Back to Journals » Patient Preference and Adherence » Volume 18
Trajectory of Caregiver Burden and Associated Factors in Family Caregivers of Individuals with Colorectal Cancer: A Longitudinal, Observational Multicenter Study
Authors Wang J, Duan Y , Geng L, Li X, Yue S, Liu H
Received 23 November 2023
Accepted for publication 11 April 2024
Published 18 April 2024 Volume 2024:18 Pages 879—892
DOI https://doi.org/10.2147/PPA.S451487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Jing Wang,1,* Yi Duan,2,* Liangrong Geng,2 Xiaoyu Li,3 Shujin Yue,2 Hongxia Liu2
1Department of Nursing, Peking University Third Hospital, Beijing, People’s Republic of China; 2School of Nursing, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Center for Treatment of Undiagnosed Diseases, the First Affiliated Hospital of Nanchang University, Jiangxi Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shujin Yue, Beijing University of Chinese Medicine, No. 11 North 3rd Ring East Road, Chao-Yang District, Beijing, 100029, People’s Republic of China, Email [email protected]
Purpose: To (1) investigate the changes in 5 domains (lack of family support, impact on finance, impact on daily schedule, impact on health, and self-esteem) of family caregiver (FC) burden and overall burden for first diagnosed colorectal cancer; (2) exploring changes in FC burden for colorectal cancer patients over time and analyze the trajectory and sub-trajectories of FC burden; and (3) identify the FC‐related and patient‐related factors most associated with the overall FC burden and each of its sub-trajectories.
Patients and methods: This study is a descriptive longitudinal study. A convenience sampling method was used to recruit patients with colorectal cancer and their primary FCs from seven hospitals.
Results: A total of 185 pairs of first diagnosed colorectal cancer patient and their FC were investigated for 4 times. The results reveal the overall burden and 5 domains of burden showed a trend of increasing first and then decreasing, and the burden was the heaviest at the time in the middle of chemotherapy. In the course of time, the aspect that caused the greatest amount of burden on average transitioned from the “effect on daily schedule” (range= 3.3 and 3.9) to the “effect on finances” (range= 3.1 to 3.4).
Conclusion: Almost 88% of FCs have a either a moderate or a high level of burden. The quality of life of patients and the self-efficacy, social support and care ability of FCs have a great impact on the overall FC burden and each sub-trajectory.
Keywords: care burden, colorectal cancer, family caregiver, longitudinal study, trajectories
Introduction
Colorectal cancer is one of the most common malignant tumors of the digestive system. According to Global Cancer Statistics 2020,1 the incidence rate of colorectal cancer is the third highest in the world, and the mortality rate is second. However, the survival rate of patients with colorectal cancer is improving through surgery, radiotherapy, chemotherapy, and targeted therapy. The 5-year survival rate of colon cancer is 60% to 80%,2 while that of rectal cancer is approximately 27% to 34%.3 However, the process from diagnosis to a return to social life takes time and creates a heavy burden. During this time, for many patients, family caregivers (FCs) are often the primary caregivers for colorectal cancer patients.4
Faced with long-term and complex care work, FCs of colorectal cancer patients will encounter a series of care burdens—physical, psychological, social and economic.5,6 Patients with colorectal cancer require assistance from caregivers after surgery, as they may experience defecation dysfunction following anal preservation surgery or the use of a stoma after colostomy. They also undergo a range of physical and psychological changes.7 As a result, approximately 30% of caregivers will experience fatigue, and their fatigue level will increase with time in a year.8 Kim et al found that approximately 25% of FCs of colorectal cancer patients show moderate to severe depression within 6 months after patient diagnosis.9 In addition, the social burden of FCs of patients with colorectal cancer is not optimistic. A large number of studies have shown that in the early stages following hospital discharge of colorectal cancer patients, caregivers sometimes limit social interactions out of concern that patients may not be capable of taking care of themselves,10 and caregivers’ daily lives and work will also be greatly affected.11 Caregivers may bear the cost of treatment, and the economic burden may increase due to delay or loss of work.
At present, many studies have found that FCs of patients with colorectal cancer generally have a moderate burden.12 There are two main factors that affect the burden of FCs of patients with colorectal cancer. First, patient-related factors affect FCs. The research results show that the caregivers for patients who were female, younger and had higher levels of education experienced a lower psychological burden, while the caregivers of patients with complications experienced a heavier care burden.13–15 Second, factors related to the caregivers, including self-efficacy and psychological status, affect their care burden. Caregivers who are older, female, employed and have lower levels of education face a heavier burden; caregivers with low self-efficacy and psychological barriers also experience an increased care burden.13,16,17
Care burden is a dynamic multidimensional process, and the level of care burden of FCs depends on the disease stage, treatment stage and different care needs of the patients.18 Recent research indicates that the majority of primary caregivers for colorectal cancer patients experience a significant care burden, with many reporting moderate to severe levels of burden over an extended period of time following the patient’s surgery. In order to provide targeted interventions, thus, it is necessary to investigate the changes in different domains and sub-trajectories of burden of FC. Through a longitudinal study, we can analyze the potential categories of care burden and determine the proportion of people with different types of care burden and the influencing factors. At present, the meaningful times that colorectal cancer patients’ FCs face changes in burden is surgery, chemotherapy, recurrence and metastasis. Studies on the different stages of care burden are mostly cross-sectional studies, which only describe the level of care burden and its influencing factors in a single stage of surgery or chemotherapy. However, we cannot understand the changes in caregivers’ burden trajectory from surgery to chemotherapy (recurrence/metastasis) or compare the level of care burden in different stages.
