Back to Journals » Breast Cancer: Targets and Therapy » Volume 16
Therapy for Hormone Receptor-Positive, Human Epidermal Growth Receptor 2-Negative Metastatic Breast Cancer Following Treatment Progression via CDK4/6 Inhibitors: A Literature Review
Authors Ye M, Xu H, Ding J , Jiang L
Received 2 September 2023
Accepted for publication 16 January 2024
Published 10 April 2024 Volume 2024:16 Pages 181—197
DOI https://doi.org/10.2147/BCTT.S438366
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pranela Rameshwar
Meixi Ye,1 Hao Xu,1,* Jinhua Ding,2,* Li Jiang3
1The Affiliated Lihuili Hospital, Ningbo University, Ningbo, 315040, People’s Republic of China; 2Department of Breast and Thyroid Surgery, Ningbo Medical Center Lihuili Hospital, Ningbo, 315040, People’s Republic of China; 3Department of General Practice, Ningbo Medical Center Lihuili Hospital, Ningbo, 315040, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Jiang, Department of General Practice, Ningbo Medical Center Lihuili Hospital, Road Jiangnan, Yinzhou Distract, Ningbo, 315040, People’s Republic of China, Tel +86 13957498692, Email [email protected]
Abstract: Endocrine therapy (ET) with a cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) is currently the first-line standard treatment for most patients with hormone receptor-positive (HR+) and human epidermal growth receptor 2-negative (HER2-) metastatic or advanced breast cancer. However, the majority of tumors response to and eventually develop resistance to CDK4/6is. The mechanisms of resistance are poorly understood, and the optimal postprogression treatment regimens and their sequences continue to evolve in the rapidly changing treatment landscape. In this review, we generally summarize the mechanisms of resistance to CDK4/6is and ET, and describe the findings from clinical trials using small molecule inhibitors, antibody-drug conjugates and immunotherapy, providing insights into how these novel strategies may reverse treatment resistance, and discussing how some have not translated into clinical benefit. Finally, we provide rational treatment strategies based on the current emerging evidence.
Keywords: breast cancer, human epidermal growth receptor 2, hormone receptor, cyclin- dependent kinases 4 and 6 inhibitor, endocrine resistance, molecular mechanism
Introduction
In 2020, breast cancer became the most common malignancy worldwide, with 2.3 million newly diagnosed cases, accounting for approximately 11.7% of all newly diagnosed cancers.1 Among the four molecular subtypes of breast cancer, hormone receptor-positive and human epidermal growth receptor 2-negative (HR+/HER2-) breast cancer constitute approximately 70%.2,3 Although endocrine therapy (ET) is the mainstay therapy for HR+/HER2- metastatic breast cancer (mBC) or advanced breast cancer (ABC),4 a large proportion of patients eventually develop resistance to ET.5 Due to the expanding body of knowledge on the biological complexity of breast cancer, treatment options for HR+/HER2- mBC have increased. Currently, three CDK4/6 inhibitors (CDK4/6i) palbociclib (Ibrance), ribociclib (Kisqali), and abemaciclib (Verzenio) have been approved by the Food and Drug Administration (FDA) due to their greater efficacy, oral availability, and relatively low toxicity profile.
As the standard of care for first-line therapy, the combination of CDK4/6i with an aromatase inhibitor (AI) significantly prolonged the median progression-free survival (mPFS) from 12–14 months to more than 25 months (as observed in PALOMA-2, MONALEESA-2 and MONARCH-3 studied).6–8 The MONARCH-2, PALOMA-3 and MONALEESA-3 studies demonstrated that the combination of fulvestrant with CDK4/6is doubled the mPFS compared to that of fulvestrant alone after progression on prior AIs.9–11 However, most patients whose tumors ever respond to CDK4/6i eventually develop acquired resistance.12 After progression, there are still no established guidelines for the optimal treatment options, because the mPFS is five to seven months when ET plus targeted therapy, including the inhibitors to the PI3K-AKT-mTOR signaling pathway, CDK4/6 inhibitor not the same as the prior. Generally, post-CDK4/6i therapies include the continuation of CDK4/6 inhibition, novel ET agents, and combinations of ET with other targeted agents, including everolimus, alpelisib and so on, chemotherapy and antibody-drug conjugate (ADC).
In this review, we will generally summarize the possible mechanisms of CDK4/6i resistance, mainly focusing on therapeutic strategies for reported clinical trials that have the potential to transform our treatment paradigm over the next decade. Treatment-related adverse effects and potential predictive biomarkers are also explored in this review.
Resistance Mechanisms to CDK4/6i
In the first-line therapy for the patients with HR+/HER2- mBC, the efficacy of three CDK4/6is including palbociclib, ribociclib, and abemaciclib is similar, with the mPFS of 25–28 months and hazard ratios (HRs) of 0.54–0.58 compared to AI alone.6–8 Several clinical studies have attempted to identify molecular biomarkers that can predict the response to the combination of CDK4/6i with ET in HR+/HER2- ABC patients, and a number of candidates have ever been identified; however, none of these methods have been sufficiently validated for clinical use. The mechanisms of resistance to CDK4/6i blockade are mainly studied in vitro and preclinical, and no specific mechanism was revealed between three CDK4/6is. Generally, the underlying mechanisms include aberrations affecting cell cycle progression and the activation of other signaling pathways (Figure 1). The alterations that affect cell cycle mediators include loss-of-function alterations in retinoblastoma (RB)13–15 and upregulation or overexpression of CDK6,16 cyclin E1/E2,12 CDK2,13,15,17 E2F,18 CDK4,19–21 WEE1,22 MDM2,23 and INK4,24 loss of FZR1,25 and FAT1.26 Activation of the PAM signal pathway is known to be a crucial factor in resistance to ET with CDK4/6i.13,27 Additionally, the loss of ER or PR expression,25 increased transcriptional activity of AP-1,13,15,17,27 Smad 3 suppression,28 autophagy activation,29 and immune-related mechanisms are also suggested to be involved in the resistance to CDK4/6i. Given the differential properties of the three CDK4/6is, which may influence the mode of action, the underlying mechanisms of resistance may potentially vary. Data from such research are awaited. The biomarker analysis of the MAINTAIN and PACE trials may reveal the potential differences in different CDK4/6is. Notably, the continuous dosing of CDK4/6 inhibitors may be required to maintain cell cycle arrest and proliferation inhibition.
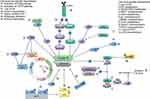 |
Figure 1 The key mechanisms for the resistance to CDK4/6 inhibitors. |
Possible Strategies to Overcome Resistance
Continuation of CDK4/6i Treatment with ET
The combination of CDK4/6is with ET is well accepted as the preferred option for HR+/HER2-mBC patients without visceral crisis or life-threatening disease.30–32 It is currently unclear whether continuation of CDK4/6 inhibition after prior therapy with CDK4/6is and ET is beneficial, given the previous results were inconsistent.
