Back to Journals » Clinical Interventions in Aging » Volume 19
Validation and Improvement of the Saga Fall Risk Model: A Multicenter Retrospective Observational Study
Authors Tago M , Hirata R , Katsuki NE, Nakatani E , Tokushima M, Nishi T, Shimada H, Yaita S, Saito C , Amari K, Kurogi K, Oda Y, Shikino K , Ono M, Yoshimura M, Yamashita S , Tokushima Y, Aihara H, Fujiwara M, Yamashita SI
Received 20 September 2023
Accepted for publication 28 December 2023
Published 7 February 2024 Volume 2024:19 Pages 175—188
DOI https://doi.org/10.2147/CIA.S441235
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Masaki Tago,1 Risa Hirata,1 Naoko E Katsuki,1 Eiji Nakatani,2 Midori Tokushima,1 Tomoyo Nishi,1 Hitomi Shimada,3 Shizuka Yaita,1 Chihiro Saito,4 Kaori Amari,5 Kazuya Kurogi,6 Yoshimasa Oda,7 Kiyoshi Shikino,8,9 Maiko Ono,10 Mariko Yoshimura,11 Shun Yamashita,1 Yoshinori Tokushima,1 Hidetoshi Aihara,1 Motoshi Fujiwara,1 Shu-ichi Yamashita1
1Department of General Medicine, Saga University Hospital, Saga, Japan; 2Graduate School of Public Health, Shizuoka Graduate University of Public Health, Shizuoka, Japan; 3Shimada Hospital of Medical Corporation Chouseikai, Saga, Japan; 4Shizuoka General Hospital, Shizuoka, Japan; 5Department of Emergency Medicine, Saga-Ken Medical Centre Koseikan, Saga, Japan; 6Department of General Medicine, National Hospital Organization Ureshino Medical Center, Saga, Japan; 7Department of General Medicine, Yuai-Kai Foundation and Oda Hospital, Saga, Japan; 8Department of General Medicine, Chiba University Hospital, Chiba, Japan; 9Department of Community-Oriented Medical Education, Chiba University Graduate School of Medicine, Chiba, Japan; 10Department of General Medicine, Karatsu Municipal Hospital, Saga, Japan; 11Safety Management Section, Saga University Hospital, Saga, Japan
Correspondence: Masaki Tago, Department of General Medicine, Saga University Hospital, 5-1-1 Nabeshima, Saga, 849-8501, Japan, Tel +81 952 34 3238, Fax +81 952 34 2029, Email [email protected]
Purpose: We conducted a pilot study in an acute care hospital and developed the Saga Fall Risk Model 2 (SFRM2), a fall prediction model comprising eight items: Bedriddenness rank, age, sex, emergency admission, admission to the neurosurgery department, history of falls, independence of eating, and use of hypnotics. The external validation results from the two hospitals showed that the area under the curve (AUC) of SFRM2 may be lower in other facilities. This study aimed to validate the accuracy of SFRM2 using data from eight hospitals, including chronic care hospitals, and adjust the coefficients to improve the accuracy of SFRM2 and validate it.
Patients and Methods: This study included all patients aged ≥ 20 years admitted to eight hospitals, including chronic care, acute care, and tertiary hospitals, from April 1, 2018, to March 31, 2021. In-hospital falls were used as the outcome, and the AUC and shrinkage coefficient of SFRM2 were calculated. Additionally, SFRM2.1, which was modified from the coefficients of SFRM2 using logistic regression with the eight items comprising SFRM2, was developed using two-thirds of the data randomly selected from the entire population, and its accuracy was validated using the remaining one-third portion of the data.
Results: Of the 124,521 inpatients analyzed, 2,986 (2.4%) experienced falls during hospitalization. The median age of all inpatients was 71 years, and 53.2% were men. The AUC of SFRM2 was 0.687 (95% confidence interval [CI]:0.678– 0.697), and the shrinkage coefficient was 0.996. SFRM2.1 was created using 81,790 patients, and its accuracy was validated using the remaining 42,731 patients. The AUC of SFRM2.1 was 0.745 (95% CI: 0.731– 0.758).
Conclusion: SFRM2 showed good accuracy in predicting falls even on validating in diverse populations with significantly different backgrounds. Furthermore, the accuracy can be improved by adjusting the coefficients while keeping the model’s parameters fixed.
Keywords: accidental falls, inpatients, validation study, accident prevention
Introduction
Falls in hospitals and long-term care facilities have become a significant issue in healthcare and caregiving,1–3 making fall prevention highly desirable. Multiple fall prediction models have been developed to prevent falls.4–6 These models have often been designed for specific settings, including community-dwelling individuals (outpatients),7 older inpatients,8 acute care hospitals,9 tertiary hospitals,10 rehabilitation wards,11,12 patients with liver cirrhosis,13 patients with chronic obstructive pulmonary disease,14 and long-term care facilities.15,16 These prediction models are typically intended for specific populations and settings. Therefore, a single fall prediction model that can target a wide range of settings would be highly convenient and valuable for healthcare and caregiving.