Considering most of the areas investigated in the above study belong to western countries, it’s important to note that China’s medical conditions, patients’ economic situation, and attitudes toward cancer differ significantly from those of Western countries. Additionally, previous studies have predominantly focused on caregivers of cancer patients, with minimal exploration of the burden on family caregivers of colorectal cancer patients. Given the unique characteristics and caregiving needs associated with colorectal cancer, particularly in the context of Chinese society, this study aims to leverage the findings from existing research as a framework to investigate the burden on family caregivers of colorectal cancer patients in the Chinese social context.
Based on the conceptual model of caregiver adaptation which mainly covers and explains the relationship of the background and situation of care, the process of care evaluation and the feeling of care,19 this study hypothesizes that the caregiver burden is associated with 2 types of stressors: patient‐related and FC‐related. Patient‐related factors include demographic characteristics such as age, gender, self-care level, and clinical conditions such as diagnosis, complications, physical functioning and symptoms. FC‐related factors include demographic characteristics such as employment status, relationship with patient and coping style, self-efficacy, social support and caring ability. According to the Cancer Family Caregiving Model which mainly consists of three parts (cancer trajectory, background factors and pressure process),18 we selected four time points: after surgery (T1), in the middle of chemotherapy (T2), end of chemotherapy (T3) and 3 months after chemotherapy (T4). Therefore, the theoretical framework of this study (Figure 1) is formed based on the above two theoretical frameworks, and appropriate indicators are selected according to this framework, which were described in detail in the method section.
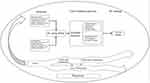 |
Figure 1 Theoretical framework of this study. |
This study aimed to (1) investigate the changes in 5 domains (lack of family support, impact on finance, impact on daily schedule, impact on health, and self-esteem) of FC burden for first diagnosed colorectal cancer from the immediate postoperative period to 3 months after chemotherapy; (2) exploring changes in FC burden for first diagnosed colorectal cancer patients over time to analyze the trajectory and sub-trajectories of FC burden; and (3) identify the FC‐related and patient‐related factors that associated with the overall FC burden and each of its sub-trajectories.
Methods
Study Design
This study was a descriptive longitudinal study. Participants completed questionnaires at four points in time: after surgery (T1), in the middle of chemotherapy (T2), end of chemotherapy (T3) and 3 months after chemotherapy (T4).
Participants and Settings
A convenience sampling method was used to recruit patients with colorectal cancer and their primary FCs from seven hospitals in Beijing, Jiangsu, Henan, and Inner Mongolia from December 2018 to January 2020. The written informed consent has been signed by all participants. Patients’ inclusion criteria were (1) first diagnosed with colorectal cancer, (2) over 18 years old, and (3) able to complete the questionnaire independently or under the guidance of researchers. Eligible FCs (1) were spending ≥ 4 hours per day taking care of the patient (2) over 18 years old and volunteered to participate in the study. The exclusion criteria of patients and FCs were (1) cancer recurrence, (2) severe physical disease, and (3) psychiatric disorder diagnosed by psychiatrists.
According to the theory of multilevel mediation modeling of continuous dependent variables in longitudinal data,20 in order to make the study more credible, this study was conducted with a mediation effect test validity of 80%, using the method of maximum likelihood (ML), and in this study, the intragroup correlation coefficient (ICC) was set to be 0.7, and the number of replications was set to be 4 times. The sample size obtained by software calculation was 138 pairs of patient-FC. Considering the high loss to follow-up rate in longitudinal studies, we increased the sample size by 30%. Finally, the sample size of this study was 180 pairs of patient-FC.
In this study, of the 205 patient‐FC dyads, 203 met the inclusion and exclusion criteria and completed the first (T1) assessment. Ten, five and three participants dropped out in T2, T3 and T4, respectively. Finally, 185 (91.1%) participants completed all four assessments. The main reason for loss to follow-up was that the contact information of colorectal cancer patients and their family caregivers was wrong and deterioration of the patient’s condition.
Data Collection
First Time Data Collection
Before the investigation, the researcher gave unified training to the team members. The researcher described the content and significance of each item and inform them of the precautions. Ensure that team members use unified guidelines to guide participants to finish questionnaire, and that team members can use more consistent language to explain any questions arising from the process. When encountering relevant problems during the survey, the researcher timely communicated with the team members by telephone, and held a video conference with the team members every two weeks to discuss and solve problems during the process.