A real-world study (RWS) investigated 839 patients who received CDK4/6i as a first-line treatment.33 The unadjusted mPFS for patients who received CDK4/6i as second-line therapy was 8.25 months, and that for those who received chemotherapy, fulvestrant, or everolimus was 3.71, 3.25 and 3.32 months, respectively. Notably, 74.5% (229/308) of patients in this cohort continued to receive the same CDK4/6i regimen in the second-line setting, as was in the first-line treatment. Similarly, a single-institution study reported an estimated 10.3-month mPFS with palbociclib-fulvestrant as the second-line therapy among patients who had progressed on palbociclib-AI treatment.34 In a multicentre cohort, after progression on palbociclib, 71.3% of patients received non-sequential therapy with another CDK4/6i abemaciclib, and the mPFS was 5.3 months and the mOS was 17.2 months, which were notably similar to the results of abemaciclib monotherapy in heavily pretreated, CDK4/6i-naïve, HR+/HER2- patients in the MONARCH-1 study.35
The Phase II MAINTAIN study (NCT02632045) assessed the ongoing use of ribociclib or not plus fulvestrant or exemestane after progression on CDK4/6i. Palbociclib and ribociclib were previously used by 87% and 13%, respectively, of the patients in this study. The ribociclib-based regimen demonstrated a clinical benefit rate (CBR) of 43% and an mPFS of 5.29 months, while fulvestrant or exemestane alone had a CBR of 25% and an mPFS of 2.76 months, and these two regimens were significantly different (HR=0.57, 95% CI=0.39–0.95, p=0.006).36 In a subset analysis, the PFS benefit of ribociclib in the second line was statistically significant in patients who had palbociclib as first-line therapy (n = 103, HR 0.58, 95% CI 0.38–0.90) but not in patients who had received ribociclib in the first line (n = 14, HR 0.50 95% CI 0.15–1.70).
However, in the phase II PACE study (NCT03147287), patients who were receiving a CDK4/6i first-line therapy for at least 6 months were randomized to receive fulvestrant (Group A) or fulvestrant and palbociclib (Group B) or fulvestrant, palbociclib, and avelumab (Group C), and there was no significant difference in the mPFS between Group A (4.8 months) and Group B (4.6 months).37 Notably, 94.5% and 91.9% of patients in Group A and Group B respectively, had previously received palbociclib therapy.
The phase II BioPER single-arm study enrolled 33 HR+/HER2-mBC patients who had prior clinical benefit from palbociclib plus ET. Patients continued palbociclib but had switched their ET, and the CBR was 34%, with an mPFS of 2.6 months.38 Two other studies (NCT03809988 and NCT02738866) also evaluated the effect of the continuation of the same CDK4/6i regimen as the previously used and switched to different ET drugs.
Currently, it is unclear whether it would be more appropriate to continue treatment with the same CDK4/6i, or switch to another CDK4/6i in the second-line setting. The PALOMA-2 and PACE data suggest that palbociclib may have different effects than other CDK4/6is. It may be reasonable to continue CDK4/6is at the time of progression, especially in patients treated with palbociclib in the first-line setting; however, additional data are needed to confirm this as a treatment strategy. Further understanding of tumor mutations and mechanisms of resistance to endocrine resistance may allow us to target patients who would benefit from continuing CDK4/6i therapy.
Continuation of CDK4/6i with Targeted Therapy
TRINITI-1 is a Phase II, multicentre, single-arm study used to explore the effect of triple therapy (25mg exemestane, 300mg ribociclib, and 2.5mg everolimus daily) after progression on a CDK4/6i in HR+/HER2- mBC.39 The clinical benefit rate (CBR) among 95 efficacy-evaluable patients at week 24 was 41.1% (95% CI, 31.1–51.6%), which met the primary endpoint, and the mPFS was 5.7 months. The acceptable safety profile and preliminary efficacy results of the TRINITI-1 study provide support for further investigations of larger sample sizes, randomized, controlled trials.
The phase II PACE study (NCT03147287) is also designed to evaluate the efficacy of ongoing treatment with CDK4/6i plus fulvestrant with or without avelumab following progression on CDK4/6i plus AI. The participants were randomly assigned to three groups: Group A (fulvestrant alone), Group B (fulvestrant plus palbociclib), Group C (fulvestrant, palbociclib and avelumab combination).37 The results showed an mPFS of 8.1 (95% CI 3.2–10.7) months in Group C and 4.8 (95% CI 2.1–8.2) months in Group A (p=0.23). There was no significant difference between Group C and Group A; however, a numerical increase in the mPFS indicated that the benefit may result from the addition of avelumab and palbociclib, which needs to be further validated in a randomized, controlled, Phase III study. Table 1 shows clinical trials or retrospective studies that target cyclin D1-CDK4/6 complex after CDK4/6i progression in the patients with HR+/HER2- mBC.
 |
Table 1 Clinical Trials or Retrospective Studies Targeting cyclinD1-CDK4/6 Complex |
Inhibitors Targeting the PAM Signaling Pathway
PI3K Inhibitors
Thus far, the most successful PI3K inhibitor clinically available is alpelisib. Phase IB trials have shown that treatment with combination of alpelisib with ET results in clinical benefit in HR+/HER2- mBC patients with acquired resistance to ET, particularly in the PIK3CA mutated cancers.40,41 The phase III SOLAR-1 trial showed that, in the patients with PIK3CA mutations, the mPFS was longer in the alpelisib plus fulvestrant group than in the fulvestrant monotherapy group (11.0 vs 5.7 months, HR=0.65; 95% CI=0.50–0.85; p<0.001).42 However, in this study, only 20 patients were previously treated with CDK4/6i.
The BYLieve trial assessed the activity of the combination of alpelisib with ET in HR+/HER2- mBC patients with PIK3CA mutations who were previously exposed to CDK4/6is.43 Cohort A included 121 patients who had progressed on prior CDK4/6i plus AI, and were treated with alpelisib plus fulvestrant. The results showed that 54.6% of patients were free from progression at 6 months, and the mPFS was 7.2 (95% CI 5.3–9.2) months. Efficacy data of alpelisib in cohort A compared well with that of SOLAR-1, where 44.4% of patients were free of progression at 6 months. These data indicate that the PI3K inhibitor alpelisib is an ideal option in the patients with PIK3CA mutations who had a progression on CDK4/6i therapy.
mTOR Inhibitors
In a Phase III, multicentre, randomized BOLERO-2 trial, in the patients with HR+/HER2- mBC progression on a nonsteroidal AI, everolimus plus exemestane significantly improved the mPFS compared to exemestane plus placebo (10.6 vs 4.1 months).44,45 However, none of patients in the trial had previously been treated with CDK4/6i.
To date, only two retrospective studies have evaluated the efficacy of everolimus plus exemestane in the patients with HR+/HER2- mBC who were pretreated with CDK4/6i. However, the results are controversial. In a small-sample study, the mPFS of patients who used prior CDK4/6i (n=16) or not (n=27) was 3.6 vs 4.2 months (HR=1.22, 95% CI=0.65–2.28, p=0.538), respectively; and the mOS was 15.6 vs 11.3 months (HR=0.70, 95% CI=0.35–1.40, p=0.308), respectively.46 However, in the study containing 192 patients with HR+/HER2- mBC, 79 patients received prior CDK4/6i use, while 113 patients did not. Patients with prior CDK4/6i use had a shorter mPFS of 3.8 (95% CI: 3.4–4.7) months than patients without prior CDK4/6i use (5.4 (95% CI: 3.9–6.2) months), with an HR for progression of 1.46 (95% CI: 1.08 to 1.97, p = 0.013). Moreover, the mOS was not significantly different between groups.47
The above results suggest that an mPFS of 4 months may be the most likely benefit of everolimus for patients with prior CDK4/6i use. Compared to patients with an mPFS of 7 months or more who benefit from PI3K inhibitors or AKT inhibitors, it seems reasonable that everolimus should be used in later line.
