Back to Journals » Journal of Pain Research » Volume 17
The Efficacy of Neuromodulation Interventions for Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis
Authors Xu R, Yu C, Zhang X, Zhang Y, Li M, Jia B, Yan S, Jiang M
Received 10 November 2023
Accepted for publication 19 March 2024
Published 12 April 2024 Volume 2024:17 Pages 1423—1439
DOI https://doi.org/10.2147/JPR.S448528
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Michael A Ueberall
Runbing Xu,1,* Changhe Yu,2,* Xinyu Zhang,1 Yipin Zhang,1 Mengfei Li,1 Bei Jia,1 Shiyan Yan,3 Miao Jiang1,4
1Hematology and Oncology Department, Beijing University of Chinese Medicine Affiliated Dongzhimen Hospital, Beijing, People’s Republic of China; 2Tuina and Pain Management Department, Beijing University of Chinese Medicine Affiliated Dongzhimen Hospital, Beijing, People’s Republic of China; 3School of Acupuncture-Moxibustion and Tuina, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 4School of Life Science, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Miao Jiang, School of Life Science, Beijing University of Chinese Medicine, Gongchen Street, Beijing, People’s Republic of China, Tel +8613511072205, Email [email protected] Shiyan Yan, School of Acupuncture-Moxibustion and Tuina, Gongchen Street, Beijing University of Chinese Medicine, Beijing, People’s Republic of China, Tel +8613521436209, Email [email protected]
Purpose: To determine the efficacy and safety of a neuromodulation intervention regimen in the treatment of chemotherapy-induced peripheral neuropathy (CIPN).
Patients and Methods: Systematic searches were conducted in seven English databases. Randomized controlled trials of all neuromodulation interventions (both invasive and non-invasive) for the treatment of CIPN were selected. Group comparisons of differences between interventions and controls were also made. We divided the outcomes into immediate-term effect (≤ 3 weeks), short-term effect (3 weeks to ≤ 3 months), and long-term effect (> 3 months).
Results: Sixteen studies and 946 patients with CIPN were included. Among immediate-term effects, neuromodulation interventions were superior to usual care for improving pain (SMD=− 0.77, 95% CI − 1.07~ 0.47), FACT-Ntx (MD = 5.35, 95% CI 2.84~ 7.87), and QOL (SMD = 0.44, 95% CI 0.09~ 0.79) (moderate certainty); neuromodulation loaded with usual care was superior to usual care for improving pain (SMD=− 0.47, 95% CI − 0.71 ~ − 0.23), and QOL (SMD = 0.40, 95% CI 0.12 ~ 0.69) (moderate certainty). There were no statistically significant differences between the neuromodulation interventions regimen vs usual care in short- and long-term outcomes and neuromodulation vs sham stimulation from any outcome measure. There were mild adverse events such as pain at the site of stimulation and bruising, and no serious adverse events were reported.
Conclusion: Neuromodulation interventions had significant immediate-term efficacy in CIPN but had not been shown to be superior to sham stimulation; short-term and long-term efficacy could not be determined because there were too few original RCTs. Moreover, there are no serious adverse effects of this therapy.
Keywords: chemotherapy, peripheral neuropathy, neuromodulation, systematic review, meta-analysis
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is the most common dose-limiting side effect during cancer therapy. Various conventional cytotoxic drugs can cause CIPN, such as paclitaxel, platinum, periwinkle alkaloids, proteasome inhibitors, thalidomide and so on.1,2 The symptoms of CIPN commonly occur in the hands and feet, while the symptoms are more severe in the lower extremities than the upper extremities. The typical clinical features include tingling, numbness, burning, symmetrical “socking type”, and other paresthesias and dysesthesias.3,4 Meanwhile, some patients may experience ataxia and other motor symptoms.5,6 CIPN seriously affects the quality of life for cancer patients, and symptoms may continue for extended periods. The treatment’s efficacy may be compromised if the chemotherapy dose is lowered or stopped too soon due to a severe adverse effect.7,8
The conventional CIPN treatments are pharmacological interventions and non-pharmacological interventions.4,9 According to the American Society of Clinical Oncology and the European Society for Medical Oncology guidelines, duloxetine is recommended for CIPN pain management with moderate evidence.2–4 However, a recent meta-analysis shows that duloxetine’s efficacy in treating CIPN is not considerably more remarkable than the placebo effect, and caution should be used concerning its side effects.10,11 Based on the above discussion, the search for a proven treatment is critical.
Non-pharmacological interventions for CIPN have become a research hotspot. Neuromodulation interventions, as a rapidly evolving multidisciplinary non-pharmacological therapy, are now widely applied in pain management.12,13 The International Neuromodulation Society defines it as: “The alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation or chemical agents, to specific neurological sites in the body”.13,14 Common neuromodulation interventions include invasive and non-invasive ways, with invasive interventions including spinal cord stimulation (SCS), peripheral nerve stimulation (PNS), and acupuncture.15–17 Non-invasive interventions include neurofeedback (NF), scrambler therapy, and TENS.18–20
Current research on the mechanisms of CIPN involves the peripheral nerve, spinal nerve, and brain levels.21–23 Neuronal overexcitation, imbalance of nerve cell metabolic homeostasis, as well as brain hyperactivity, reduced GABAergic inhibition, neuroinflammation, and overactivation of the GPCR/MAPK pathway may all contribute to the onset and development of CIPN.23 Therefore, neuromodulation interventions based on the nerve or brain level are promising, and these interventions act directly or indirectly on the nerves and the brain through various stimulation modalities to improve their functions and enhance peripheral nerve regeneration processes.22
The benefits of neuromodulation interventions in pain alleviation and improved quality of life have recently been demonstrated by several high-quality RCTs.24–26 Neuromodulation interventions have shown promise in the management of CIPN, according to several guidelines2,4 and reviews.3,9 However, the efficacy of neuromodulation therapy is still unknown because there are not many large-scale clinical trials, there are contradicting findings in the present research, and the methodologies are not all of high quality (eg, some studies did not report the details of the random grouping process; small sample sizes; no blinding was used in the intervention process or in assessing outcomes, etc.). The majority of the original research included in previous systematic reviews of neuromodulation treatment for treating CIPN lacked quantitative analysis.18,27 Therefore, this study provided a comprehensive assessment of the therapeutic effects of neuromodulation interventions for CIPN through a systematic review and meta-analysis utilizing neurophysiological tests and patient-reported outcome measures (PROMs). The aim was to in order to fill the gaps in existing studies and to determine the efficacy and safety of neuromodulation as a stand-alone treatment or in combination in reducing pain, relieving symptoms, and improving the quality of life of patients with CIPN.