Since study start, team members have entered the seven hospitals in Beijing, Inner Mongolia, Jiangsu and Henan in advance. The team members daily screened patients and their FCs who met the inclusion exclusion criteria through their medical records with the permission of the department physicians. When the patients finished the operation, the patient’s condition is stable after doctor evaluation, the team members will explain the purpose, significance and implementation of this study. After the patients and their FCs had a full understanding of the study, they signed an informed consent and began the face-to-face survey by the trained team members during their hospitalization.
Follow-Up Data Collection
After the participant was discharged from the hospital, the research members contacted them by phone or WeChat to schedule the next follow-up appointment and participants were reinterviewed in person. The patients and their FCs who met the inclusion and exclusion criteria were investigated four times after surgery (T1), in the middle of chemotherapy (T2), at the end of chemotherapy (T3) and 3 months after chemotherapy (T4). For those who did not want to continue the study, or who interrupted or abandoned treatment due to changes in their condition, the data collection was terminated after recording their reasons. Participants were provided a $10 incentive for completion of each survey.
Measures
Participants’/FC Demographic and Clinical Characteristics
The patient and FC characteristics were measured using a researcher‐designed and participant‐completed basic information form, which asked for age, gender, educational level, marriage status, and salary. The patients’ characteristics were obtained from medical records, including cancer stage, surgery time, and medical treatment received.
Patients’ Physical Functioning and Symptoms
We used the 30‐item European Organization for Research and Treatment of Cancer QLQ‐C30 (EORTC QLQ-C30) to measure patients’ physical functioning and symptoms.21 The survey includes 5 function dimensions (physical, role, emotional, cognitive, and social), 9 symptom dimensions, and a global QOL dimension, and the items were scored on a 4-point scale from 1 (not at all) to 4 (very much), and the scores of each field were standardized from 0 to 100, with higher scores indicating better function. The symptom dimensions included fatigue, nausea/vomiting, pain, dyspnoea, diarrhoea, financial difficulties, insomnia, lack of appetite, and constipation, with higher scores indicating more severe symptoms. After more than ten years of clinical use, the Chinese version of the EORTC QLQ-C30 has been widely used in cancer patients in China with good reliability and validity.22 The Cronbach’s α coefficients of all domains are >0.7 except for cognitive function dimension (0.49).
FCs’ Self-Efficacy
FC self-efficacy was measured by the General Self Efficacy Scale (GSES).23 The Chinese version of the scale was translated and revised by Wang et al24 and has been proved to have good reliability and validity.25 It has been used to measure FCs’ self-efficacy in caring for patients with various cancers and it is used to measure whether an individual has self-efficacy in the face of different situations, such as “I can handle whatever comes my way”, and “I can always solve problems if I try” and so on. The GSES has 10 items and is scored from 0 (no confidence at all) to 4 (totally confident). The higher the score is, the more confident the caregiver is in taking care of the patient. The Cronbach’s α coefficients of the scale was 0.92, which had good reliability and validity.
FCs’ Social Support
The social support rating scale designed by Chinese scholar Shuiyuan Xiao was used for measurement.26 A higher score means higher social support. The score range of the scale is 12–66: <22 is low level, 23–44 is medium level, 45–66 is high level. At present, it has been widely used in the field of social support for cancer patients. Cronbach’s α coefficients of the total scale was 0.90.
FCs’ Coping Strategies
The Ways of Coping Questionnaire (WCQ) was used to assess how FCs cope with cancer.27 The WCQ contains 20 items that can be divided into two subscales: twelve-item positive response and eight-item negative response. Each item was answered on a 0–3 point scale, and the subscale with the highest average score was the coping mechanism chosen by the FC. The WCQ has good reliability and validity in cancer patients and general population.28 The retest reliability of the Chinese version of WCQ was 0.89, and the Cronbach’s α coefficients of total scale was 0.9.
FCs’ Caring Ability
We use the Family Caregiver Task Inventory (FCTI) to assess FCs’ caring ability.29 The FCTI contained 25 items that can be divided into 5 subscales. The five subscales are adaptation to the role of care, coping and providing assistance, dealing with personal emotional needs, assessing family and community resources, and adjusting personal life and care needs. Each item was answered from 0 (no difficulty) to 3 (very difficult). Higher scores indicated that FCs thought the care task was difficult and their caring ability was lower. Cronbach’s α coefficients of the Chinese version of FCTI was 0.93, and the internal reliability of the five subscales was between 0.67 and 0.86, which proved that the FCTI has good reliability and validity in FCs of patients with cancer in China.30
Caregivers’ Care Burden
The 24-item Caregiver Reaction Assessment (CRA) was used to assess the care burden of FC.31 The CRA has 5 domains: positive caregiving experiences (self‐esteem) and negative caregiving experiences (lack of family support, impact on finances, impact on daily schedule, and impact on health). The “lack of family support” domain means that other family members support family caregivers in their work and life. Also, the “impact on finances” domain refers to the economic pressure felt by family caregivers. While the “impact on daily schedule” refers to the degree of influence on the daily life, work and leisure time arrangement of family caregivers. As the same, the “impact on health” domain means the effect of care tasks on the physiology and health of family caregivers. However, the positive domain “self‐esteem” means family caregivers have higher positive feelings in the care process. Caregivers make the patients more comfortable because of their own efforts, and at the same time, they earned respect and gratitude from patients’ relatives. From this experience, they could also get a great sense of satisfaction and recognize self-worth.