AKT Inhibitor (AKTi)
Capivasertib (AZD5363), a novel oral pan-Akt kinase inhibitor, has demonstrated promising results in preclinical studies. In a Phase I study, capivasertib plus fulvestrant had an mPFS of 2.7 months in patients with PTEN-mutant ER+ mBC, and an objective response rate (ORR) of 16% in all ER+ mBC patients.48 In a phase II FAKTION study (NCT01992952), the combination of capivasertib with fulvestrant significantly extended the mPFS in patients with endocrine-resistant HR+/HER2- mBC. The mPFS was 10.3 months for capivasertib and 4.8 months for the placebo (HR=0.57; 95% CI=0.39–0.84; one-sided p=0.0017; two-sided 0.0035).49,50
The phase III study CAPItello-291 (NCT04305496) confirmed the results of FAKTION. In the ITT population (n=708), the mPFS was 7.2 (95% CI 5.5–7.4) months in the capivasertib plus fulvestrant group versus 3.6 (95% CI 2.8–3.7) months in the fulvestrant group; HR=0.60 (95% CI 0.51–0.71), p<0.001; and in the AKT pathway-altered population (n=289), the mPFS was 7.3 (95% CI 5.5–9.0) and 3.1 (95% CI 2.0–3.7) months in the cavasertib plus fulvestrant group and fulvestrant group, respectively; and HR=0.50 (95% CI 0.38–0.65), p<0.001.51 Importantly, 69% (n=489) of patients in the ITT population and 70.6% (n=204) of patients in the AKT pathway- altered population had previously received CDK4/6i for advanced disease. These results suggest that capivasertib plus fulvestrant has the potential to be a future treatment option for patients with HR+ ABC who have progressed on an endocrine-based regimen that included CDK4/6i.
Another AKTi, ipatasertib, was also evaluated in the patients with HR+/HER2- mBC following progression on prior CDK4/6i use. In the phase I TAKTIC study (NCT03959891), the patients were treated with ipatasertib plus AI, or ipatasertib plus fulvestrant, or ipatasertib, fulvestrant plus palbociclib. In the phase III FINER study (NCT04650581), patients with HR+/HER2- mBC who received a CDK4/6i as first line therapy were randomized to the ipatasertib plus fulvestrant group or control group. The above two trials are still ongoing, and the results may reveal the real value of the AKT inhibition in the patients who progressed on prior CDK4/6i therapy.
However, the regimen containing AKTi, CDK4/6i and ET should also be evaluated in this standard population who are resistant to first-line CDK4/6i treatment, because of the side effect of certain ATKi is also well-tolerable. Table 2 shows the clinical trials or retrospective studies that target the PAM signaling pathway after CDK4/6i progression in patients with HR+/HER2- mBC.
 |
Table 2 Clinical Trials or Retrospective Studies of Targeting PI3K-AKT-mTOR Pathway |
Antibody Drug Conjugate (ADC)
ADC is a kind of molecule that consists of a recombinant monoclonal antibody that is bound to a cytotoxic drug (called a payload) via a synthetic linker.52 Currently, several ADCs are under clinical investigation for breast cancer treatment, and the targets are as follows: HER2 (drug: T-DM1, trastuzumab deruxtecan (T-DXd), SYD985, A166, ARX788, RC-48), HER3 (drug: U3-1402), zinc transporter LIV1 (drug: SGN-LIV1A), trophoblast cell-surface antigen (Trop-2) (drug: sacituzumab govitecanserve, SG) and receptor tyrosine kinase-like orphan receptor 2 (drug: CAB-ROR2-ADC).
The Trop-2 protein is highly expressed in nearly 80% of breast cancers, regardless of the molecular type.53,54 SG is a first-in-class Trop-2-directed, apparent diffusion coefficient ADC, that has high antitumor activity.55–59 The phase III TROPICS-02 study assessed the clinical value of SG in HR+/HER2- mBC patients who ever received at least one line of ET and two to four lines of chemotherapy for metastatic disease. In this study, more than 98% of patients have been pretreated with CDK4/6i. The primary endpoints of mPFS were 5.5 (95% CI 4.2–7.0) and 4.0 (95% CI 3.1–4.4) months in patients with SG and with TPC, respectively, and there was a significant difference (HR=0.66, 95% CI 0.53–0.83, p=0.0003).60 At the second interim analysis, which was presented at the 2022 European Society for Medical Oncology (ESMO) annual meeting, there was an absolute benefit of 3.2 (14.4 [95% CI 13.0–15.7]-11.2 [95% CI 10.1–12.7]) months with a 21% reduction in the risk of death between the two groups, and the difference was significant. Additionally, the safety profile of SG was manageable and consistent with that in previous studies. Therefore, SG demonstrated a statistically significant and clinically meaningful benefit and should be considered as a potential treatment option in heavily pretreated patients with limited treatment options. This is especially true for patients who have received CDK4/6i for metastatic disease.
In a phase Ib study, a cohort of 54 patients with low HER2 expression were treated with T-DXd, and an mPFS of 11.1 months and an ORR of 37.0% were reported.61 A phase III DESTINY-Breast04 study (NCT03734029) was conducted to evaluate the role of T-DXd in mBC patients with HER2-low expression who received 1–2 lines prior lines of chemotherapy in the metastatic settings.62 Among 480 HR+/HER2-low expression mBC patients, approximately 70% had received CDK4/6i for metastatic disease. The mPFS in this population was 10.1 versus 5.4 months in the T-DXd group and TPC group, respectively, and the difference was statistically significant (HR=0.51, 95% CI=0.40–0.64, p<0.001).62 In addition, the confirmed ORR in HR+/HER2-low patients treated with T-DXd was 52.6%, which was significantly greater than that in patients treated with TPC (16.3%). Therefore, the DESTINY-Breast04 study established T-DXd as the standard of care for HR+/HER2-low mBC patients, especially for those who received CDK4/6i and 1–2 prior lines of chemotherapy for metastatic setting.
Oral Selective Estrogen Receptor Degrader (SERD)
Fulvestrant shows efficacy in tamoxifen-refractory patients, and patients with ESR1-mutated HR+ mBC who have progressed on prior AIs.63 Emerging data have shown that Fulvestrant is the most effective endocrine monotherapy for patients with HR+/HER2-naive mBC.64 However, the role of fulvestrant in HR+/HER2-refractory mBC patients who experience disease progression after treatment with CDK4/6is has rarely been investigated in clinical studies. Oral SERDs are a promising class of drugs because of their ability to overcome ESR1 mutations, oral delivery, and improved bioavailability, and they are being investigated as monotherapies and in combination with CDK4/6i and other drugs such as everolimus (Table 3).
 |
Table 3 Other Clinical Trials on CDK4 Inhibitors Progression |
EMERALD is an international, multi-center, randomized, open-label, phase III study that enrolled postmenopausal patients with ER+/HER2- mBC via randomization to the elacestrant group or standard of care (SOC) group.65 Progression on previous CDK4/6is was needed. Elacestrant patients demonstrated a significant improvement in mPFS compared with SOC both in the overall population (2.8 vs.1.9 months, HR=0.70; 95% CI=0.55–0.88; p=0.002) and in patients with ESR1 mutations (3.8 vs.1.9 months, HR=0.55; 95% CI, 0.39–0.77; p=0.0005).