Materials and Methods
We had registered on PROSPERO with the number CRD42023413430. This review was reported adhering to the PRISMA guidelines (Supplementary Material, Table S1).28
Data Sources and Literature Search
We searched PubMed, Embase, Cochrane, Web of Science, PsyclNFO, and CINAHL from the database inception to February 2023. The search language is limited to English, and the search term is shown in Supplementary Material, Table S2. Two authors (Xu and Yu) independently screened the title, abstract, and full-text articles in turn. Disputes are resolved by third parties (Yan).
Inclusion Criteria
The inclusion criteria are formulated from five aspects: ①population (P): Patients with CIPN, ②interventions measures (I): Neuromodulation Interventions Program, ③control group (C): Usual care and sham stimulation, ④outcome (O): Patient-reported Outcomes Measures and neurophysiological examinations, ⑤study design (S): RCTs. (see Supplementary Material, Table S3 for specific details)
Exclusion Criteria
The exclusion criteria included: (1) the participant with cause peripheral neuropathy due to other diseases; (2) Cross-over trials; (3) non-English published articles; (4) Repeated publication or inability to obtain literature in full text (If there were duplicates, the most recent/comprehensive paper was selected for inclusion in the study).
Data Extraction
Two investigators (Zhang and Jia) independently extracted the literature data. Disputes are resolved by third parties (Jiang). Information was extracted as follows: (1) Characteristics information: authors, publication year, patients’ characteristics (age, chemotherapy drug, chemotherapy status, etc.), sample size, details of interventions/control measures; (2) outcome data: pain intensity, FACT-Ntx, EORTC QLQ-CIPN20, quality of life, and NCS; (3) risk of bias assessment required for key elements.
We divided the outcomes into immediate-term effect (≤3 weeks), short-term effect (3 weeks to ≤3 months), and long-term effect (>3 months).29–31 In research with multiple time points, we chose data for the point closest to 3 weeks and the third month. The point longer than 3 months was also extracted.
Risk of Bias Assessment
The Revised Cochrane Risk of Bias Tool for Randomized Trials (RoB2)32 was conducted to assess the methodological quality of each study in five aspects: (1) randomization process, (2) deviations from the intended interventions, (3) missing outcome data, (4) measurement of the outcome, (5) the selection of the reported result. For each included study, three determinations, “high risk”, “unknown risk”, and “high risk”, were made. This process was assessed independently by two researchers (Zhang and Li).
Statistical Methods
Statistical analysis was performed using Review Manager 5.4.1. The types of data in this study were measurement data, which were expressed using standardized mean differences (SMD) or mean differences (MD), and 95% confidence intervals (CIs) were calculated. Subgroups were analyzed according to follow-up time or intervention protocol (e.g, use of neuromodulation interventions alone, neuromodulation loaded with usual care). The statistical heterogeneity of the results for different groups was analyzed using the Q test, combining the P value with the judgment of heterogeneity: P>0.10 and I2≤50% represent good homogeneity within the group, so the fixed-effect model was used for meta-analysis; P≤0.10 and I2>50% represent greater statistical heterogeneity within the group, so the random-effect model was used.33 When heterogeneity is high, subgroup analysis was implemented to trace the sources. The publication bias was performed by funnel plots with Egger’s test.
Level of Evidence
Use the Grading of Recommendations Assessment Development and Evaluation (GRADE) to evaluate the level of evidence for outcome indicators.34 Five factors (risk of bias, inconsistency, indirectness, imprecision, and publication bias) that may lower the level of evidence were evaluated using GRADEpro GDT (https://gradepro.org/). The final results will be divided into four levels: high, moderate, low, or very low.
Results
Literature Search
The initial database search obtained 1449 publications, and 1625,26,35–48 RCTs were finally included in the literature for meta-analysis. The details are shown in Figure 1.
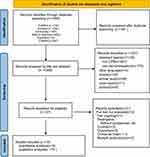 |
Figure 1 Flowchart of systematic review. |
Characteristics of Included Literature
Sixteen studies were included from the United States,25,38,46,48 China,26,37,40,42 Canada,49 Germany,41,47 the United Kingdom,35 South Korea,36 Australia,44 Sweden,45 and Brazil.43 One of the studies was a three-arm study,46 so there were 17 comparisons in the review. A total of 946 patients were involved, including 461 patients in the experimental group and 465 patients in the control group. According to the variations in interventions between the experimental and control groups, the aforementioned 17 comparisons were classified into three categories: Neuromodulation interventions in comparison to usual care (including standard care and usage of medicines advised by guidelines);38,41,42,48,49 Neuromodulation interventions versus sham stimulation,25,36,40,43–47 and Neuromodulation interventions combined with usual care versus usual care.26,35,37,46
Outcome indicators for these RCTs include: 1. pain level (scales including NRS, VAS, BPI-SF) 2. quality of life (scales including SF-36, EORTC QLQ-C30, FACT-G, WHOQOL-BREF) 3. scales for the evaluation of symptoms and functions in patients with CIPN: the Functional Assessment of Cancer Therapy Neurotoxicity (FACT-NTX);50 European Organization for Research and Treatment of Cancer Chemotherapy Induced Peripheral Neuropathy Questionnaire (EORTC QLQ-CIPN20).51 A total of 10 RCTs25,35–37,40,42,43,45,46,48 measured pain intensity, 8 RCTs26,35,38,40,41,44,48,49 reported quality of life, 7 RCTs26,37,38,40,44,46,49 measured FACT-Ntx, 5 RCTs25,35,36,44,49 reported EORTC QLQ-CIPN20 and 5 RCTs26,37,41,42,47 reported NCS. All RCTs had immediate effect measures for outcomes at the end of the last interventions; a total of 8 RCTs27,28,38,41,42,44,46,49 reported short-term effect outcomes ranging from 4 weeks to 80 days; a total of 1 RCT45 reported long-term effects. The details are shown in Table 1.