Each item was scored on a 5-point Likert scale from 1 (strongly disagree) to 5 (strongly agree). For self-esteem, a higher score means a lower care burden. The higher the score of the other 4 dimensions, the heavier the care burden. Previous research in caregivers of cancer patients in China showed the Cronbach’s α coefficients of the five domains is between 0.61 to 0.73 and the assessment has good reliability and validity.32
Ethical Considerations
The study complies with the Declaration of Helsinki and was approved by Medical Ethics Committee of Beijing university of Chinese medicine(2018BZHYLL0106).
Data Analysis
SPSS 26.0 software was used to input data and to describe the participants’ demographic and clinical characteristics. Five domains of FC burden and overall caregiver burden were analyzed in terms of their means, standard deviations (SD), number, and frequency. We used Mplus 7.0 software to identify the sub-trajectories of the overall FC burden by a Growth Mixture Modeling (GMM). The trajectory of FC overall burden over time was analyzed by repeated measurement ANOVA and semi‐parametric, group‐based trajectory modelling, which can explore similar trajectories of overall care burden. We also used Bayesian and Akaike information criteria and entropy for model selection to examine the potential categories of overall burden sub-trajectories by goodness‐of‐fit. Finally, the factors most strongly related to changes in trajectories of the overall FC burden and its sub-trajectories were identified separately by generalized estimating equation (GEE).
Results
Participant Characteristics
The patients (n=185) with colorectal cancer were 60.0% male, with an average age of 59.2 years (SD=12.0). Their BMI was 22.8 (SD=2.8). Most of the patients’ education levels (88.6%) were junior high school or above, and almost half were retired (48.1%). A total of 44.9% of the patients’ monthly incomes exceeded 3000 yuan; 60.00% of the patients lived with their spouses, and 54.6% of the patients needed assistance in activities of daily life (ADL). Among the patients’ diagnoses, rectal cancer accounted for 47.6%, colon cancer accounted for 52.4%, and most of the patients were moderately differentiated (60.5%). A total of 125 patients had undergone colostomy (Table 1).
 |
Table 1 Demographic and Clinical Characteristics of Participants (N = 185) |
Many FCs were female (51.4%), with a mean age of 45.3 years (SD=12.0); 67.00% of them were employed, and most of the FCs had an education level of junior high school or above (91.9%) and a monthly income of more than 3000 yuan (64.9%). They spent approximately 10.0 hours (SD= 5.7) per day taking care of patients. Approximately 75.6% of the FCs had 1–2 people to share the care task, while 51.40% had to take care of others. More than half of FCs were children of the patient (55.1%) (Table 1).
Changes in Five Domains of FC Burden (Based on CRA Five Subscales) Over Time
We standardized the care burden score to make the overall burden and the 5 domains of burden comparable. After that, the higher the score of each dimension was, the heavier the care burden for FCs. The change trend of overall care burden and the five domains of burden were tested by repeated measurement ANOVA (P = 0.000) (Table 2). At T1 (after surgery) and T2 (in the middle of chemotherapy), the highest level of burden on average was “impact on daily schedule” over time (range = 3.3‐3.9). At T3 (end of chemotherapy) and T4 (3 months after chemotherapy), the highest level of burden was “impact on finances” over time (range = 3.1–3.4). The overall burden and 5 domains of burden showed a trend of increasing first and then decreasing, and the burden was the heaviest at T2 (Figure 2).
 |
Table 2 Repeated Measures ANOVA of Overall and 5 Domains of Burden Trends |
 |
Figure 2 The Changes of 5 Domains and Overall Burden. |
Changes in the Overall FC Burden and Its Sub-Trajectories Over Time
The FC burden trajectories are inscribed for each individual and random effects are estimated, which show differences in the level of and changes in FC burden across individuals. Therefore, growth mixed model analysis was used to identify the heterogeneity of FC burden (Table 3). Finally, with the smallest BIC (2315.80), AIC (2325.00) and entropy (1) values, 3 sub-trajectories of FC burden were identified. According to the relative relationship between the 3 sub-trajectories and the overall FC burden, we named them high burden, moderate burden, and low burden. Most FCs belonged to the moderate burden trajectory group (55.1%), with a moderate burden level (range = 2.5‐3.4); 31.9% of FCs belonged to the high burden trajectory group, with a high mean burden over time (range =2.9–3.5); and 13.0% of FCs belonged to the low burden trajectory group (range = 2.1‐3.2). Three sub-trajectories increased at T2 (in the middle of chemotherapy) and decreased dramatically at T3 (end of chemotherapy) (Figure 3).