The SERENA-2 study (NCT04214288) was designed to explore the efficacy and safety of a range of camizestrant doses administered once daily as a monotherapy in comparison with fulvestrant. Among the patients who received CDK4/6i as a previous systemic therapy for metastatic disease, the mPFS was 5.5 (95% CI 3.7–10.9), 3.8 (95% CI 2.0–7.6) and 2.1 (95% CI 1.9–3.7) months in the camizestrant 75mg group, camizestrant 150mg group and fulvestrant 500mg group, respectively. A clinically meaningful improvement in PFS was observed in patients post-CDK4/6i, which requires to be further validated in more patients who progressed on CDK4/6is.66
In contrast, two other phase II AMEERA-3 and aceIERA trials showed no significant difference in PFS between patients with oral SERDs and those with TPC.67,68 Notably, approximately 80% of patients in the AMEERA-3 trial and 40% of patients in the aceIERA trial were treated with CDK4/6i therapy previously for metastatic disease. Thus, additional clinical trials are needed to further investigate the role of oral SERDs in the patients with a progression on CDK4/6i.
The above trials involved oral SERD monotherapy, and there was heterogeneity across trials in terms of the proportion of patients with ESR1 mutations. The ESR1-mutant subgroup derived a greater benefit than the ESR1-wild-type subgroup,68 which suggests that the ESR1 mutation may be a potential biomarker for identifying ongoing ER dependence in this pretreated population of patients. Additionally, since the continuation of CDK4/6 inhibition and oral SERDs has been proven effective in CDK4/6- pretreated HR+/HER2- ABC, the combination of CDK4/6i and oral SERD should also be further investigated in such populations.
Chemotherapy
In the metastatic setting, chemotherapy alone or in combination with a PD-1/PD-L1 inhibitor or PARP inhibitor is the preferred treatment for triple-negative breast cancer, and is often used in combination with HER2-targeted therapy for HER2-positive breast cancer. Although CDK4/6i plus ET is considered as the standard of care for HR+/HER2- mBC, most of patients whose tumors are ever sensitive to this therapy eventually develop to CDK4/6i resistance. In addition to the drugs mentioned above, chemotherapy is still an important and essential option for the patients who progress on CDK4/6i. As observed in the main clinical trials of CDK4/6is, which include PALOMA-2/3, MONALEESA-2/3, and MONARCH-2/3, more than 40% of patients choose chemotherapy as their next-line therapy.6–11 In addition, in the DESTINY-Breast 04 study and TROPICS-02 study, the patients had received 1–2 lines or 2–4 lines of chemotherapy before entering the studies. Above all these findings demonstrate that chemotherapy is an effective therapeutic option for HR+/HER2- mBC patients even if they have been previously pretreated with CDK4/6i. Classic drugs, such as anthracyclines69 and taxen,70 and new chemotherapeutics such as capecitabine plus vinorelbine, eribulin and utidelone, have been shown to be effective for treating ABC in clinical trials.71–73 Therefore, we now have more choices for first- or later-line chemotherapy.
Other Potential Strategies
Poly (ADP-Ribose) Polymerase Inhibitor (PARPi)
PARPis such as olaparib and talazoparib have been approved by the FDA for mTNBC patients with gBRCA mutations, due to their meaningful therapeutic effects and manageable side effects in clinical studies.74,75 However, the patients enrolled in these studies had not previously received any prior CDK4/6i. Recently, the phase II TBCRC 048 study evaluated the activity of olaparib in 54 mBC patients (75% ER+/HER2-, 19% TNBC, and 6% other subtypes) with germline mutations associated with homologous recombination defects (HRDs) other than those in BRCA (cohort 1) and somatic mutations, including somatic BRCA1/2 mutations (cohort 2).76 Confirmed responses were observed only for germline PALB2 (ORR, 82%) and somatic BRCA1/2 (ORR, 50%) mutations. Notably, 93% (38/41) of the HR+/HER2- mBC patients received CDK4/6i before tumor progression, and the ORR was 77.7% (7/9) for patients with germline PALB2 mutations and 40% (4/10) for those with somatic BRCA1/2 mutations, respectively. The study suggested that PARPis are effective treatment for mBC patients with gPALB2 or sBRCA1/2 mutations, even if they have been treated with CDK4/6i.
CDK2 Inhibitor
Recent studies have demonstrated the role of the cyclin E-CDK2-Rb cascade, which regulates the late G1/S cell cycle transition, in BC tumorigenesis and endocrine resistance.77–79 Compared with palbociclib and letrozole alone, preclinical studies have demonstrated increased efficacy of the non-selective CDK2 inhibitor dinaciclib concurrently with palbociclib and letrozole.80 More specific CDK2 inhibitors are currently under evaluation in early-phase trials enrolling patients with solid tumors including ER+/HER2- breast cancer. These included PF-07104091 (NCT04553133 and NCT05262400), BLU-222 (NCT05252416), and a CDK2/4/6 inhibitor PF-06873600 (NCT03519178).
CDK7 Inhibitor
CDK7 has dual roles in regulating the cell cycle and transcription; thus, it is considered as a potential target for cancer therapy. Several selective inhibitors of CDK7, including SY-1365, SY-5609, and XL102, have now progressed to phase I/II clinical trials for mBC. NCT03134638 is a phase I study used to evaluate the safety and antitumor activity of SY-1365 in patients with select solid tumors including breast cancer, ovarian cancer and other solid cancers. Cohort 5 included 12 patients with HR+ mBC who progressed on CDK4/6i plus ET treatment, and investigated the benefit of combining SY-1365 with fulvestrant in this setting. However, the study was terminated for business reasons. NCT04247126 is a study that explored the role of SY-5609 in metastatic solid cancer. In Group 2 Part 1, patients with HR+/HER2-mBC post-CDK4/6i treatment were enrolled, and the study is ongoing. Another ongoing study (NCT04726332) on the CDK7 inhibitor XL102 is also to evaluate in HR+/HER2- mBC patients who progressed on CDK4/6i.
BCL2 Inhibitor
B cell lymphoma 2 (BCL2) is overexpressed in approximately 80% and 70% of ER+ primary and metastatic breast cancers, respectively.81,82 A preclinical study showed that venetoclax (a kind of BCL2 inhibitor) may be effective for treating HR+ mBC.83 The phase II VERONICA study (NCT03584009) compared venetoclax plus fulvestrant with fulvestrant alone in patients with ER+/HER2- LABC or mBC, following disease progression on a CDK4/6i and ET. The results showed that venetoclax plus fulvestrant did not significantly improve the CBR (11.8% vs 13.7%) or the mPFS (2.69 vs 1.94 months) when compared with fulvestrant alone.84 These results do not support for the clinical utility of venetoclax plus fulvestrant in treating CDK4/6i-refractory mBC. However, additional studies are still needed to explore the role of BCL2 inhibitors in CDK4/6 resistant HR+/HER2- mBC.