 |
Table 1 Characteristics of the Included Studies |
Quality Assessment
Among the included studies, 6 RCTs25,26,36,40,46,49 were evaluated as low risk, 7 RCTs35,41–45,47 were evaluated as unknown risk, and 3 RCTs37,38,48 were evaluated as high risk. Selection bias: 3 studies37,45,47 did not report details of the random grouping process, while the grouping method of the rest of the studies was by computer-generated random numbers, and 5 studies36,40–42,48 did not report details of allocation concealment methods. Implementation bias: Because of the characteristic of the neuromodulation interventions, trial performers and participants are difficult to blind to the interventions (eg, needling). Six studies26,38,46–49 did not blind trial performers and participants, and 3 studies26,46,47 blinded subjects only. No bias occurred due to the study setting in the included studies. Three studies37,42,48 were evaluated as high risk or some concern because of inappropriate methods for intervention effect analysis. Withdraw bias: 2 studies38,48 were evaluated as high risk because many cases were lost to follow-up, and no reasonable reason was given. Measurement bias: 6 studies35,37,38,41,44,48 did not clearly report assessor blindness. Given that the outcomes were mostly subjective, it is possible that the outcome assessors were impacted by the absence of blindness (details in Figure 2).
 |
Figure 2 Risk of bias summary. |
Immediate-Term Effect
Four outcomes of CIPN pain intensity, FACT-Ntx, EORTC-CIPN20 and quality of life were meta-analyzed. NCS outcomes were quantitatively described in the three groups according to the inclusion of RCT interventions and outcomes. The results of the GRADE evaluation are shown in Supplementary Material, Figure S1.
Nerve conduction study (ncs)
The 5 studies26,37,41,42,47 revealed the results for immediate-term effect of nerve conduction studies involving the common peroneal nerve, sural nerve, median nerve, ulnar nerve, and tibial nerve. Among them, 3 studies26,41,42 fully reported distal nerve latency, amplitude, and conduction velocity, while the other two37,47 only reported nerve conduction velocity.
For neuromodulation with usual care, the results of one study26 showed that the included population was in the normal range of neurophysiological examinations at baseline, and there was no significant difference for endpoints compared to baseline. However, another study37 showed a significant improvement in nerve conduction velocities of peroneal nerve sensory at the end of the treatment when neuromodulation interventions with usual care compared to usual care (P<0.01), and this intervention regimen was also statistically significant in a within-group comparison of MCV in the bilateral median nerve and the peroneal nerve.
For the use of neuromodulation alone, one study42 showed significant differences in the improvement of the amplitude of the sural nerve, MCV of the peroneal nerve, and the amplitude and the MCV of the tibial nerve when compared to usual care. However, another study showed41 that at the end of the treatment, all outcomes were not statistically significant when compared to the usual care. Rick showed that47 there was no statistically significant improvement in NCV of the peroneal nerve with neuromodulation compared to sham groups, and there was a significant difference in conduction velocities of peroneal nerve after 3 weeks of interventions (P=0.021), whereas the difference was not statistically significant after 3 months of interventions.
Pain Intensity
Twelve studies reported the immediate effect on the improvement of CIPN pain intensity, including 734 patients. Because the included studies had different scales for assessing pain intensity but the same purpose and tendency, the results are combined using standardized mean differences (details in Figure 3A).
Four studies38,42,46,48 compared neuromodulation interventions with usual care and showed that there was a significant difference in the reduction of pain intensity with neuromodulation interventions compared with usual care (moderate evidence; fixed-effect model; N = 187; SMD = −0.77, 95% CI −1.07~ 0.47, P<0.00001; I2=0%).
The combined results of the 6 studies25,36,40,43,45,46 showed that there is no difference between neuromodulation interventions and sham stimulation in interventions (high evidence; fixed-effects model; N = 265; SMD = −0.21, 95% CI −0.45~ −0.04, P = 0.1; I2 = 36%).
Three studies26,35,37 showed that the difference between neuromodulation interventions combined with usual care and usual care in terms of pain reduction was statistically significant (moderate evidence; fixed-effects model; N = 282; SMD = −0.47, 95% CI −0.71~ −0.23, P = 0.0001; I2= 41%).
FACT-Ntx
Six studies reported the immediate effect on the improvement of the FACT-Ntx neurotoxicity index, including 734 patients. Only two groups were included in the number of studies, and the results were analyzed in the form of descriptive analysis combined with meta-analysis (details in Figure 3B).
Three studies38,46,49 compared neuromodulation interventions with usual care and showed that there was a significant difference in the improvement of FACT-Ntx neurotoxicity index with neuromodulation interventions compared with usual care (moderate evidence; fixed-effect model; N = 122; MD = 5.35, 95% CI 2.84~ 7.87, P < 0.0001; I2= 48%).
The combined results of the 2 studies40,46 showed that the difference between neuromodulation interventions and sham stimulation in terms of sham stimulation was not statistically significant (low evidence; fixed-effects model; N = 71; MD = 1.23, 95% CI −1.70~ 4.16, P = 0.41; I2= 0%). Only 1 study26 showed that there was a significant difference in the improvement of FACT-Ntx neurotoxicity indexes between neuromodulation interventions combined with usual care compared with usual care (MD = 0.55, 95% CI 0.12~0.97, P = 0.01).
EORTC QLQ-CIPN20
Five RCTs reported between-group differences on the EORTC QLQ-CIPN20 scale. The outcomes were analyzed through a combination of descriptive and meta-analysis (details in Figure 3C).
One RCT49 showed no statistical difference between neuromodulation interventions and usual care in improving QLQ-CIPN20 neurotoxicity indicators (MD=−0.34, 95% CI −1.05~ 0.37, P=0.35); 1RCT35 compared neuromodulation interventions combined with usual care to usual care, and the results showed that neuromodulation interventions combined with usual care were more effective than usual care, but not statistically significant overall (MD=−0.35, 95% CI −0.74~ 0.03, P=0.07).
Three studies25,36,44 comparing the difference in QLQ-CIPN20 neurotoxicity indicators between neuromodulation interventions and sham stimulation, combined results showed no statistical significance between the two groups (moderate evidence; fixed-effect model; N = 151; SMD = 0.27, 95% CI −0.05~ 0.6, P = 0.10; I2= 0%).
Quality of Life
Seven studies with outcomes reporting between-group differences in quality of life (details in Figure 3D).
Four studies38,41,48,49 compared neuromodulation interventions with usual care and showed a significant difference in the improvement of quality of life with neuromodulation interventions compared with usual care (moderate evidence; fixed-effect model; N = 162; SMD = 0.44, 95% CI 0.09~ 0.79, P = .01; I2= 0%).