 |
Table 3 Significant Factors Related to Overall FC Burden and Subtrajectories by GEE Analysis |
 |
Figure 3 Changes in Sub-trajectories and Overall FC Burden. |
Factors Related to the Changes in Overall FC Burden and FC Burden Sub-Trajectories
The factors related to overall burden included patient factors, including gender (β = −1.490, P = 0.036), tumor differentiation (β = 1.261, P = 0.007), physical function (β = −0.050, P = 0.026), role function (β = −0.035, P = 0.041), social function (β = −0.015, P = 0.047), fatigue (β = 0.065, P = 0.000), nausea and vomiting (β = 0.061, P = 0.000), and sleep disturbance (β = 0.022, P = 0.010). Another related factor was FC, including alternative care (β = −1.491, P = 0.000), self-efficacy (β = −0.202, P = 0.005), social support (β = −0.255, P = 0.000), care ability (β = 0.235, P = 0.000), and negative coping (β = 0.360, P = 0.000). (See Supplementary materials Table S1 for details)
The high FC burden sub-trajectory was significantly related to changes in patient self-care (β = 1.348, P = 0.047), global health (β = −0.097, P = 0.012), fatigue (β = 1.072, P = 0.013), nausea and vomiting (β = 0.064, P = 0.010), pain (β = 0.084, P = 0.016), FC age (β = −0.322, P = 0.029), care time (β = 0.273, P = 0.001), FC alternative care (β = −2.501, P = 0.002), FC self-efficacy (β = −0.207, P = 0.045), FC social support (β = −0.312, P = 0.025) and care ability (β = 0.354, P = 0.000). (See Supplementary materials Table S2 for details)
For the moderate FC burden group, the FC burden was significantly related to patient physical function (β = −0.054, P = 0.023) and role function (β = −0.070, P = 0.001). Social function (β = −0.016, P = 0.046), fatigue (β = 0.085, P = 0.000), nausea and vomiting (β = 0.080, P = 0.000), sleep disturbance (β = 0.029, P = 0.005), financial impact (β = 0.030, P = 0.001), FC alternative care (β = −1.133, P = 0.002), FC social support (β = −0.309, P = 0.000), FC care ability (β = 0.202, P = 0.002), and FC negative coping (β = 0.348, P = 0.001). (See Supplementary materials Table 3 for details)
The lower FC burden sub-trajectory was significantly related to changes in patient age (β = −1.501, P = 0.002), gender (β = 5.714, P = 0.020), self-care (β = 5.463, P = 0.003), fatigue (β = 0.079, P = 0.011), appetite loss (β = 3.101, P = 0.009), FC age (β = 3.364, P = 0.000), education level (β = −2.206, P = 0.000), care hour (β = 3.932, P = 0.000), FC self-efficacy (β = −0.273, P = 0.000) and FC care ability (β = 1.710, P = 0.000). (See Supplementary materials Table S4 for details)
Discussion
The results of this study show that during the follow-up period, the 5 domains of burden and overall burden of FCs of patients with colorectal cancer showed a trend of increasing first and then decreasing. At T2 (in the middle of chemotherapy), the FC burden was more serious, and the FC burden became lighter at T4 (3 months after chemotherapy). The “impact on daily schedule” domain of FC burden was the most severe burden at T1 (after surgery) and T2 (in the middle of chemotherapy), which is similar to a previous study.33,34 However, financial strain was another serious burden at T3 (end of chemotherapy) and T4 (3 months after chemotherapy). This may be due to the higher treatment costs of surgery and chemotherapy, and the patients and caregivers also need to bear the cost of missed work and accommodations.35 Under the Chinese health care system, some of the expenses incurred by surgery and chemotherapy need to be paid by the patient’s family due to the limitation of medical care insurance system, and the 1-year average treatment cost of colorectal cancer patients is up to be 60,845 Chinese Yuan and the proportion of self-financing cost reached 35% according to the statistics,36 which is several times of the annual per capita disposable income in the locality and shows that the economic burden of disease for colorectal cancer patients is very heavy.
Three sub-trajectories of the FC burden were identified: high, moderate, and low. More than half (55.14%) of FCs were at the moderate level of burden, and 31.89% of FCs perceived a high-level burden, which is basically consistent with the research results,33 suggesting that both groups need support from healthcare professionals. The three sub-trajectories all rose first and then decreased with time (P < 0.05). The FC burden was the most serious at T2 (in the middle of chemotherapy). This result suggested that nurses should be alert to the burden and health of FCs at T2 (in the middle of chemotherapy) and carry out health education and related nursing skills training in the early stages.
The different sub-trajectories were related to different FC‐related and patient‐related factors, which implies that they probably require different intervention strategies. Patient fatigue and FC care ability were the strongest factors related to overall FC burden and the various sub-trajectories. When patients with colorectal cancer feel tired, FCs undertake more activities to take care of patients, such as helping them with ADL and changing ostomy bags, which will increase their burden. Therefore, clinicians should assess patients’ fatigue status, identify the influencing factors, and carry out pertinent treatment to relieve patients’ fatigue and reduce the burden of caregivers. Furthermore, the low care ability of FCs will increase the FC burden. The care ability of FCs can be improved by interventions, which include five domains: adapting to the role, responding to help, dealing with personal emotions, assessing family and community resources, and adjusting life to meet the patient’s care needs. According to the five domains, clinicians can implement different interventions to relieve FC burden.