Loss of RB,13,15,17,85 CDK6 overexpression,16 CCNE1 overexpression,76 p16 amplification,86 fibroblast growth factor receptor 1 (FGFR1) amplification,87,88 mitotic aurora kinase (AURKA) amplification,89 E2F amplification,90 and cyclin-dependent kinase 2 (CDK2) overexpression13,15,17,91 have also been reported to be associated with CDK4/6i resistance. Hence, the inhibition of these targets may be effective for patients with CDK4/6i resistance, and the inhibitors for the above targets associated with CDK4/6i resistance are still in development in clinical trials. Table 3 demonstrates the ongoing or completed clinical trials on special targets in addition to the cyclin-D1/CDK4/6 complex and the PAM signaling pathway.
Predict Response of Biomarkers
After progression on CDK4/6i, increasing amounts of attention has been focused on the PAM signaling pathway, because it is activated in approximately >70% of HR+ mBC. This pathway includes PI3K mutation (hyperactivation of the alpha isoform (p110α) of phosphatidylinositol 3-kinase),92,93 AKT1 mutations, loss of PTEN function, or loss of the regulatory function of proteins TSC1/TSC2. Thus, biomarker analyses on this signaling pathway may provide definite evidence for targeted therapy.
In the phase III SOLAR-1 trial, the mPFS in the patients with PIK3CA mutations was 11.0 (95% CI, 7.5–14.5) and 5.7 (95% CI, 3.7–7.4) months in the alpelisib-fulvestrant group and control group, respectively; moreover, there were significant differences (HR=0.65; 95% CI=0.50–0.85; p<0.001); however, the mPFS in the patients without PIK3CA mutation was 7.4 (95% CI, 5.4–9.3) and 5.6 (95% CI, 3.9–9.1) months in the alpelisib-fulvestrant group and control group, with an HR of 0.85, 95% CI, 0.58–1.25).42 The alpelisib-based regimen showed greater therapeutic benefit in HR+/HER2- mBC patients with PIK3CA mutations than in those without PIK3CA mutations. Encouraged by the success of the SOLAR-1 study, additional prospective studies of PI3K inhibitors are in progress for the patients with PIK3CA mutation, including the BYLieve study (NCT03056755), the TAKTIC study (NCT03959891), are in progress for patients with PIK3CA mutations, and the FINER study (NCT04650581).
The results of the FAKTION study revealed an mPFS of 9.5 months in the pathway-altered subgroup for patients receiving capivasertib (n=28), compared with an mPFS of 5.2 months in the placebo group (n=31; HR 0.59 [95% CI 0.34–1.03]; p=0.0064); and an mPFS of 10.3 months in the pathway non-altered subgroup for patients receiving capivasertib (n=43), compared with an mPFS of 4.8 months in the placebo group (n=38; HR 0.56 [95% CI=0.33–0.96]; p=0.0035). These results indicate that patients with HR+/HER2- mBC who progress after receiving prior AI therapy may benefit from the addition of capivasertib to fulvestrant regardless of pathway status. However, the primary analysis identified PI3K/AKT/PTEN pathway-altered tumors through tests that had suboptimal sensitivity or were unable to identify all activating PIK3CA mutations (ddPCR), and 59 (42%) of the patients were identified as pathway-altered participants. In the updated analysis, an exploratory subgroup analysis was performed in which an expanded genetic testing panel, next- generation sequencing (NGS), was used to identify participants with PAM pathway-altered tumors with increased accuracy. The expanded pathway-altered subgroup accounted for 76 (54%) of the 140 participants in the ITT population. Subsequent subgroup analysis revealed a significant improvement in PFS in response to capivasertib in the expanded pathway-altered subgroup (12.8 vs 4.6 months, HR=0.44, 95% CI=0.26–0.72, p=0.001); however, this improvement was not observed in the expanded pathway non-altered subgroup (7.7 vs 4.9 months, HR=0.70, 95% CI =0.40–1.25, p=0.23).50 The expanded biomarker results suggest that genomic tumour profiling will be needed to accurately identify the approximately 50% of patients whose tumors carry relevant PI3K/AKT/PTEN pathway alterations and will most benefit from capivasertib.
According to exploratory biomarker analyses of the TRINITI study, 89 efficacy-evaluable patients had a baseline ctDNA biomarker assessment.39 A panel containing 566 genes was used, and the results showed that the frequencies (exceeding 10%) of the mutations were as follows: PIK3CA (33.7%), ESR1(33.7%), TP53 (19.1%), KMT2C (15.7%), FOXP1 (12.4%), RB1 (11.2%), and ATM (10.1%). Concomitant PI3KCA and ESR1 mutations were found in 14 patients (15.7%). There was a similar CBR at week 24 (36.7% vs 36.7%) among patients with PI3KCA mutations and those with ESR1mutations, respectively. A trend towards longer mPFS was found in patients with either wild-type (wt)PIK3CA or wtESR1 at baseline compared with those who with a mutation in the respective gene; and patients with both wtPIK3CA and wtESR1 at baseline had a numerically longer median PFS than patients who had at least one mutated PIK3CA or ESR1 gene. Biomarker analysis suggested that the patients with either or both of PIK3CA and ESR1 mutations who had progressed on prior CDK4/6i treatment may had a relatively worse survival even if they received a triple combination regimen comprising CDK4/6i, an mTOR inhibitor and ET.
Treatment Related Adverse Effects (TRAEs)
Gastrointestinal disorders, including nausea, vomiting, diarrhea and constipation were the common TRAEs in all the studies. Of course, gastrointestinal disorders are more common in the patients treated with targeted therapy (PI3K-AKT-mTOR inhibitors or ADCs).42,43,46,47,49,50,60,62 Additionally, TRAEs are also specific. Hyperglycemia and hypertension are mainly exhibited in PI3K-AKT-mTOR inhibitors, whereas hematological toxicity occurs more in SG-treated patients. Any more grade of TRAEs than 10% are demonstrated in Tables 4 and 5.
 |
Table 4 Clinical Trials on Oral SERDs After CDK4/6i Progression |
 |
Table 5 Clinical Trials of ADCs and Inhibitors Targeting PI3K-AKT-mTOR |
The most common grade 3–4 AEs in were hyperglycemia (36.6%), rash (9.9%), maculopapular rash (8.8%), and diarrhea (6.7%) in the alpelisib-fulvestrant group.42,43 Similarly, the most common grade 3–4 AEs were hypertension (32%), diarrhoea (14%), and rash (20%) in the capivasertib-treated group.46,47 The AEs of everolimus seemed to be different, which showed grade 3–4 stomatitis (8%), anemia (6%), dyspnea (4%), hyperglycemia (4%), fatigue (4%), and pneumonitis (3%) in the everolimus-exemestane group.44 In the T-DXd group, the most common AEs of grade 3 or higher were neutropenia (13.7%), anemia (8.1%), and fatigue (7.5%), leukopenia (6.5%).62 In addition, interstitial lung disease (ILD) remained an important risk factor associated with T-DXd, and occurred in 45 patients (12.1%) who received T-DXd, including 13 (3.5%) had grade 1, 24 (6.5%) had grade 2, 5 (1.3%) had grade 3, and 3 (0.8%) had grade 5 events.62 In the TROPICS-02 study, the most common grade 3 or higher treatment-related AEs (>5% incidence) were neutropenia (51%), leukopenia (9%), diarrhea (9%), anemia (6%), and fatigue (6%) in the SG group.60
Discussion
Due to the amazing success of prolonging the PFS and OS, CDK4/6 inhibitors have gradually been promoted from late- to first- and second-line treatment for patients with HR+/HER2- mBC. However, in the metastatic setting, secondary resistance eventually develops regardless of the effectiveness of a CDK4/6i. Currently, treatment with CDK4/6is is unsatisfactory. Thus, how to summarize the emerging prospective clinical trials to overcome CDK4/6i resistance is an urgent and important research topic. Given the diverse mechanisms of resistance, a one-size-fits-all approach may not always be appropriate.