Two studies40,44 compared neuromodulation interventions with the sham group and showed that there was no statistically significant difference in the improvement of quality of life with neuromodulation interventions compared with usual care (very low evidence; fixed-effect model; N = 64; SMD = −0.08, 95% CI −0.59~ 0.43, P = 0.75; I2 = 0%).
Two studies26,35 comparing neuromodulation interventions combined with usual care to usual care showed a significant difference in improvement in quality of life with neuromodulation interventions combined with usual care compared to usual care (moderate evidence; fixed-effect model; N = 191; SMD = 0.40, 95% CI 0.12~ 0.69, P = 0.006; I2 =0%).
Short-Term Effect
We did a meta-analysis to evaluate the short-term effect of FACT-Ntx, EORTC QLQ-CIPN20 and QoL according to the different interventions and the reporting of outcome measures in the literature. The results of the GRADE evaluation are shown in Supplementary Material, Figure S2.
FACT-Ntx
Six studies with outcomes reporting between-group differences in FACT-Ntx scales. None of the three inter-group comparisons found that the intervention group was superior to the control group in improving the FACT-Ntx neurotoxicity index (details in the Figure 4A).
The results of the meta-analysis of the two RCTs38,49 combined showed no statistically significant results for the neuromodulation interventions compared to the usual care group (very low evidence; fixed-effect model; N = 71; SMD = 0.05, 95% CI −0.42~ 0.51, P=0.84; I2=0%).
Two RCTs44,46 combined showed no statistically significant results for the neuromodulation interventions compared to the sham group (very low evidence; fixed-effect model; N = 95; SMD = 0.02, 95% CI −0.39~ 0.44, P = 0.92; I2=0%).
Two RCTs26,46 combined showed no statistically significant results for the neuromodulation interventions combined with usual care compared to usual care (moderate evidence; fixed-effect model; N = 138; SMD = 0.30, 95% CI −0.04~ 0.63, P=0.08; I2=0%).
EORTC QLQ-CIPN20
Two studies25,44 compared neuromodulation interventions with the sham group and showed that there was no statistically significant difference in the improvement of quality of life with neuromodulation interventions compared with usual care (very low evidence; fixed-effect model; N = 79; SMD = 0.10, 95% CI −0.36~ 0.55, P = 0.68; I2=0%). (Details in Figure 4B)
Quality of Life
Two studies41,49 compared neuromodulation interventions with the usual care group and showed that there was no statistically significant difference in the improvement of quality of life in the two groups (very low evidence; fixed-effect model; N = 60; SMD = 0.22, 95% CI −0.30~ 0.73, P = 0.41; I2= 0%). (details in the Figure 4C)
Long-Term Effect
Only 1 study45 with some concerns about the risk of bias followed the long-term effects of neuromodulation therapy on the degree of pain relief compared to sham stimulation and showed no statistically significant differences between the two groups (P = 0.885 > 0.05).
Adverse Events
A total of 10 studies26,35,36,38,40,42,44,46,48,49 reported adverse events, of which 5 studies26,40,46,48,49 reported that patients did not experience adverse effects related to neuromodulation interventions. The rest of the studies had no serious adverse events, and these minor adverse symptoms resolved independently and did not require interventions.
The adverse effects associated with acupuncture were pain, bruising, bleeding, and claustrophobia with a blindfold;35,38,42,46 the possible adverse events related to electrical stimulation were diarrhoea, lymphedema, extremity oedema, and flu-like symptoms;36 and the adverse events associated with the laser interventions were tingling and hot/cold changes in temperature.44 Notably, Iravani43 reported that the usual care of CIPN (gabapentin) causes somnolence and dizziness.
Publication Bias
Funnel plots were plotted for the immediate effect of improvement in pain intensity as an outcome indicator, and Egger tests were performed to analyze for publication bias. The results showed that the funnel plots were symmetrical, and Egger tests P=0.538>0.05, indicating that there was no publication bias in the 13 pieces of literature included in this outcome indicator (details in the Supplementary Material, Figure S3).
Discussion
Main Findings
Our review’s conclusions complement those of previous systematic studies and advance their findings. The moderate evidence suggests that a treatment regimen of neuromodulation interventions with/without usual care lasting between 2 and 3 weeks is more efficacious than usual care alone in improving quality of life over the immediate-term period of 3 weeks. Furthermore, moderate evidence suggests that a treatment regimen of neuromodulation interventions lasting between 2 and 3 weeks is more efficacious than usual care in alleviating pain intensity over the immediate-term period of 3 weeks. There is moderate evidence of a significant difference in the short-term efficacy of neuromodulation interventions compared with usual care in FACT-Ntx.
The present results suggest that neuromodulation interventions could potentially be effective for treating pain, relieving symptoms, and improving QOL in individuals with CIPN. Both immediate-term improvements in QOL 0.4 points (95% CI 0.12~0.69) of neuromodulation interventions with the usual care group and 0.41 points (95% CI 0.10~0.72) of the neuromodulation intervention group exceed the minimum clinically important difference (MCID) of 0.1 points for EORTC QLQ-C30 or 0.3 points for FACT-G52,53 when compared to usual care. In addition, the immediate-term decrease in FACT-Ntx of 5.35 points (95% CI 2.84~ 7.87) achieves the MCID of 1.38 points54 in neuromodulation intervention groups when compared to usual care. However, the pain intensity of −0.77 points (95% CI −1.07~ −0.47) did not exceed the MCID of 2 points.55,56 It is worth noting that the results regarding QLQ-CIPN20, pain intensity or FACT-Ntx did not favor neuromodulation interventions over sham interventions in the immediate-, short- or long-term; thus, it is likely that further replications of this research might provide remarkably different outcomes.
This study does not restrict tumor type or chemotherapeutic regimen; this study only produces generalized results without specific analysis of the individual cases. However, since this study only intakes a small number of individual outcome indicators in the original literature, the results should be interpreted cautiously.
