Back to Journals » Vascular Health and Risk Management » Volume 19
Thromboprophylaxis in Patients Admitted to the Surgical Ward: Clinical Audit
Authors Abdalla YA , Kamil AM , Mohamed SAA , Mohamed AHA, Khalifa E , Mohamed MHA , Abdelgadir EEA, Dabora M, Awoda MSEME
Received 24 May 2023
Accepted for publication 11 September 2023
Published 22 September 2023 Volume 2023:19 Pages 651—656
DOI https://doi.org/10.2147/VHRM.S418808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Daniel Duprez
Yassin Abdelrahim Abdalla,1 Arwa Mustafa Kamil,2 Samya Abbas Abdelrazig Mohamed,3 Ahmed Hashim Ahmed Mohamed,4 Eman Khalifa,5 Mohamed Hamid Abdelsalam Mohamed,2 Eilaf Eltayeb Abdalla Abdelgadir,6 Muawiya Dabora,7 Mohammed Salah Eldin Mohammed Elshikh Awoda8
1Internal Medicine Department, Faculty of Medicine and Health Science, Omdurman Islamic University, Khartoum, Sudan; 2MBBS, Alzaiem Alazhari University, Khartoum, Sudan; 3MBBS, Al Gezira University, Wadmedani, Sudan; 4MBBS, National Ribat University, Khartoum, Sudan; 5MBBS, University of Khartoum, Khartoum, Sudan; 6MBBS, Alneelain University, Khartoum, Sudan; 7Department of Surgery, Alshuhada Teaching Hospital, Khrtoum, Sudan; 8Surgery Department, National Ribat University, Khartoum, Sudan
Correspondence: Yassin Abdelrahim Abdalla, Email [email protected]
Background: Hospital-acquired thrombosis (HAT) is associated with significant morbidity, mortality, and financial burden globally. Following trusted guidelines for VTE prevention has shown effective, safe, and satisfactory results. This prompts national collaborative efforts to maintain a consensus approach for the safe risk assessment of inpatients and the prescription of thromboprophylaxis.
Objective: This study aimed to detect and estimate deviations from international thromboprophylaxis protocols. The study also aimed to raise the quality of practice and adherence to evidence-based protocols in Alshuhada Teaching Hospital.
Methods: A cross-sectional audit of general surgical inpatients was performed from October 2021 to May 2022. The first cycle was from 1/10/2021 to 21/10/2021, and the second cycle was from 13/5/2022 to 31/5/2022. The target population was adults aged > 18 years. Data were collected via an online checklist on two separate occasions. The criteria were based on the NICE guideline for venous thromboembolism in individuals aged over 16 years: “Reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism NG89”.
Results: Forty-five surgical inpatients were included in this study: 20 in the first cycle and 25 in the second cycle. The first-cycle report showed that only 25% of VTE candidates received this regimen. In the second cycle, practice significantly improved, with 92% of admitted patients having their risk assessment tool completed within 24 h of admission. 79% of VTE prophylaxis candidates were prescribed adequate pharmacological prophylaxis within 14 h of admission.
Conclusion: The rate of adequate thromboprophylaxis for inpatients undergoing surgery was very low before clinicians received education on VTE prevention, whereas was evidently high after they had received them. The cause of non-adherence in the pre-intervention phase was a lack of adequate knowledge regarding the magnitude and burden of HAT and the importance of thromboprophylaxis, which has a potential role in preventing the majority of HAT.
Keywords: hospital acquired thrombosis, deep vein thrombosis, pulmonary embolism, venous thromboembolism
Introduction
Any venous thromboembolism (VTE) event that happens within 90 days of hospitalization is defined as hospital acquired thrombosis (HAT). HAT includes deep vein thrombosis (DVT) and pulmonary embolism (PE).1,2 HAT is significantly associated with morbidity, mortality.3 It is estimated that annual incidence of VTE in Europe reaches More than 1.5 million cases with more than 50% mortality; making VTE the third leading cause of cardiovascular death globally.1,4 For more than three decades, following trusted guidelines for VTE prevention has shown effective, safe, and satisfactory results,5 prompting national collaborative efforts to maintain a consensual approach for safe risk assessment of inpatients and prescription of thromboprophylaxis.
VTE is acknowledged as the fifth most common cause for postoperative readmissions.6 However, with the use of preventive measures such as compression stockings and anti-coagulants, 70% of HAT cases are avoidable. In spite of that, less than 50% of admitted eligible patients receive these measures. A recent study that includes 500,000 surgical operations found that only 40% of patients whose developed VTE postoperatively did so before they were discharged. The rest majority of the VTE cases (60%) occurred up to 90 days after discharge. This result suggests that relying on discharge data to record VTE incidence may underestimate the actual magnitude of the problem.7
Prevention methods for patients with VTE include pharmacological thromboprophylaxis and mechanical thromboprophylaxis (eg anti-embolism stockings or intermittent pneumatic compression). Pharmacological thromboprophylaxis includes unfractionated heparin (UFH) and low-molecular-weight heparin (LMWH).