Optimal sequencing of treatment options depends on the following issues: (1) the presence of specific molecular aberrations at a specific time point, such as acquired ESR1 mutations, or PI3K-AKT-mTOR pathway alterations; (2) the comparative efficacy of selected treatment relative to current gold standard treatment paradigms; (3) previous treatment history, duration of treatment response and patient’s physical status; and (4) the balance between maximizing survival benefits and quality of life (QOL), and financial and other toxicities during the whole treatment journey.
Therefore, based on the emerging evidence, we believe that rebiopsy of tumor is vital for future therapy. Molecular profiling, including ESR1 mutations, PI3K-AKT-mTOR pathway alterations may provide definite targets for therapy. For patients with ESR1 mutations, oral SERDs are the preferred option; for patients with PI3K-AKT-mTOR pathway alterations, the inhibitors of this signaling pathway including alpelisib, capivasertib, and everolimus-based regimens should be used. For patients without above definite mutations or alterations, or with a short duration of CDK4/6i treatment, chemotherapy and ADCs should be preferred considered. While earlier introduction of a highly efficacious drug, especially an ADC, may prolong PFS and OS to a greater extent, the impact on long-term toxicities, including financial burden, cost-effectiveness and QOL, should also be considered. Therefore, in current clinical practice, ADCs such as T-DXd and SG are often used after one or two lines of chemotherapeutic drugs.
With a better understanding of the mechanisms of CDK4/6i resistance, additional mutations leading to acquired mechanisms could be identified, and we can use these mutations as targets and develop drugs to treat these mutations, which could provide individualized treatment options. In the near future, targeted therapy to treat meaningful mutations could benefit patients more, and future treatments will be more precise.
Acknowledgments
I would like to thank Dr Yi Dai and Dr Yuxingzi Chen who have contributed substantially to the completion of this study, and they made a significant contribution to the work reported.
Funding
This work was funded by Medical Science and Technology Project of Zhejiang Province (2022KY1078 and 2023KY1030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Anderson WF, Chatterjee N, Ershler WB, et al. Estrogen receptor breast cancer phenotypes in the surveillance, epidemiology, and end results database. Breast Cancer Res Treat. 2002;76(1):27–36. doi:10.1023/A:1020299707510
3. Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107(6):djv048. doi:10.1093/jnci/djv048
4. Flaum LE, Gradishar WJ. Advances in endocrine therapy for postmenopausal metastatic breast cancer. Cancer Treat Res. 2018;173:141–154.
5. Hoffmann J, Bohlmann R, Heinrich N, et al. Characterization of new estrogen receptor destabilizing compounds: effects on estrogen-sensitive and tamoxifen-resistant breast cancer. J Natl Cancer Inst. 2004;96:210–218. doi:10.1093/jnci/djh022
6. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936. doi:10.1056/NEJMoa1607303
7. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738–1748. doi:10.1056/NEJMoa1609709
8. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi:10.1200/JCO.2017.75.6155
9. Gw S Jr, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–2884. doi:10.1200/JCO.2017.73.7585
10. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, Phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi:10.1016/S1470-2045(15)00613-0
11. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2- negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi:10.1200/JCO.2018.78.9909
12. Konecny GE, Winterhoff B, Kolarova T, et al. Expression of p16 and retinoblastoma determines response to CDK 4/6 inhibition in ovarian cancer. Clin Cancer Res. 2011;17:1591–1602. doi:10.1158/1078-0432.CCR-10-2307
13. Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi:10.1158/0008-5472.CAN-15-0728
14. Malorni L, Piazza S, Ciani Y, et al. A gene expression signature of retinoblastoma loss-of function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget. 2016;7:68012–68022. doi:10.18632/oncotarget.12010
15. Taylor-Harding B, Aspuria PJ, Agadjanian H, et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget. 2015;6:696–714. doi:10.18632/oncotarget.2673
16. Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene. 2017;36:2255–2264. doi:10.1038/onc.2016.379
17. Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget. 2014;5:6512–6525. doi:10.18632/oncotarget.2270
18. Dean J, Thangavel C, McClendon AK, et al. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29(28):4018–4032. doi:10.1038/onc.2010.154
19. Cen L, Carlson BL, Schroeder MA, et al. p16-Cdk4-Rb axis controls sensitivity to a cyclin-dependent kinase inhibitor PD0332991 in glioblastoma xenograft cells. Neuro Oncol. 2012;14(7):870–881. doi:10.1093/neuonc/nos114
20. Wu A, Wu B, Guo J, et al. Elevated expression of CDK4 in lung cancer. J Transl Med. 2011;9(1):38. doi:10.1186/1479-5876-9-38
21. Olanich ME, Sun W, Hewitt SM, et al. CDK4 amplification reduces sensitivity to CDK4/6 inhibition in fusion-positive rhabdomyosarcoma. Clin Cancer Res. 2015;21(21):4947–4959. doi:10.1158/1078-0432.CCR-14-2955
22. Matheson CJ, Backos DS, Philip Reigan P. Targeting WEE1 kinase in cancer. Trends Pharmacol Sci. 2016;37:872–881. doi:10.1016/j.tips.2016.06.006
23. Wander SA, Cohen O, Gong X, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor- positive metastatic breast cancer. Cancer Discov. 2020;10:1174–1193. doi:10.1158/2159-8290.CD-19-1390
24. Fujita T, Liu W, Doihara H, et al. Regulation of Skp2-p27 axis by the Cdh1/anaphase-promoting complex pathway in colorectal tumorigenesis. Am J Pathol. 2008;173(1):217–228. doi:10.2353/ajpath.2008.070957
25. Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell. 2018;34(6):893–905. doi:10.1016/j.ccell.2018.11.006
26. Li Q, Jiang B, Guo J, et al. INK4 tumor suppressor proteins mediate resistance to CDK4/6 kinase inhibitors. Cancer Discov. 2022;12(2):356–371. doi:10.1158/2159-8290.CD-20-1726
27. Jansen VM, Bhola NE, Bauer JA, et al. Kinome-Wide RNA interference screen reveals a role for PDK1 in acquired resistance to CDK4/6 inhibition in ER-positive breast cancer. Cancer Res. 2017;77(9):2488–2499. doi:10.1158/0008-5472.CAN-16-2653
28. Zelivianski S, Cooley A, Kall R, et al. CDK4-mediated phosphorylation inhibits Smad3 activity in cyclin D overexpressing breast cancer cells. Mol Cancer Res. 2010;8(10):1375–1387. doi:10.1158/1541-7786.MCR-09-0537
29. Vijayaraghavan S, Karakas C, Doostan I, et al. CDK4/6 and autophagy inhibitors induce senescence in Rb positive cytoplasmic cyclin E negative synergisticallycancers. Nat Commun. 2017;8:15916. doi:10.1038/ncomms15916
30. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol. 2018;29(8):1634–1657. doi:10.1093/annonc/mdy192
31. Boér K. Fulvestrant in advanced breast cancer: evidence to date and place in therapy. Ther Adv Med Oncol. 2017;9(7):465–479. doi:10.1177/1758834017711097