FC self-efficacy is an important factor in determining the overall FC burden and is associated with the low- and high-burden groups. The results show that low self-efficacy can add FC burden. Thus, medical staff can provide health education, affirm FCs’ efforts, and help them formulate clear nursing plans and rehabilitation goals, and introduce past successful cases so FCs can learn from others’ experience, feel hopeful and find the strength to improve self-efficacy.
FCs’ low social support and “not having alternative caregivers” were also related to overall FC burden and moderate and high burden groups. Health professionals can encourage other family members to share care tasks, suggest FCs participate in social activities, remind them to seek help to relieve pressure and develop health education and regular follow-ups to reduce FCs’ burden.37
The trajectories of the overall burden and middle burden groups were similar, so there were many of the same influencing factors. Patients’ physical state, role and social function were worse, and symptoms of nausea, vomiting and sleep disturbance were more serious, which means that the FCs needed to expend more time and energy taking care of patients’ ADL and rehabilitation exercises, so the FC burden became heavier. This is consistent with the research results of scholars Hanly.13
The results of this study also show that negative coping styles will increase the FC burden. Although adopting, weakening, avoiding and other negative coping styles can help FCs escape care tasks and alleviate negative emotions short term, they cannot effectively solve problems over the long term and may lead to psychological problems such as anxiety and depression.38
Each sub-trajectory has unique factors. When patients are male and their tumor differentiation is worse, this can result in a heavier overall burden on FCs. According to Chinese tradition, men are often the main source of the family’s income, and when they are ill and unable to work, this will increase the financial burden of FCs. At the same time, patients whose tumor differentiation is higher often deteriorate quickly and seriously, and their treatment costs more. The prognosis of the patients is poor, and the disease easily metastasizes, recurs, and patients have a 5-year survival rate of only 6%. When taking care of patients, FCs not only bear the high cost of treatment but also the psychological burden of losing relatives at any time, which further aggravates the burden of care.
Patients’ global health is an independent risk factor affecting the FC burden, which is consistent with the results of the high burden group. After surgery and supportive treatment, the physiological function, psychological function and social function of patients are damaged to varying degrees, and their global health is poor. Therefore, caregivers need to exercise more strength and patience, which increases the burden of care. Additionally, FCs who are younger experience a higher burden. This may be because young FCs not only need to take care of ADL but also their children and parents. They have to bear the pressure of conflict between work and care time.39 FCs will not be able to cope with heavy care tasks, which will increase the burden of care.
For the moderate burden group, patients’ financial impact also increased FCs’ burden. This may be because the family has to bear the cost of surgery and chemotherapy and also the cost of food and accommodations, loss of work income and so on. In addition, patients with stoma use high-price stoma care products for a long time. If there are stoma complications, they need to spend more. Families with financial difficulties have greater stress, which will cause caregivers to feel negative emotions and increase their psychological burden.
For the low burden group, younger patients had a higher FC burden. On the one hand, the malignant degree of young patients is relatively high, and their prognosis is poor. It is difficult for their families to accept the fact, and they are more likely to have restless and pessimistic ideas.40 On the other hand, young adults are the main economic pillar of the family, and if they are ill, FCs have to bear the impact of cancer and undertake the main care tasks and medical expenses. In addition, FCs who are undereducated suffer a high burden. Considering that FCs with higher education have better comprehension ability, they can better master and understand disease knowledge and nursing skills.41 Schandl, et al42 suggests that FCs with lower levels of education are more likely to have uncertainties about the disease and anxiety and depression, and they may have limited ability to find or use helpful resources.
This study used a longitudinal study design to explore the FC burden for colorectal cancer patients. Compared with cross-sectional studies, this study was able to summarize the changes in FC burden over time and make a more convincing argument for causation. Moreover, this study used GMM to identify three sub-trajectories of the burden of care, which explored the changes and influencing factors of FC burden in more depth.
Implications for Practice
This study examined the changes and the factors influencing in the FC burden for patients with colorectal cancer. Tailored support programs can be developed to address the specific needs of FCs at different stages of the cancer journey, potentially reducing their burden and improving their well-being. Recognizing the dynamic nature of FC burden, healthcare teams can offer more comprehensive care that includes both patient and caregiver support, addressing the psychosocial and physical challenges both parties face.
Limitations
There are some limitations in this study. First, the study participants were selected from tertiary hospitals in different regions, resulting in the influencing factors explored in this study only applying to FCs in the same conditions. Second, the short follow-up period in this study does not provide a complete picture of changes in FC burden. Future studies could lengthen the length of follow-up and expand the sample size by selecting participants within different levels of hospitals to make the findings more representative and comprehensive.
