitoneally with ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg). Blood was taken as much as 1 mL from each rat that had been anesthetized previously intracardially in the left ventricular area using a 19–21 G needle and then the blood was put into the sample tube.12 Euthanasia of the mice using an anesthetic agent overdose with 2–3 times the dose of anesthetic agent intravenously. Following euthanasia, an abdominal-pelvic laparotomy was performed for the collection of the ventral prostate and adipose tissue, which were then weighed and processed for subsequent analysis.
Sample Processing and Histology
Tissue was fixed in formalin solution for 24 h, washed in 70% alcohol, dehydrated, diaphonized, para-plasticized, and cut at a thickness of 5 μm for analysis. The blades were stained with hematoxylin and eosin (H&E) to analyze histological changes by differentiating cells from surrounding connective tissue and to identify cytoplasm and nuclei. The stereology (acini, epithelium, and stroma) of prostate cross-sections was quantified using a Weibel reticle of 160 points13 and 10 images per slide were quantified. Tissue was analyzed using a light microscope (Leica Microsystems) with x40 magnification were captured with Nikon Eclipse TS100 (Nikon, Tokyo, Japan) and analysed with ImageJ to calculate the hyperplasia prostate at the Department of Pathological Anatomy of our institution.
Body Weight and Obesity Analysis
The body weight of the rats in each group was measured and recorded every day. Rat’s obesity was determined based on Lee’s index.14
Rats are declared obese if the index value of Lee >0.3.
Statistical Analysis
All data obtained are processed using the SPSS version 22 program (Armonk, NY: IBM Corp.). The statistical analysis carried out in this study consisted of descriptive statistical calculations, frequency distribution, and statistical tests such as Fisher’s Exact Test and Spearman Correlation Test. The statistical test results are significant if the p-value is <0.05.
Results
Analysis of the Effect of Fat Intake on the Incidence of Obesity
The impact of fat intake on obesity both at week-8 and week-16 saw in Table 1.
 |
Table 1 The Relationship of Fat Intake with Obesity |
Analysis from Table 1 shows that ND is not at risk of causing obesity, while HFD is at risk of causing obesity; Both on week-8 (4 obese among 6 heads) and week-16 (4 obese among 6) (p=0.005).
Analysis of the Effect of Fat Intake on MCP-1 Circulatory Levels as Well as the Percentage of the Prostate Gland That Has Cystic Dilatation
Changes in MCP-1 levels of circulation as well as the percentage of the prostate gland that experienced cystic dilatation due to fat intake can be seen in Table 2.
 |
Table 2 Differences in MCP-1 Levels and Cystic Dilatation of the Prostate Gland Between HFD and ND Groups |
From Table 2, it can be seen that MCP-1 levels of circulation and the percentage of the prostate gland that experienced cystic dilatation were significantly higher in rats that obtained HFD than ND; well at week-8 (MCP-1: 18.87 vs 15.66; Prostate gland undergoing cystic dilatation: 63.46% vs 47.24%) as well as week-16 (MCP-1: 21.27 vs 21.27; Prostate gland undergoing cystic dilatation: 67.79% vs 56.39%). It was concluded that HFD increases circulatory MCP-1 levels and the percentage of the prostate gland undergoing cystic dilatation.
Correlation Analysis of MCP-1 Levels with the Percentage of Prostate Gland Undergoing Cystic Dilatation
The correlation between MCP-1 circulatory levels and the percentage of prostate glands undergoing cystic dilatation can be seen in Table 3.
 |
Table 3 Correlation Between Levels of MCP-1, Percentage of Prostate Glands Subjected to Cystic Dilatation |
From Table 3, with Spearman correlation analysis, MCP-1 levels of circulation are meaningfully correlated (p<0.05) with a percentage of the prostate gland undergoing cystic dilatation at week 16 (r=0.713, p<0. 001). At week-8, it is not statistically meaningful (r=0.406, p=0.095). This suggests that the longer exposure to high circulating MCP-1 levels, the greater the risk of the prostate gland experiencing cystic dilatation.
Analysis of MCP-1 Levels and the Percentage of the Prostate Gland That Experienced Cystic Dilatation in Mice That Got HFD
Because in mice that got ND, no one became obese, the analysis of MCP-1 levels and the percentage of the prostate gland that experienced cystic dilatation was only carried out in rats that received HFD. The analysis can be seen in Table 4.
 |
Table 4 MCP-1 Levels Circulation and Prostate Gland Percentage Experiencing Cystic Dilatation in Mice That Got HFD |
From Table 4, there was no meaningful difference in MCP-1 levels of circulation and the percentage of the prostate gland that experienced cystic dilation in rats that got HFD and became obese or not obese, either at week 8 or week-16. It can be concluded that obesity does not affect the levels of MCP-1 circulation and the incidence of prostate gland undergoing cystic dilatation.