32. Hu X, Li T, Wang B, et al. Comparison of 4th ESO-ESMO international consensus guidelines for advance breast cancer and Chinese anti-cancer association committee of Breast Cancer Society guideline. Breast. 2019;45:36–42. doi:10.1016/j.breast.2019.02.009
33. Martin JM, Handorf EA, Montero AJ, et al. Systemic Therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441–446. doi:10.1093/oncolo/oyac075
34. Samuel Eziokwu A, Varella L, Lynn Kruse M, et al. Real-world outcomes of cyclin-dependent kinase inhibitors continued beyond first disease progression in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer. 2021;21(3):205–209. doi:10.1016/j.clbc.2020.09.010
35. Wander SA, Han HS, Zangardi ML, et al. Clinical outcomes with abemaciclib after prior CDK4/6 inhibitor progression in breast cancer: a multicenter experience. J Natl Compr Canc Netw. 2021:1–8. doi:10.6004/jnccn.2020.7662
36. Kalinsky K, Accordino MK, Chiuzan C, et al. A randomized, phase II trial of fulvestrant or exemestane with or without ribociclib after progression on anti-estrogen therapy plus cyclin- dependent kinase 4/6 inhibition (CDK4/6i) in patients(pts) with unresectable or hormone receptor-positive (HR+), HER2-negative metastatic breast cancer(MBC):MAINTAIN trial. J Clin Oncol. 2022;40(17):LBA1004.
37. L ME, Ren Y, Wagle N, et al. GS3-06 palbociclib after CDK4/6i and endocrine therapy (PACE): a randomized phase II study of fulvestrant, palbociclib, and avelumab for endocrine pre-treated er+/HER2- metastatic breast cancer. In:
38. Albanell J, M P-GJ, Gil-Gil M, et al. Palbociclib rechallenge for hormone receptor- positive/HER-negative advanced breast cancer: findings from the phase II BioPER trial. Clin Cancer Res. 2023;29:1):67–80. doi:10.1158/1078-0432.CCR-22-1281
39. Bardia A, Hurvitz SA, DeMichele A, et al. Phase I/II trial of exemestane, ribociclib, and everolimus in women with HR +/HER2 - advanced breast cancer after progression on CDK4/6 inhibitors (TRINITI-1). Clin Cancer Res. 2021;27(15):4177–4185. doi:10.1158/1078-0432.CCR-20-2114
40. Mayer IA, Abramson VG, Formisano L, et al. A phase Ib study of alpelisib (BYL719), a PI3Kα- specific inhibitor, with letrozole in ER+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23(1):26–34. doi:10.1158/1078-0432.CCR-16-0134
41. Juric D, Janku F, Rodón J, et al. Alpelisib plus fulvestrant in PIK3CA-altered and PIK3CA- wild type estrogen receptor-positive advanced breast cancer: a phase 1b clinical trial. JAMA Oncol. 2019;5(2):e184475. doi:10.1001/jamaoncol.2018.4475
42. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-mutated, hormone receptor- positive advanced breast cancer. N Engl J Med. 2019;380(20):1929–1940. doi:10.1056/NEJMoa1813904
43. Rugo HS, Lerebours F, Ciruelos E, et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): one cohort of a Phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22(4):489–498. doi:10.1016/S1470-2045(21)00034-6
44. Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor- positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi:10.1056/NEJMoa1109653
45. Yardley DA, Noguchi S, Pritchard KI, et al. Everolimus plus exemestane in Postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis. Adv Ther. 2013;30(10):870–884. doi:10.1007/s12325-013-0060-1
46. Cook MM, Al Rabadi L, Kaempf AJ, et al. Everolimus plus exemestane treatment in metastatic hormone receptor-positive breast cancer patients previously treated with CDK4/6 inhibitor therapy. Oncologist. 2021;26(2):101–106. doi:10.1002/onco.13609
47. Mo H, Renna CE, Moore HCF, et al. Real-world outcomes of everolimus and exemestane for the treatment of metastatic hormone receptor-positive breast cancer in patients previously treated with CDK4/6 inhibitors. Clin Breast Cancer. 2022;22(2):143–148. doi:10.1016/j.clbc.2021.10.002
48. Smyth LM, Batist G, Meric-Bernstam F, et al. Selective AKT kinase inhibitor capivasertib in combination with fulvestrant inPTEN-mutant ER-positive metastatic breast cancer. NPJ Breast Cancer. 2021;7(1):44. doi:10.1038/s41523-021-00251-7
49. Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21(3):345–357. doi:10.1016/S1470-2045(19)30817-4
50. Howell SJ, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor- positive, HER2-negative breast cancer (FAKTION): overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022;23(7):851–864. doi:10.1016/S1470-2045(22)00284-4
51. Turner N, Oliveira M, J HS, et al. Capivasertib in hormone receptor-positive advanced breast cancer. N Engl J Med. 2023;388(22):2058–2070. doi:10.1056/NEJMoa2214131
52. Beck A, Goetsch L, Dumontet C, et al. Strategies and challenges for the next generation of antibody-drug conjugates. Nat Rev Drug Discov. 2017;16(5):315–337. doi:10.1038/nrd.2016.268
53. Ambrogi F, Fornili M, Boracchi P, et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9(5):e96993. doi:10.1371/journal.pone.0096993
54. Trerotola M, Cantanelli P, Guerra E, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32(2):222–233. doi:10.1038/onc.2012.36
55. Goldenberg DM, Sharkey RM. Sacituzumab govitecan, a novel, third-generation, antibody- drug conjugate (ADC) for cancer therapy. Expert Opin Biol Ther. 2020;20(8):871–885. doi:10.1080/14712598.2020.1757067
56. Nagayama A, Vidula N, Ellisen L, et al. Novel antibody-drug conjugates for triple negative breast cancer. Ther Adv Med Oncol. 2020;12:1758835920915980. doi:10.1177/1758835920915980
57. Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget. 2015;6(26):22496–22512. doi:10.18632/oncotarget.4318
58. Cardillo TM, Govindan SV, Sharkey RM, et al. Sacituzumab govitecan (IMMU-132), an anti- trop-2/SN-38 antibody-drug conjugate: characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–931. doi:10.1021/acs.bioconjchem.5b00223
59. Govindan SV, Cardillo TM, Sharkey RM, et al. Milatuzumab-SN-38 conjugates for the treatment of CD74+ cancers. Mol Cancer Ther. 2013;12(6):968–978. doi:10.1158/1535-7163.MCT-12-1170
60. Rugo HS, Bardia A, Marmé F, et al. Sacituzumab govitecan in hormone receptor- positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2022;40(29):3365–3376. doi:10.1200/JCO.22.01002
61. Modi S, Park H, Murthy RK, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi:10.1200/JCO.19.02318
62. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2- low advanced breast cancer trial investigators. N Engl J Med. 2022;387(1):9–20. doi:10.1056/NEJMoa2203690
63. Turner NC, Swift C, Kilburn L, et al. ESR1 mutations and overall survival on fulvestrant versus exemestane in advanced hormone receptor-positive breast cancer: a combined analysis of the phase III SoFEA and EFECT trials. Clin Cancer Res. 2020;26(19):5172–5177. doi:10.1158/1078-0432.CCR-20-0224
64. Robertson JFR, Bondarenko IM, Trishkina E, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. doi:10.1016/S0140-6736(16)32389-3
65. Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD Trial. J Clin Oncol. 2022;40(28):3246–3256. doi:10.1200/JCO.22.00338
66. Oliveira M, Pominchuck D, Nowecki Z, et al. GS3-02 Camizestrant, a next generation oral SERD vs fulvestrant in post-menopausal women with advanced ER-positive HER2- negative breast cancer: results of the randomized, multi-dose Phase 2 SERENA-2 trial.