Conclusions
In conclusion, the overall burden of FC for colorectal cancer patients showed an increasing and then decreasing trend from surgery to 3 months after chemotherapy. The burden of FC was highest during the time in chemotherapy. Healthcare professionals and the community need to focus on FC at this point in time and provide some professional advice and support. However, there is heterogeneity in the overall burden, which can be statistically categorized into three distinct groups, known as sub-trajectories. Almost 88% of FCs have a either a moderate or a high level of burden. In general, the quality of life of patients and the self-efficacy, social support and care ability of FCs have a great impact on the overall FC burden and each sub-trajectory. The results suggest that healthcare professionals should take targeted measures to address these influencing factors to help FCs reduce their burden and improve their quality of life.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Ethic Approval
Name of ethics committee: Beijing University of Chinese Medicine
Ethic approval number: 2018BZHYLL0106
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (2022-JYB-JBZR-026).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA a Cancer J Clinicians. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Ca a Cancer J Clinicians. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca a Cancer J Clinicians. 2018;68(6):394–424. doi:10.3322/caac.21492
4. Wan SW, Chong CS, Jee XP, Pikkarainen M, He HG. Perioperative experiences and needs of patients who undergo colorectal cancer surgery and their family caregivers: a qualitative study. Supportive Care Cancer. 2022;30(6):5401–5410. doi:10.1007/s00520-022-06963-1
5. Fletcher BS, Paul SM, Dodd MJ, et al. Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. J Clin Oncol. 2008;26(4):599–605. doi:10.1200/jco.2007.12.2838
6. Osse BH, Vernooij-Dassen MJ, Schadé E, Grol RP. Problems experienced by the informal caregivers of cancer patients and their needs for support. Cancer Nursing. 2006;29(5):378–388. doi:10.1097/00002820-200609000-00005
7. Tran BT, Pham NH, Nguyen TX, et al. Measurement of Health-Related Quality of Life Among Colorectal Cancer Patients Using the Vietnamese Value Set of the EQ-5D-5L. Patient Preference Adherence. 2020;14:2427–2437. doi:10.2147/ppa.S281500
8. Traa MJ, De Vries J, Roukema JA, Den Oudsten BL. The association between patient’s and partner’s fatigue in couples coping with colorectal cancer: a longitudinal study. Supportive Care Cancer. 2016;24(10):4113–4121. doi:10.1007/s00520-016-3226-y
9. Kim Y, Kashy DA, Evans TV. Age and attachment style impact stress and depressive symptoms among caregivers: a prospective investigation. J Cancer Survivorship. 2007;1(1):35–43. doi:10.1007/s11764-007-0011-4
10. Mosher CE, Champion VL, Azzoli CG, et al. Economic and social changes among distressed family caregivers of lung cancer patients. Supportive Care Cancer. 2013;21(3):819–826. doi:10.1007/s00520-012-1585-6
11. Gray TF, Azizoddin DR, Nersesian PV. Loneliness among cancer caregivers: a narrative review. Palliative Supportive Care. 2020;18(3):359–367. doi:10.1017/s1478951519000804
12. Palma E, Simonetti V, Franchelli P, Pavone D, Cicolini G. An observational study of family caregivers’ quality of life caring for patients with a stoma. Gastroenterol nursing. 2012;35(2):99–104. doi:10.1097/SGA.0b013e31824c2326
13. Hanly P, Maguire R, Hyland P, Sharp L. Examining the role of subjective and objective burden in carer health-related quality of life: the case of colorectal cancer. Supportive Care Cancer. 2015;23(7):1941–1949. doi:10.1007/s00520-014-2551-2
14. Maguire R, Hanly P, Hyland P, Sharp L. Understanding Caregiver Burden in Colorectal Cancer: what Role Do Patient And Carer Factors Play? Value Health. 2014;17(7):A660. doi:10.1016/j.jval.2014.08.2418
15. Shaffer KM, Kim Y, Carver CS. Physical and mental health trajectories of cancer patients and caregivers across the year post-diagnosis: a dyadic investigation. Psychol Health. 2016;31(6):655–674. doi:10.1080/08870446.2015.1131826
16. Kim Y, Shaffer KM, Carver CS, Cannady RS. Prevalence and predictors of depressive symptoms among cancer caregivers 5 years after the relative’s cancer diagnosis. J Consulting Clin Psychol. 2014;82(1):1–8. doi:10.1037/a0035116
17. Wadhwa D, Burman D, Swami N, Rodin G, Lo C, Zimmermann C. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psycho-Oncology. 2013;22(2):403–410. doi:10.1002/pon.2104
18. Fletcher BS, Miaskowski C, Given B, Schumacher K. The cancer family caregiving experience: an updated and expanded conceptual model. Eur j Oncol Nursing. 2012;16(4):387–398. doi:10.1016/j.ejon.2011.09.001
19. Kramer BJ. Gain in the caregiving experience: where are we? What next? Gerontologist. 1997;37(2):218–232. doi:10.1093/geront/37.2.218
20. Preacher KJ. Multilevel SEM Strategies for Evaluating Mediation in Three-Level Data. Multivariate Behav Res. 2011;46(4):691–731. doi:10.1080/00273171.2011.589280
21. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J National Cancer Inst. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365
22. Wan C, Meng Q, Yang Z, et al. Validation of the simplified Chinese version of EORTC QLQ-C30 from the measurements of five types of inpatients with cancer. Anna Oncol. 2008;19(12):2053–2060. doi:10.1093/annonc/mdn417
23. Schwarzer R. The Assessment of Optimistic Self-beliefs--Comparison of the Chinese, Indonesian, Japanese, and Korean Versions of the General Self-Efficacy Scale. Psychologia Oriental Int J Psychol. 1997;40(1):1–13.