Discussion
This study assesses the influence of HFD on the incidence of obesity and inflammatory factors (monocyte chemoattractant protein-1/MCP-1 levels) on the histopathological picture of the prostate. This study was conducted on Wistar mice, a model of experimental animals commonly used to induce obesity with HFD. Obesity is known as a risk factor for BPH. The excessive intake of a high-fat diet and the lack of daily exercise potentiates the obesity development. In this study, we found that there is a relationship of fat intake with obesity. According to Lee’s criteria occurred in mice given HFD both at week-8 (4 mice obese from 6 rats) and at week-16 (4 rats obese from 6 rats) (p=0.030), while in rats given ND, none of them became obese (Table 1). Rats given HFD showed an increase in body weight of 20–30%, depending on the percentage of HFD given and the type of rat. Human studies have shown that HFD (≥30% of energy from fat) easily triggers obesity. Animal studies try to induce obesity commonly using fat intake of as much as 30–78% of total energy.15
One of the pathologies for the occurrence of BPH is inflammatory factors. This study found MCP-1 levels of circulation and the percentage of the prostate gland that experienced cystic dilatation were significantly higher in Wistar mice that obtained HFD than ND. MCP-1 levels of circulation and the percentage of the prostate gland that undergoes cystic dilatation are significantly higher (p<0.05) in rats yang obtained HFD compared to ND; well at week-8 (MCP-1: 18.87 vs 15.66; Prostate gland undergoing cystic dilatation: 63.46% vs 47.24%) as well as week-16 (MCP-1: 21.27 vs 21.27; Prostate gland undergoing cystic dilatation: 67.79% vs 56.39%). It was concluded that HFD increases MCP-1 levels circulation and the percentage of prostate gland undergoing cystic dilatation.
To determine the effect of HFD on the occurrence of obesity and kidney disorders and one of the underlying mechanisms, Decleves et al16 examined C57BL/6J rats given HFD (60% of total calories came from fat) and ND (5% of total calories came from fat). After one week, there was an increase in the excretion of hydrogen peroxidase (H2O2) and MCP-1 urine, both of which are markers of inflammation. At the end of week 12, there was a significant increase in MCP-1 gene expression in the kidneys, of mice given HFD compared to ND (1.00±0.26 in ND and 7.53±2.32 in HFD). There is a decrease in the activity of the enzyme AMP-activated protein kinase (AMPK) in the kidneys. AMPK is the primary energy sensor in eukaryote cells, which is reported to decrease activity in all organs after HFD. After the first week, there have been no morphological changes in the kidneys, but after 12 weeks glomerulomegaly and an increase in the extracellular matrix of the glomerulus.16 In follow-up studies, mice were given HFD and AMPK enzyme activators to increase enzyme activity. It was found that increased excretion of H2O2 and MCP-1 urine and the occurrence of glomerulomegaly could be prevented, but the body weight of rats still increased. This suggests that inflammation and damage to the target organs due to HFD, one of the mechanisms through the role of the AMPK enzyme, is independent of the occurrence of obesity.16 High-fat intake increases ROS through increased expression of NADPH oxidase which causes activation of NF-kB, in this study HFD causes an increase in circulating MCP-1 levels which are pro-inflammatory cytokines.17
The consumption of foods rich in animal fats not only leads to obesity, impaired glucose tolerance, and insulin resistance but also increases the levels of ataxin in adipose tissue leading to increased production of lysophosphatidic acid. This compound has been shown to play a direct role in inducing the prostate gland hyperplastic and neoplastic growth, which plays a role in BPH and prostate cancer progressivity through pro-inflammatory mechanisms.18 Cai et al19 examined Sprague-Dawley mice fed high intake of saturated fatty acids for 1 and 3 months and found a 15% increase in the weight of the ventral prostate area. Vykhovanets et al17 report that NF-kB reporters who were given HFD were similar in composition to the Western diet and obtained increased intraprostatic inflammation, cell proliferation, and prostate gland size. The study also found that HFD causes an increase in prostate tissue that undergoes cystic degeneration, a sign of prostate hyperplasia.
Inflammation is one of the pathomechanisms for the occurrence of BPH. One of the inflammatory markers studied in this study was MCP-1. This study found that MCP-1 levels correlated meaningfully with the percentage of the prostate gland undergoing cystic dilatation, particularly at week-16 (r=0. 713; p<0.001). Whereas in week-8 it is not statistically meaningful (r=0.406; p=0.095) (Table 3). This suggests that the more prolonged exposure to high levels of MCP-1 circulation, the greater the risk of getting a prostate gland with cystic dilatation.
Robert et al13 assessed inflammation by examining tissue microarrays in 282 patients undergoing prostate surgery, and it was found that 81% had T lymphocyte markers (CD3), 52% B lymphocytes (CD20), and 82% macrophage markers. There was also an increase in prostate volume (77 cm3 vs 63 cm3; p=0.002) in patients with chronic inflammation compared to those who did not have the rash.
Zlotta et al20 examined the relationship between inflammatory degrees and prostatic hyperplasia of prostate tissue from surgery in 220 Caucasian men and 100 Asian men. Chronic inflammation was found in >70% of cases. Patients with chronic inflammation were 6.8 times more at risk of getting a higher BPH score than those with a lower BPH score.