The interventions we included were categorized into central neuromodulation techniques (eg, EEG neurofeedback) and peripheral neuromodulation techniques (including TENS, scrambler therapy, acupuncture, photobiomodulation, etc.)18 In our inclusion of EEG neurofeedback, biofeedback training is done by monitoring the EEG activity of CIPN patients and providing feedback.48 This therapy may reduce a patient’s perception of discomfort by tuning sensory areas of the CIPN patient’s brain, enhancing the brain’s ability to filter signals such as pain or affecting pain-related neural pathways.57–59 The mechanisms of peripheral neuromodulation techniques for the treatment of CIPN mainly include improving limb microcirculation,60,61 reducing oxidative stress and inflammatory reactions,62–64 inhibiting the release of pain signals through gate-control theory,65–67 and regulating the release of neurotransmitters.68,69
There was no statistically significant improvement in various indicators between the neuromodulation intervention group and the sham group. The possible mechanisms may be due to the specificity of neuromodulation interventions;44 thus, it is difficult to achieve absolute non-stimulation of placebo control. The sham group included in this study had minor stimulation,25,40,43,46 which enhanced the placebo effect. Secondly, the neuromodulation intervention treatment process involves a high level of doctor–patient interaction, which can produce certain neurobiological effects.70,71 Thirdly, patient expectations are another primary source of the placebo effect for analgesia.72–75 A secondary analysis76 of one study concluded that it may be due to higher expectations of baseline outcomes among subjects in the sham acupuncture group.
Nerve conduction studies (NCS) is a generalized CIPN assessment method, and it has been shown that the sensitivity of the NCS to different types of drug-induced CIPN may vary.77,78 Chemotherapy drug toxicity is prone to affect Aδ fibres and C fibres, while NCS is more sensitive to detecting lesions in Aα large nerve fibres.79 The individual evidence suggests that neuromodulation interventions improve MCV of the common peroneal nerve, MCV of the tibial nerve and its wave amplitude, SCV of the ulnar nerve, SCV of the peroneal nerve, and wave amplitude in immediate effects. However, other studies contradict these results. Because of the clinical heterogeneity and the small number of included studies, the above results cannot present evidence-based support for clinical use.
Comparison of Similar Studies
There are currently 2 systematic reviews18,27 of neuromodulation interventions and 4 studies80–83 of acupuncture for CIPN similar to ours. There are similarities in the results, suggesting that such therapies reduce neuropathic pain27,83,84 and improve patient quality of life.81,83 It can also be combined with usual care to improve outcomes.82 However, unlike other studies: we found that neuromodulation interventions significantly improved CIPN symptoms (FACT-Ntx and EORTC QLQ-CIPN20) compared with usual care, but there was no significant difference in efficacy compared with sham stimulation across all outcomes.
At the same time, our study conducted a meta-analysis and evidence pooling of all randomized controlled trials of neuromodulation interventions, expanding the sample size of evidence and complementing the overall evidence on neuromodulation interventions. A categorical assessment of the effects of neuromodulation interventions over time refines the evidence support for clinical practice.
Strengths and Limitations
The review’s strengths include the study of a wide variety of neuromodulation interventions. The moderate quality data were sufficient to conclude that neuromodulation interventions, whether with or without usual care, are beneficial to usual care on immediate-term self-reported symptoms (pain, FACT-Ntx) and quality of life. Other strengths of this research include the adoption of rigorous procedures involving synthesizing and summarizing the quality of the evidence by the GRADE system.
This study is especially crucial since it provides a thorough assessment and identification of critical knowledge gaps in this field, which will help guide clinical practices and future research. These studies must be appropriately powered, have low bias risks, and have long-term follow-up. To increase transparency and minimize bias, future RCTs should adhere to the Consolidated Standards of Reporting Trials guideline when designing studies and presenting research results.
One limitation of this study is the possibility of language bias because only English publications were included. Studies with small sample sizes, which are more vulnerable to bias, were also included. Due to the characteristics of interventions, it is difficult to blind the intervenor and patients, and 6 studies did not blind the intervenor or subjects, thus potentially creating the bias due to deviations from the intended interventions. Few studies could be pooled due to the significant degree of heterogeneity between trials, such as types of cancer, cycles of chemotherapy drugs, stimulus intensity of interventions, and course of treatment. Moreover, harms associated with treatment were often not reported or inconsistently reported.
Implications for Future Research
Our study will provide the following implications for future research: first, there is a need for larger, high-quality pilot studies of the long-term effects of neuromodulation interventions. Second, future trials should concentrate on providing a thorough description (eg, stimulation intensity, frequency, waveform, lead position and coil orientation) of the intervention itself, as well as a report of standardized outcomes, to make future meta-analysis easier to do. We also recommend analyzing different mechanisms or modalities of neuromodulation, such as those based on the peripheral and central nervous systems. Third, our findings suggest that expectations and placebo effects may play a larger role in the non-specific effects of sham stimulation, and thus patients’ outcome expectations could be assessed in future studies using specialized multiscale or assessment methods.85 Mechanistic studies of placebo effects and expectations could also be undertaken. Fourth, for trials comparing neuromodulation interventions with usual care, efforts should be strengthened to address issues such as treatment nonadherence, crossover, and blinding difficulties.
Conclusions
There is moderate-quality evidence that neuromodulation interventions are an immediately superior therapeutic strategy to usual care in relieving immediate pain, reducing CIPN symptoms and improving quality of life. However, no positive results were obtained for its comparative sham stimulation. Also, given the limited sample size, it is suggested that more extensive clinical trials of neuromodulation interventions for the treatment of CIPN could be conducted subsequently. Because of the severe impact of CIPN on the quality of survival of cancer patients and the paucity of current first-line therapies, evidence-based support for alternative therapies is, therefore, urgently needed.
Data Sharing Statement
The data used to support the findings of this study are available from the first author upon request.