Assessing thromboembolic risk factors according to standardized risk assessment tools aids risk stratification. Another point worthy of mention is assessment of the risk of bleeding and possible contraindications to antithrombotic medications before starting. Unfortunately, only 40–50% of medical patients and 60–75% of surgical patients receive adequate thromboprophylaxis despite intensive national and international recommendations to apply sufficient thromboprophylaxis guidelines to patients admitted to hospital. Hence, regular audits can reinforce and establish consistent use of venous thromboembolism prophylaxis.8
One analysis of cost-effectiveness found that the ACCP guidelines for surgical patients were affordable yet effective in individuals admitted for hospital risk assessment, compared to those who did not underwent risk assessment.9
This study aimed to detect and estimate deviations from international thromboprophylaxis protocols. The study also aimed to raise the quality of practice and adherence to evidence-based protocols in Alshuhada Teaching Hospital.
Materials and Methods
The cross-sectional audit was conducted between October 2021 and May 2022. It included all patients admitted to the General Surgery Department of Alshuhada Teaching Hospital located in the province of Bahri, Khartoum State. The first cycle was from 1/10/2021 to 21/10/2021, the second cycle was from 13/5/2022 to 31/5/2022.
The study aim is to detect and estimate the deviation from international protocols of thromboprophylaxis, and to upgrade practices and ensure adherence to evidence-based protocols.
The targeted population included all adults (age >18 years) admitted to the surgical ward for elective or emergency interventions and/or operations during the mentioned period. Pediatric cases and those who were treated as outpatients or in short-stay wards had been excluded.
Surgical staff at all levels (house officers, medical officers, and registrars) did not know about the collection, either before or after the results were presented. The sample consisted of 45 patients, of which 20 were baseline examples of prevalent contemporary practices and the remaining 25 were obtained after a discussion of the national guidelines.
The data for the two cycles were collected electronically via an online checklist on two separate occasions, 2 months apart, to ensure maintenance of good practice. The checklists were filled by the authors themselves as one to one interview for each patient separately, before which a verbal consent was taken.The cause of admission and type of operation for each patient were documented, and the criteria extracted from the NICE guidelines (mentioned below) were checked.
The data were analysed using the Statistical Package of Social Science SPSS version 26.0, and descriptive analysis data are presented as tables and reported as frequencies and figures.
Intervention
We conducted a local presentation after collecting and analysing data from the first cycle. The presentation focused on raising awareness among the staff regarding the international VTE prophylaxis protocol. The attendees included hospital administrators, interns, registrars, and a consultant. The results of the study and areas of defects were clarified, and options for better practice were discussed.
Moreover, posters illustrating the summary of the guidelines and important information, such as the dose of pharmacological prophylaxis, duration of treatment, and statistics on the HAT burden, were distributed across the hospital.
The staff were also trained to use the risk assessment tool which was later attached to the file of each patient in the hospital. The risk assessment tool contains a standard template, in addition to the assessor’s name and the time of assessment.
Audit Standards
The criteria are based on the NICE guidelines for venous thromboembolism in individuals aged over 16: “Reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism NG89”.
The following criteria were used to assess the practice:
- Use of the VTE risk assessment tool within 24 hours of admission.
- Prescribe pharmacological measures.
- Counsel patients discharged on pharmacological thromboprophylaxis about the correct usage and dose.
- Counsel patients discharged on pharmacological thromboprophylaxis regarding the necessity of taking the treatment for the recommended duration.
- Counsel the patients at risk about their risk.
- Counsel the patients at risk about the consequences of DVT.
- Counsel the patients at risk about the importance of prophylaxis.
- Counsel the patients at risk about possible side effects of the prophylactic agent.
- Counsel the patients at risk about how to reduce their risk.
- Upon discharge, counsel patients at high risk about the features of DVT and PE.
- Counsel patients discharged on pharmacological thromboprophylaxis about the side effects and when to seek medical help.
Ethical Consideration
Ethical approval was obtained from hospital administration before starting. A verbal informed consent was taken from each individual patient separately upon collecting data. Personal data were excluded from the study.
Results
Forty-five admitted patients were included in this audit, 20 in the first cycle and 25 in the second cycle.
A total 29% of patients were managed conservatively (Table 1). The types of operation are presented in Table 2.
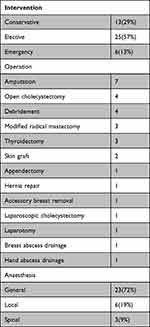 |
Table 1 Type of Intervention |
 |
Table 2 Risk Factors for HAT |
Upon application of the risk assessment tool, 75% of patients required pharmacological prophylaxis (34 patients).
Risk for VTE is shown in Table 2.