24. Wang C, Hu Z, Liu Y. Evidences of Reliability and Validity of the Chinese Version of General Self Efficacy Scale. Chin J Appl Psychol. 2001;1(1):37–40.
25. Cao X, Yang H. Mediating Effect of Mental Resilience and Self-efficacy on Social Support and Positive Emotions of Primary Caregivers in Malignant Tumor Patients. Chin J Social Med. 2019;36(05):480–483.
26. Liu M, Liu L, Zhang S, Li T, Ma F, Liu Y. Fear of cancer recurrence and hope level in patients receiving surgery for non-small cell lung cancer: a study on the mediating role of social support. Supportive Care Cancer. 2022;30(11):9453–9460. doi:10.1007/s00520-022-07318-6
27. Corti EJ, Johnson AR, Gasson N, Bucks RS, Thomas MG, Loftus AM. Factor Structure of the Ways of Coping Questionnaire in Parkinson’s Disease. Parkinson’s Dis. 2018;2018:7128069. doi:10.1155/2018/7128069
28. Gustafsson M, Edvardsson T, Ahlström G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Supportive Care Cancer. 2006;14(12):1205–1212. doi:10.1007/s00520-006-0080-3
29. Clark NM, Rakowski W. Family caregivers of older adults: improving helping skills. Gerontologist. 1983;23(6):637–642. doi:10.1093/geront/23.6.637
30. Lee RL, Mok ES. Evaluation of the psychometric properties of a modified Chinese version of the Caregiver Task Inventory--refinement and psychometric testing of the Chinese Caregiver Task Inventory: a confirmatory factor analysis. J Clin Nurs. 2011;20(23–24):3452–3462. doi:10.1111/j.1365-2702.2011.03729.x
31. Given CW, Given B, Stommel M, Collins C, King S, Franklin S. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nursing Health. 1992;15(4):271–283. doi:10.1002/nur.4770150406
32. Zheng Y, Lou Y, Wang H. Validity and reliability research of Chinese edition of Caregiver Reaction Assessment. Chin J Nurs. 2008;1(9):856–859.
33. Lee YH, Liao YC, Shun SC, et al. Trajectories of caregiver burden and related factors in family caregivers of patients with lung cancer. Psycho-Oncology. 2018;27(6):1493–1500. doi:10.1002/pon.4678
34. Milbury K, Badr H, Fossella F, Pisters KM, Carmack CL. Longitudinal associations between caregiver burden and patient and spouse distress in couples coping with lung cancer. Supportive Care Cancer. 2013;21(9):2371–2379. doi:10.1007/s00520-013-1795-6
35. Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prevention. 2020;29(7):1304–1312. doi:10.1158/1055-9965.Epi-19-1534
36. Shi JF, Wang L, Ran JC, et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: overall design and results from a multicenter retrospective epidemiologic survey. Cancer. 2021;127(11):1880–1893. doi:10.1002/cncr.33445
37. Glajchen M, Goehring A, Johns H, Portenoy RK. Family Meetings in Palliative Care: benefits and Barriers. Curr Treatment Options Oncol. 2022;23(5):658–667. doi:10.1007/s11864-022-00957-1
38. Unsar S, Erol O, Ozdemir O. Caregiving burden, depression, and anxiety in family caregivers of patients with cancer. Eur j Oncol Nursing. 2021;50:101882. doi:10.1016/j.ejon.2020.101882
39. Sánchez-Gundín J, Fernández-Carballido AM, Torres-Suárez AI, Barreda-Hernández D. Quality of life in non-metastasic colorectal cancer patients in FOLFOX or XELOX therapy. Farmacia hospitalaria. 2019;43(2):56–60. doi:10.7399/fh.11156
40. Thana K, Lehto R, Sikorskii A, Wyatt G. Informal caregiver burden for solid tumour cancer patients: a review and future directions. Psychol Health. 2021;36(12):1514–1535. doi:10.1080/08870446.2020.1867136
41. Kudrick LD, Baddour K, Wu R, et al. Longitudinal Analysis of Caregiver Burden in Head and Neck Cancer. JAMA Otolaryngol. 2023;149(8):681–689. doi:10.1001/jamaoto.2023.1283
42. Schandl A, Ringborg C, Mälberg K, Johar A, Lagergren P. Caregiver burden and health-related quality of life among family caregivers of oesophageal cancer patients: a prospective nationwide cohort study. Acta oncologica. 2022;61(10):1186–1191. doi:10.1080/0284186x.2022.2119098
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