There was no meaningful difference (p>0.05) in serum MCP-1 levels and the percentage of the prostate gland that had cystic dilatation in mice that got HFD and became obese or not obese, either at week-8 or week-16 (Table 4). It was concluded from this study that HFD-induced obesity did not cause an increase in pro-inflammatory status and incidence of the prostate gland undergoing cystic dilatation.
Fowke et al21 of the 169 men who underwent prostate biopsy found that the prostate volume of 7.19 mL was more significant in men with a BMI of 30.8 compared to a BMI of 25.9, but did not get a link between pro-inflammatory cytokines and the size of the prostate gland.
This study did not find a difference in MCP-1 levels as a marker of inflammation, and the incidence of the prostate gland experiencing cystic dilatation between obese mice compared to non-obese was likely because the length of time of obesity was not enough to cause the prostate gland to undergo cystic dilatation.
Conclusion
High-fat intake has been shown to increase the risk of obesity, but obesity does not increase inflammatory status and incidence of the prostate gland experiencing cystic dilatation. On the other hand, high fat intake increases the inflammatory rate, leading to an increase in the incidence of the prostate gland experiencing cystic dilatation.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We acknowledge Muhammad Faruk for his help in providing us with the linguistic assistance for this original research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: an endocrine society scientific statement. Endocr Rev. 2017;38(4):267–296. doi:10.1210/ER.2017-00111
2. Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. 2021;12. doi:10.3389/fendo.2021.706978
3. Wondmkun YT. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611. doi:10.2147/DMSO.S275898
4. Wang M, Yang Y, Liao Z. Diabetes and cancer: epidemiological and biological links. World J Diabetes. 2020;11(6):227. doi:10.4239/WJD.V11.I6.227
5. Liu J, Ramakrishnan SK, Khuder SS, et al. High-calorie diet exacerbates prostate neoplasia in mice with haploinsufficiency of PTEN tumor suppressor gene. Mol Metab. 2015;4(3):186–198. doi:10.1016/J.MOLMET.2014.12.011
6. Narita S, Nara T, Sato H, et al. Research evidence on high-fat diet-induced prostate cancer development and progression. J Clin Med. 2019;8(5):597. doi:10.3390/jcm8050597
7. Lokeshwar SD, Harper BT, Webb E, et al. Epidemiology and treatment modalities for the management of benign prostatic hyperplasia. Transl Androl Urol. 2019;8(5):529. doi:10.21037/TAU.2019.10.01
8. Mathey LIP. Benign prostatic hyperplasia: epidemiology, pathophysiology, and clinical manifestations. Mol Mech Cancer. 2022. doi:10.5772/INTECHOPEN.104823
9. Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate. 2010;70(5):473. doi:10.1002/PROS.21081
10. Syarif RH, Aman M, Lawrence GS, Lawrence GS. High-fat diet increases the level of circulating Monocyte Chemoattractant Protein-1 in Wistar rats, independent of obesity. Ann Med Surg. 2021;65. doi:10.1016/j.amsu.2021.102266
11. Laidding SR, Josh F. Combination of platelet-rich plasma and stromal vascular fraction on the level of transforming growth factor-$\upbeta$ in rat subjects experiencing deep dermal burn injury. Ann Med Surg. 2020;60:737–742. doi:10.1016/j.amsu.2020.11.088
12. Kurniawan VR, Islam AA, Adhimarta W, et al. The role of diphenhydramine HCl on tumor necrosis factor-α levels in Wistar rats with traumatic brain injury: an in vivo study. Ann Med Surg. 2022;81. doi:10.1016/j.amsu.2022.104399
13. Robert G, Descazeaud A, Nicolaïew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009;69(16):1774–1780. doi:10.1002/pros.21027
14. Damasceno DC, Sinzato YK, Bueno A, et al. Metabolic profile and genotoxicity in obese rats exposed to cigarette smoke. Obesity. 2013;21(8):1596–1601. doi:10.1002/oby.20152
15. Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23(2):270–299. doi:10.1017/S0954422410000168
16. Declèves A-E, Mathew AV, Cunard R, Sharma K. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol. 2011;22(10):1846–1855. doi:10.1681/ASN.2011010026
17. Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S. High-fat diet increases NF-κB signaling in the prostate of reporter mice. Prostate. 2011;71(2):147–156. doi:10.1002/pros.21230
18. Kulkarni P, Getzenberg RH. High-fat diet, obesity and prostate disease: the ATX–LPA axis? Nat Rev Urol. 2009;6(3):128–131. doi:10.1038/ncpuro1311
19. Cai X, Haleem R, Oram S, et al. High fat diet increases the weight of rat ventral prostate. Prostate. 2001;49(1):1–8. doi:10.1002/pros.1112
20. Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of inflammation and benign prostatic hyperplasia on autopsy in Asian and Caucasian men. Europ Urol. 2014;66(4):619–622. doi:10.1016/j.eururo.2014.06.026
21. Fowke JH, Koyama T, Fadare O, Clark PE, Hurst R. Does inflammation mediate the obesity and BPH relationship? An epidemiologic analysis of body composition and inflammatory markers in blood, urine, and prostate tissue, and the relationship with prostate enlargement and lower urinary tract symptoms. PLoS One. 2016;11(6):e0156918. doi:10.1371/journal.pone.0156918
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.