Acknowledgment
This review was supported by grants from the National Natural Science Foundation of China (No. 81973667 and 81803956) and Beijing University of Chinese Medicine Special Funding for Basic Research Operating Costs of Central Universities (2022-JYB-JBZR-022).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Burgess J, Ferdousi M, Gosal D, et al. Chemotherapy-induced peripheral neuropathy: epidemiology, pathomechanisms and treatment. Oncol Ther. 2021;9(2):385–450. doi:10.1007/s40487-021-00168-y
2. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325–3348. doi:10.1200/JCO.20.01399
3. Desforges AD, Hebert CM, Spence AL, et al. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: an update. Biomed Pharmacother. 2022;147:112671. doi:10.1016/j.biopha.2022.112671
4. Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31(10):1306–1319. doi:10.1016/j.annonc.2020.07.003
5. de Arenas-Arroyo S N, Cavero-Redondo I, Torres-Costoso A, Reina-Gutiérrez S, Lorenzo-García P, Martínez-Vizcaíno V. Effects of exercise interventions to reduce chemotherapy-induced peripheral neuropathy severity: a meta-analysis. Scand J Med Sci Sports. 2023;33(7):1040–53. doi:10.1111/sms.14360
6. Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772–781. doi:10.1002/ana.24951
7. Havrilesky LJ, Reiner M, Morrow PK, Watson H, Crawford J. A review of relative dose intensity and survival in patients with metastatic solid tumors. Crit Rev Oncol Hematol. 2015;93(3):203–210. doi:10.1016/j.critrevonc.2014.10.006
8. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev. 2014;40(7):872–882. doi:10.1016/j.ctrv.2014.04.004
9. Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): a narrative review. Br J Anaesth. 2017;119(4):737–749. doi:10.1093/bja/aex229
10. Chow R, Novosel M, So OW, et al. Duloxetine for prevention and treatment of chemotherapy-induced peripheral neuropathy (CIPN): systematic review and meta-analysis. BMJ Support Palliat Care. 2023;13(1):27–34. doi:10.1136/spcare-2022-003815
11. Aghabozorgi R, Hesam M, Zahed G, Babaee M, Hashemi M, Rayegani SM. Efficacy of Duloxetine on electrodiagnostic findings of Paclitaxel-induced peripheral neuropathy, does it have a prophylactic effect? A randomized clinical trial. Anticancer Drugs. 2023;34(5):680. doi:10.1097/CAD.0000000000001429
12. Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet Lond Engl. 2021;397(10283):1505–1518. doi:10.1016/S0140-6736(20)32342-4
13. Knotkova H, Hamani C, Sivanesan E, et al. Neuromodulation for chronic pain. Lancet Lond Engl. 2021;397(10289):2111–2124. doi:10.1016/S0140-6736(21)00794-7
14. International neuromodulation society neuromodulation, or neuromodulatory effect; 2018. Avalable from: https://www.neuromodulation.com/neuromodulation-defined.
15. Thompson T, Heathcote LC, Hobson H, Solmi M. Editorial: neuromodulatory Interventions for Pain. Front Neurosci. 2021;15:746328. doi:10.3389/fnins.2021.746328
16. Magee DJ, Schutzer-Weissmann J, Pereira EAC, Brown MRD. Neuromodulation techniques for cancer pain management. Curr Opin Support Palliat Care. 2021;15(2):77–83. doi:10.1097/SPC.0000000000000549
17. Zhi JF, Liao QH, He YB, Xu WW, Zhu DW, Shao LH. Superior treatment efficacy of neuromodulation rehabilitation for upper limb recovery after stroke: a meta-analysis. Expert Rev Neurother. 2022;22(10):875–888. doi:10.1080/14737175.2022.2137405
18. Wang M, Yin Y, Yang H, Pei Z, Molassiotis A. Evaluating the safety, feasibility, and efficacy of non-invasive neuromodulation techniques in chemotherapy-induced peripheral neuropathy: a systematic review. Eur J Oncol Nurs. 2022;58:102124. doi:10.1016/j.ejon.2022.102124
19. Wischnewski M, Alekseichuk I, Opitz A. Neurocognitive, physiological, and biophysical effects of transcranial alternating current stimulation. Trends Cognit Sci. 2023;27(2):189–205. doi:10.1016/j.tics.2022.11.013
20. D’Souza RS, Alvarez GAM, Dombovy-Johnson M, Eller J, Abd-Elsayed A. Evidence-based treatment of pain in chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep. 2023;27(5):99–116. doi:10.1007/s11916-023-01107-4
21. Albin B, Adhikari P, Tiwari AP, Qubbaj K, Yang IH. Electrical stimulation enhances mitochondrial trafficking as a neuroprotective mechanism against chemotherapy-induced peripheral neuropathy. iScience. 2024;27(3):109052. doi:10.1016/j.isci.2024.109052
22. Jin MY, Weaver TE, Farris A, Gupta M, Abd-Elsayed A. Neuromodulation for peripheral nerve regeneration: systematic review of mechanisms and in vivo highlights. Biomedicines. 2023;11(4):1145. doi:10.3390/biomedicines11041145
23. Omran M, Belcher EK, Mohile NA, et al. Review of the role of the brain in chemotherapy-induced peripheral neuropathy. Front Mol Biosci. 2021;8:693133. doi:10.3389/fmolb.2021.693133
24. Seidman AD, Piulson L, Vertosick E, et al. A phase IIA trial of acupuncture to reduce chemotherapy-induced peripheral neuropathy severity during neoadjuvant or adjuvant weekly paclitaxel chemotherapy in breast cancer patients. Eur J Cancer. 2018;101:12–19. doi:10.1016/j.ejca.2018.06.008
25. Smith TJ, Razzak AR, Blackford AL, et al. A pilot randomized sham-controlled trial of MC5-A scrambler therapy in the treatment of chronic chemotherapy-induced peripheral neuropathy (CIPN). J Palliat Care. 2020;35(1):53–58. doi:10.1177/0825859719827589
26. Molassiotis A, Suen LKP, Cheng HL, et al. A randomized assessor-blinded wait-list-controlled trial to assess the effectiveness of acupuncture in the management of chemotherapy-induced peripheral neuropathy. Integr Cancer Ther. 2019;18:153473541983650. doi:10.1177/1534735419836501
27. D’Souza RS, Her YF, Jin MY, Morsi M, Abd-Elsayed A. Neuromodulation therapy for chemotherapy-induced peripheral neuropathy: a systematic review. Biomedicines. 2022;10(8):1909. doi:10.3390/biomedicines10081909
28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
29. Traeger AC, Gilbert SE, Harris IA, Maher CG. Spinal cord stimulation for low back pain. Cochrane Database Syst Rev. 2023;3(3):CD014789. doi:10.1002/14651858.CD014789.pub2
30. Verville L, Hincapié CA, Southerst D, et al. Systematic Review to Inform a World Health Organization (WHO) clinical practice guideline: benefits and harms of transcutaneous electrical nerve Stimulation (TENS) for chronic primary low back pain in adults. J Occup Rehabil. 2023;33(4):651–660. doi:10.1007/s10926-023-10121-7
31. Huang JF, Zheng XQ, Chen D, et al. Can acupuncture improve chronic spinal pain? A systematic review and meta-analysis. Glob Spine J. 2021;11(8):1248–1265. doi:10.1177/2192568220962440
32. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
33. Cochrane Handbook for systematic reviews of interventions, version 5.1.0; 2011. Cochrane Collaboration website. Available from: https://training.cochrane.org/handbook.
34. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi:10.1016/j.jclinepi.2010.07.015
35. Stringer J, Ryder WD, Mackereth PA, Misra V, Wardley AM. A randomised, pragmatic clinical trial of Acupuncture plus standard care versus standard care alone for Chemotherapy Induced peripheral Neuropathy (ACUFOCIN). Eur J Oncol Nurs. 2022;60:102171. doi:10.1016/j.ejon.2022.102171
36. Song SY, Park JH, Lee JS, et al. A randomized, placebo-controlled trial evaluating changes in peripheral neuropathy and quality of life by using low-frequency electrostimulation on breast cancer patients treated with chemotherapy. Integr Cancer Ther. 2020;19:1534735420925519. doi:10.1177/1534735420925519
37. Han X, Wang L, Shi H, et al. Acupuncture combined with methylcobalamin for the treatment of chemotherapy-induced peripheral neuropathy in patients with multiple myeloma. BMC Cancer. 2017;17(1):40. doi:10.1186/s12885-016-3037-z
38. Lu W, Giobbie-Hurder A, Freedman RA, et al. Acupuncture for chemotherapy-induced peripheral neuropathy in breast cancer survivors: a randomized controlled pilot trial. Oncologist. 2020;25(4):310–318. doi:10.1634/theoncologist.2019-0489
39. D’Alessandro EG, Nebuloni Nagy DR, de Brito CMM, Almeida EPM, Battistella LR, Cecatto RB. Acupuncture for chemotherapy-induced peripheral neuropathy: a randomised controlled pilot study. BMJ Support Palliat Care. 2022;12(1):64–72. doi:10.1136/bmjspcare-2018-001542
40. Huang CC, Ho TJ, Ho HY, et al. Acupuncture relieved chemotherapy-induced peripheral neuropathy in patients with breast cancer: a pilot randomized sham-controlled trial. J Clin Med. 2021;10(16):3694. doi:10.3390/jcm10163694
41. Rostock M, Jaroslawski K, Guethlin C, Ludtke R, Schröder S, Bartsch HH. Chemotherapy-induced peripheral neuropathy in cancer patients: a four-arm randomized trial on the effectiveness of electroacupuncture. Evid Based Complement Alternat Med. 2013;2013:1–9. doi:10.1155/2013/349653
42. Iravani S, Kazemi Motlagh AH, Emami Razavi SZ, et al. Effectiveness of acupuncture treatment on chemotherapy-induced peripheral neuropathy: a pilot, randomized, assessor-blinded, controlled trial. Pain Res Manag. 2020;2020:1–11. doi:10.1155/2020/2504674
43. Tonezzer T, Caffaro LAM, Menon KRS, et al. Effects of transcutaneous electrical nerve stimulation on chemotherapy-induced peripheral neuropathy symptoms (CIPN): a preliminary case-control study. J Phys Ther Sci. 2017;29(4):685–692. doi:10.1589/jpts.29.685
44. Teng C, Egger S, Blinman PL, Vardy JL. Evaluating laser photobiomodulation for chemotherapy-induced peripheral neuropathy: a randomised Phase II trial. Support Care Cancer. 2023;31(1):52. doi:10.1007/s00520-022-07463-y
45. Lindblad K, Bergkvist L, Johansson AC. Evaluation of the treatment of chronic chemotherapy-induced peripheral neuropathy using long-wave diathermy and interferential currents: a randomized controlled trial. Support Care Cancer. 2016;24(6):2523–2531. doi:10.1007/s00520-015-3060-7
46. Bao T, Baser R, Chen C, et al. Health-related quality of life in cancer survivors with chemotherapy-induced peripheral neuropathy: a randomized clinical trial. Oncologist. 2021;26(11):e2070–e2078. doi:10.1002/onco.13933
47. Rick O, von Hehn U, Mikus E, Dertinger H, Geiger G. Magnetic field therapy in patients with cytostatics-induced polyneuropathy: a prospective randomized placebo-controlled phase-III study: magnetic field therapy in chemotherapy-induced peripheral neuropathy. Bioelectromagnetics. 2017;38(2):85–94. doi:10.1002/bem.22005
48. Prinsloo S, Novy D, Driver L, et al. Randomized controlled trial of neurofeedback on chemotherapy-induced peripheral neuropathy: a pilot study: neurofeedback for Neuropathy. Cancer. 2017;123(11):1989–1997. doi:10.1002/cncr.30649
49. Al Onazi MM, Yurick JL, Harris C, et al. Therapeutic ultrasound for chemotherapy-related pain and sensory disturbance in the hands and feet in patients with colorectal cancer: a pilot randomized controlled trial. J Pain Symptom Manage. 2021;61(6):1127–1138. doi:10.1016/j.jpainsymman.2020.10.028
50. Calhoun EA, Welshman EE, Chang CH, et al. Psychometric evaluation of the functional assessment of cancer therapy/gynecologic oncology group-neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int J Gynecol Cancer off J Int Gynecol Cancer Soc. 2003;13(6):741–748. doi:10.1111/j.1525-1438.2003.13603.x
51. Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer Oxf Engl. 2005;41(8):1135–1139. doi:10.1016/j.ejca.2005.02.012
52. Musoro JZ, Sodergren SC, Coens C, et al. Minimally important differences for interpreting the EORTC QLQ-C30 in patients with advanced colorectal cancer treated with chemotherapy. Colorectal Dis. 2020;22(12):2278–2287. doi:10.1111/codi.15295
53. Luckett T, King MT, Butow PN, et al. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol. 2011;22(10):2179–2190. doi:10.1093/annonc/mdq721
54. Cheng HL, Lopez V, Lam SC, et al. Psychometric testing of the functional assessment of cancer therapy/gynecologic oncology group—neurotoxicity (FACT/GOG-Ntx) subscale in a longitudinal study of cancer patients treated with chemotherapy. Health Qual Life Outcomes. 2020;18(1):246. doi:10.1186/s12955-020-01493-y
55. Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi:10.1016/S0304-3959(01)00349-9
56. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
57. Hesam‐Shariati N, Chang W, Wewege MA, et al. The analgesic effect of electroencephalographic neurofeedback for people with chronic pain: a systematic review and meta‐analysis. Eur J Neurol. 2022;29(3):921–936. doi:10.1111/ene.15189
58. Caro XJ, Winter EF. EEG biofeedback treatment improves certain attention and somatic symptoms in fibromyalgia: a pilot study. Appl Psychoph Biofeed. 2011;36(3):193–200. doi:10.1007/s10484-011-9159-9