67. Tolaney SM, Chan A, Petrakova K, et al. AMEERA-3: randomized Phase II study of amcenestrant (oral selective estrogen receptor degrader) versus standard endocrine monotherapy in estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. J Clin Oncol. 2023;41(24):4014–4024. doi:10.1200/JCO.22.02746
68. Martin Jimenez M, Lim E, Chavez Mac Gregor M, et al. 211MO Giredestrant (GDC-9545) vs physician choice of endocrine monotherapy (PCET) in patients (pts) with ER+, HER2-locally advanced/metastatic breast cancer (LA/mBC): primary analysis of the phase II, randomised, open-label acelERA BC study. Ann Oncol. 2022;33:S633–S634. doi:10.1016/j.annonc.2022.07.250
69. Robert NJ, Vogel CL, Henderson IC, et al. The role of the liposomal anthracyclines and other systemic therapies in the management of advanced breast cancer. Semin Oncol. 2004;31(6 Suppl 13):106–146. doi:10.1053/j.seminoncol.2004.09.018
70. Mauri D, Kamposioras K, Tsali L, et al. Overall survival benefit for weekly vs. three-weekly taxanes regimens in advanced breast cancer: a meta-analysis. Cancer Treat Rev. 2010;36(1):69–74. doi:10.1016/j.ctrv.2009.10.006
71. Petrelli F, Di Cosimo S, Lonati V, et al. Vinorelbine with capecitabine, an evergreen doublet for advanced breast cancer: a systematic literature review and pooled-analysis of Phase II-III studies. Clin Breast Cancer. 2016;16(5):327–334. doi:10.1016/j.clbc.2016.05.002
72. Kaufman PA, Awada A, Twelves C, et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2015;33(6):594–601. doi:10.1200/JCO.2013.52.4892
73. Zhang P, Sun T, Zhang Q, et al. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: a multicentre, open-label, superiority, phase 3, randomised controlled trial. Lancet Oncol. 2017;18(3):371–383. doi:10.1016/S1470-2045(17)30088-8
74. Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi:10.1056/NEJMoa1706450
75. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi:10.1056/NEJMoa1802905
76. Tung NM, Robson ME, Ventz S, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol. 2020;38(36):4274–4282. doi:10.1200/JCO.20.02151
77. Doostan I, Karakas C, Kohansal M, et al. Cytoplasmic cyclin E mediates resistance to aromatase inhibitors in breast cancer. Clin Cancer Res. 2017;23(23):72887300. doi:10.1158/1078-0432.CCR-17-1544
78. Karakas C, Biernacka A, Bui T, et al. Cytoplasmic cyclin E and phospho–cyclin-dependent kinase 2 are biomarkers of aggressive breast cancer. Am J Pathol. 2016;186(7):1900–1912. doi:10.1016/j.ajpath.2016.02.024
79. Hunt KK, Karakas C, Ha MJ, et al. Cytoplasmic cyclin E predicts recurrence in patients with breast cancer. Clin Cancer Res. 2017;23(12):2991–3002. doi:10.1158/1078-0432.CCR-16-2217
80. J A-QA, Alves CL, Ehmsen S, et al. Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer. NPJ Precis Oncol. 2022;6(1):68. doi:10.1038/s41698-022-00311-6
81. Dawson SJ, Makretsov N, Blows FM, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103(5):668–675. doi:10.1038/sj.bjc.6605736
82. Lok SW, Whittle JR, Vaillant F, et al. A phase IB dose-escalation and expansion study of the BCL2 inhibitor venetoclax combined with tamoxifen in ER and BCL2-positive metastatic breast cancer. Cancer Discov. 2019;9(3):354–369. doi:10.1158/2159-8290.CD-18-1151
83. Vaillant F, Merino D, Lee L, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24(1):120–129. doi:10.1016/j.ccr.2013.06.002
84. Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: randomized Phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors - efficacy, safety, and biomarker results. Clin Cancer Res. 2022;28(15):3256–3267. doi:10.1158/1078-0432.CCR-21-3811
85. Guarducci C, Bonechi M, Benelli M, et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. NPJ Breast Cancer. 2018;4:38. doi:10.1038/s41523-018-0092-4
86. Liu Y, Zhong X, Wan S, et al. p16INK4a expression in retinoblastoma: a marker of differentiation grade. Diagn Pathol. 2014;9(1):180. doi:10.1186/s13000-014-0180-1
87. Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10(1):1373. doi:10.1038/s41467-019-09068-2
88. Drago JZ, Formisano L, Juric D, et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor-positive (HR+) breast cancer. Clin Cancer Res. 2019;25(21):6443–6451. doi:10.1158/1078-0432.CCR-19-0138
89. Gong X, Du J, Parsons SH, et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 2019;9(2):248–263. doi:10.1158/2159-8290.CD-18-0469
90. Teh JLF, Cheng PF, Purwin TJ, et al. In vivo E2F reporting reveals efficacious schedules of MEK1/2-CDK4/6 targeting and mTOR-S6 resistance mechanisms. Cancer Discov. 2018;8(5):568–581. doi:10.1158/2159-8290.CD-17-0699
91. De Leeuw R, McNair C, Schiewer MJ, et al. MAPK reliance via acquired CDK4/6 inhibitor resistance in cancer. Clin Cancer Res. 2018;24(17):4201–4214. doi:10.1158/1078-0432.CCR-18-0410
92. Anderson EJ, Mollon LE, Dean JL, et al. A systematic review of the prevalence and diagnostic workup of PIK3CA mutations in HR+/HER2-metastatic breast cancer. Int J Breast Cancer. 2020;2020:3759179. doi:10.1155/2020/3759179
93. Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N Engl J Med. 2018;379(21):2052–2062. doi:10.1056/NEJMra1704560
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