The risk assessment tool was not used for the patients in the first cycle. However, the tool was used in 92% of patients in the second cycle. Pharmacological prophylaxis was used in 25% of the eligible patients in the first cycle; however, the percentage increased markedly to 79% in the second cycle. Other parameters of the standard criteria are presented in Table 3.
 |
Table 3 Standard Criteria Application |
Discussion
HAT is a catastrophic condition with high morbidity and mortality rates. Although it is preventable in nearly 70% of high-risk patients through application of thromboprophylaxis guidelines, this condition still carries a significant burden for patients at risk and the health system. This reflects the poor adherence of medical staff to thromboprophylaxis measures.
The aim of this audit was to critically assess the local practice and application of these measures and to identify potential defects to guide best practices.
In the first cycle, only 25% of patients with VTE received this regimen. This result is not surprising as multiple studies have reported a low prescription proportion. A study by Li et al reported that only 29% of surgical patients received adequate thromboprophylaxis.10 Additionally, a global study involving 32 countries conducted by Cohen et al reported that only 58% of surgical patients at risk received VTE prophylaxis.11
The cause of non-adherence in the pre-intervention phase was a lack of adequate knowledge about the magnitude and burden of HAT and the importance of thromboprophylaxis, which has a potential role in preventing the majority of HAT. These issues were clearly addressed in the presentations and posters.
The second reason was the unavailability of the risk assessment tool in the patient’s admission file, which was later attached to be filled by interns. The decision to add thromboprophylaxis to the patient’s regimen was made by a resident under the supervision of a specialist.
In the second cycle, the practice was significantly improved, with 92% of admitted patients having their risk assessment tool checklist completed within 24 h of admission. Additionally, 79% of VTE prophylaxis candidates were administered adequate pharmacological prophylaxis within 14 h of admission.
Those measures used by us to improve the practice were comparably similar to the measures done by Nimeri et al12 who reported substantial improvement in practice after introduction of these policies from below 80% prior to intervention to 100%.
This being a cross-sectional study that documented the practice at a single point of time for a patient who had already been admitted did not allow the researcher to know whether the thromboprophylaxis dose was given within 24 h of admission, with a delay beyond 24 h linked to an increased mortality rate. However, the time of filling in the risk assessment tool along with the time of admission was documented.
This study was self-funded by the authors.
Ethical Consideration
Ethical clearance was obtained from the Ethical Review Committee in Alshuhada hospital, Privacy and confidentiality of collected patient’s information were ensured. Informed consent was taken from each patient prior to collection of data according to the current revision of the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
1. Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(10):756–764. doi:10.1160/TH07-03-0212
2. Sweetland S, Green J, Liu B, et al. Duration and magnitude of the postoperative risk of venous thromboembolism in middle aged women: prospective cohort study. BMJ. 2009;339(dec03 1):b4583. doi:10.1136/bmj.b4583
3. Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost. 2002;28 Suppl 2(s2):3–13. doi:10.1055/s-2002-32312
4. Goldhaber SZ. Pulmonary embolism thrombolysis: a clarion call for international collaboration. J Am Coll Cardiol. 1992;19(2):246–247. doi:10.1016/0735-1097(92)90473-Z
5. Clagett GP, Anderson FA Jr, Levine MN, Salzman EW, Wheeler HB. Prevention of venous thromboembolism. Chest. 1992;102(4 Suppl):391S–407S. doi:10.1378/chest.102.4_Supplement.391S
6. Merkow RP, Ju MH, Chung JW, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015;313(5):483–495. doi:10.1001/jama.2014.18614
7. Kahn S, Morrison D, Cohen J, et al. Interventions for implementation of thromboprophylaxis in hospitalized medical and surgical patients at risk for venous thromboembolism. Cochrane Database Syst Rev. 2013;7:CD008201.
8. Zeidan AM, Streiff MB, Lau BD, et al. Impact of a venous thromboembolism prophylaxis “smart order set”: improved compliance, fewer events. Am J Hematol. 2013;88(7):545–549. doi:10.1002/ajh.23450
9. Warlow C. venous thromboembolism after stroke. Am Heart J. 1978;96(3):283–285. doi:10.1016/0002-8703(78)90037-6
10. Cathy L, Smith RE, Berry BR. Retrospective Clinical Audit of Adherence to a Protocol for Prophylaxis of Venous Thromboembolism in Surgical Patients. C JHP. 2008;61(3):567.
11. Cohen AT, Tapson VF, Bergmann J-F, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387–394. doi:10.1016/S0140-6736(08)60202-0
12. Nimeri AA, Gamaleldin MM, Mckenna KL, Turrin NP, Mustafa BO. Reduction of Venous Thromboembolism in Surgical Patients Using a Mandatory Risk-Scoring System: 5-Year Follow-Up of an American College of Surgeons National Surgical Quality Improvement Program. Clin Appl Thrombosis. 2017;23(4):392–396. doi:10.1177/1076029615614396
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