59. Sharpe L, Ianiello M, Dear BF, Perry KN, Refshauge K, Nicholas MK. Is there a potential role for attention bias modification in pain patients? Results of 2 randomised, controlled trials. Pain. 2012;153(3):722–731. doi:10.1016/j.pain.2011.12.014
60. Kim M, Kim EM, Oh PS, et al. Usefulness of cyclic thermal therapy and red blood cell scintigraphy in patients with chemotherapy-induced peripheral neuropathy. Korean J Pain. 2021;34(4):427–436. doi:10.3344/kjp.2021.34.4.427
61. Hsieh YL, Fan YC, Yang CC. Low-level laser therapy alleviates mechanical and cold allodynia induced by oxaliplatin administration in rats. Support Care Cancer. 2016;24(1):233–242. doi:10.1007/s00520-015-2773-y
62. Caylor J, Reddy R, Yin S, et al. Spinal cord stimulation in chronic pain: evidence and theory for mechanisms of action. Bioelectron Med. 2019;5:12. doi:10.1186/s42234-019-0023-1
63. He Y, Guo X, May BH, et al. Clinical evidence for association of acupuncture and acupressure with improved cancer pain: a systematic review and meta-analysis. JAMA Oncol. 2020;6(2):271. doi:10.1001/jamaoncol.2019.5233
64. Tella BA, Oghumu SN, Gbiri CAO. Efficacy of transcutaneous electrical nerve stimulation and interferential current on tactile acuity of individuals with nonspecific chronic low back pain. Neuromodulation Technol Neural Interface. 2022;25(8):1403–1409. doi:10.1111/ner.13522
65. Robijns J, Censabella S, Bulens P, Maes A, Mebis J. The use of low-level light therapy in supportive care for patients with breast cancer: review of the literature. Lasers Med Sci. 2017;32(1):229–242. doi:10.1007/s10103-016-2056-y
66. Min YG, Baek HS, Lee KM, Hong YH. Differential response to scrambler therapy by neuropathic pain phenotypes. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-89667-6
67. Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85(4):355–375. doi:10.1016/j.pneurobio.2008.05.004
68. Uchida H, Nagai J, Ueda H. Lysophosphatidic acid and its receptors LPA1 and LPA3 mediate paclitaxel-induced neuropathic pain in mice. Mol Pain. 2014;10:71. doi:10.1186/1744-8069-10-71
69. Chen Y, Li D, Li N, et al. Role of nerve signal transduction and neuroimmune crosstalk in mediating the analgesic effects of acupuncture for neuropathic pain. Front Neurol. 2023;14:1093849. doi:10.3389/fneur.2023.1093849
70. Rossettini G, Campaci F, Bialosky J, Huysmans E, Vase L, Carlino E. The biology of placebo and nocebo effects on experimental and chronic pain: state of the art. J Clin Med. 2023;12(12):4113. doi:10.3390/jcm12124113
71. Atlas LY. How instructions, learning, and expectations shape pain and neurobiological responses. Annu Rev Neurosci. 2023;46:167–189. doi:10.1146/annurev-neuro-101822-122427
72. Colloca L. The placebo effect in pain therapies. Annu Rev Pharmacol Toxicol. 2019;59:191–211. doi:10.1146/annurev-pharmtox-010818-021542
73. Hohenschurz-Schmidt D, Draper-Rodi J, Vase L, et al. Blinding and sham control methods in trials of physical, psychological, and self-management interventions for pain (article I): a systematic review and description of methods. Pain. 2023;164(3):469–484. doi:10.1097/j.pain.0000000000002723
74. Prady SL, Burch J, Vanderbloemen L, Crouch S, MacPherson H. Measuring expectations of benefit from treatment in acupuncture trials: a systematic review. Complement Ther Med. 2015;23(2):185–199. doi:10.1016/j.ctim.2015.01.007
75. Jepma M, Koban L, van Doorn J, Jones M, Wager TD. Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nat Hum Behav. 2018;2(11):838–855. doi:10.1038/s41562-018-0455-8
76. Li X, Zhi L, Han KY, et al. Impact of baseline expectancy on outcome prediction of real and sham acupuncture for persistent chemotherapy-induced peripheral neuropathy pain in solid tumor survivors: a secondary analysis of a randomized clinical trial. Integr Cancer Ther. 2023;22:15347354221149992. doi:10.1177/15347354221149992
77. Krøigård T, Schrøder HD, Qvortrup C, et al. Characterization and diagnostic evaluation of chronic polyneuropathies induced by oxaliplatin and docetaxel comparing skin biopsy to quantitative sensory testing and nerve conduction studies. Eur J Neurol. 2014;21(4):623–629. doi:10.1111/ene.12353
78. Argyriou AA, Park SB, Islam B, et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. 2019;90(12):1361–1369. doi:10.1136/jnnp-2019-320969
79. McCrary JM, Goldstein D, Boyle F, et al. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): a systematic review and Delphi survey. Support Care Cancer. 2017;25(11):3485–3493. doi:10.1007/s00520-017-3772-y
80. Hwang MS, Lee HY, Choi TY, et al. A systematic review and meta-analysis of the efficacy of acupuncture and electroacupuncture against chemotherapy-induced peripheral neuropathy. Medicine. 2020;99(17):e19837. doi:10.1097/MD.0000000000019837
81. Xu Z, Wang X, Wu Y, Wang C, Fang X. The effectiveness and safety of acupuncture for chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Front Neurol. 2022;13:963358. doi:10.3389/fneur.2022.963358
82. Pei LX, Yi Y, Guo J, et al. The effectiveness and safety of acupuncture/electroacupuncture for chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Acupunct Med. 2022:096452842210765. doi:10.1177/09645284221076512
83. Chien TJ, Liu CY, Fang CJ, Kuo CY. The efficacy of acupuncture in chemotherapy-induced peripheral neuropathy: systematic review and meta-analysis. Integr Cancer Ther. 2019;18:1534735419886662. doi:10.1177/1534735419886662
84. Wang C, Chen S, Jiang W. Treatment for chemotherapy-induced peripheral neuropathy: a systematic review of randomized control trials. Front Pharmacol. 2022;13:1080888. doi:10.3389/fphar.2022.1080888
85. Yang XY, Xia WY, Xu YY, et al. Patients’ expectancy scale of acupuncture: development and clinical performance test. Complement Ther Clin Pract. 2023;53:101797. doi:10.1016/j.ctcp.2023.101797
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