Although numerous fall prediction models have been developed, many facilities in Japan use assessment tools developed without established evidence.17 Therefore, using data from adult inpatients aged ≥20 years in a single acute care hospital in a regional city in our previous study, we developed a fall prediction model, the Saga Fall Risk Model 2 (SFRM2), which can be easily applied upon admission.18
The SFRM2 comprises eight easily assessable items at admission (age, sex, emergency admission, admission to neurosurgery, use of hypnotics, history of falls, independence of eating, and Bedriddenness ranks). Notably, it includes Bedriddenness rank, a widely used official activity of daily living (ADL) scale in healthcare and long-term care in Japan.19,20 The Bedriddenness rank can be assessed in four steps, enabling a rapid evaluation. It demonstrates excellent inter-rater reliability and criterion-related validity, making it a convenient and objective ADL scale.21,22 In a prospective validation study conducted at two acute and chronic care hospitals, the overall area under the curve (AUC) of SFRM2 was 0.793. However, it varied between hospitals, with 0.822 in the acute care hospital and 0.642 in the chronic care hospital, indicating differences in the SFRM2 accuracy between hospitals.23 Therefore, this study aimed to externally validate SFRM2 using data from various hospitals with diverse backgrounds, demonstrate its accuracy, and adjust the coefficients of the prediction model to improve its precision.
Materials and Methods
Study Design, Setting, and Participants
This retrospective observational study was conducted in multiple regions of Japan in chronic care, acute care, and tertiary hospitals. This study included eight hospitals, the characteristics of which are shown in Table S1. The study included all patients aged ≥20 years admitted from April 1, 2018, to March 31, 2021.
Data and Definitions
All data, including admission and discharge dates; age; sex; admitting department; emergency admission; Bedriddenness ranks and Cognitive function scores by the Ministry of Health, Labour, and Welfare; and ADL score, including independence of eating, prescription and name of hypnotic medications upon admission, history of falls, presence of post-stroke sequelae (I60.0–I64, I69), Parkinson’s syndrome (G20, G21), use of mobility aids, visual impairment, transportation by ambulance, referral letter, surgery operations during hospitalization, rehabilitation, and in-hospital falls, were extracted from the medical charts or health records of each hospital. The Bedriddenness rank, a public ADL scale developed by the Ministry of Health, Labour, and Welfare in Japan, has five major classes that are further divided into nine detailed categories. The Cognitive function score was classified into six major classes and subdivided into eight detailed categories.19,20 In this study, Bedriddenness ranks were categorized into five major classes (normal, J: independence/autonomy, A: housebound, B: chair-bound, and C: bed-bound), and Cognitive function scores were classified into six major classes (normal, 1, 2, 3, 4, and M). Hypnotic medications were defined as benzodiazepines and non-benzodiazepines, excluding melatonin receptor agonists and orexin receptor antagonists.18 In-hospital fall was the outcome of this study, which was defined as any unexpected fall from any height or position, regardless of injury, including falls from stairs, chairs, and beds that occurred during walking, while sitting, or when in a supine position. Fall-related data were collected from incident or accident reports documenting fall-related incidents. In hospitals where information on previous falls was unavailable, patients with a history of femoral neck fracture were defined as having a history of falls.24,25 Missing data included in the analysis were treated as independent categories.
Statistical Analysis
For all patients and the two groups of patients who experienced or did not experience falls during hospitalization, continuous and categorical variables are presented as median values (interquartile range) and absolute numbers (percentages), respectively. The fall prediction model used in this study was the SFRM2 logistic regression model developed previously. The formula for SFRM2 is as follows:
Using the eight items of the SFRM2, a logistic regression analysis using the forced entry method was conducted with in-hospital falls as the outcome, encompassing the entire study population. Additionally, individual scores for SFRM2 were calculated for each patient, and the AUC of SFRM2, 95% confidence interval (95% CI), and shrinkage coefficient were determined.
Furthermore, we performed random sampling from the entire study population at a 2:1 ratio, designating two-thirds as the test set for model readjustment and one-third as the validation set for the readjusted model. The outcome in the test set was in-hospital falls, and logistic regression analysis with a forced entry method was conducted for the eight variables of the SFRM2. A new model formula, SFRM2.1, was developed based on the obtained regression coefficients. Using SFRM2.1, we calculated the AUC and 95% CI for both the test and validation sets. For the SFRM2 scores of the entire population and the SFRM2.1 scores of the validation set, we computed fall probabilities, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for various cutoff values derived using a sensitivity of 90%, the Youden index, or a specificity of 90%. We conducted subgroup analyses, distinguishing patients aged ≥65 years from other groups and calculated the AUC for SFRM2 and SFRM2.1. All analyses were conducted using SPSS Statistics version 25 by IBM, with a significance level set at p <0.05.
Sample Size
We determined the sample size of 1,327 patients based on the effect size of 0.20 (predicted AUC of 0.70, null hypothesis AUC of 0.50), a fall rate of 3.5%, alpha error of 0.05, and beta error of 0.20, as estimated by the AUC of 0.793.23
Ethical Considerations
This study conformed to the Declaration of Helsinki and the ethical guidelines for medical and health research involving human subjects issued by the Ministry of Health, Labour, and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology in Japan. This study was approved by the Saga University Clinical Research Review Board (no. 2021–07-R-07). The study was registered with the University Hospital Medical Information Network (UMIN) at www.umin.ac.jp (ID: UMIN000045420). Consent was obtained from all patients using the hospital’s comprehensive agreement method, and their anonymity was protected. We disclosed the research information on the hospital’s website and allowed patients to opt out of the study.
Results
Patients’ Background and Incidence of Fall Events
During the study period, 162,177 patients were admitted. We excluded 11,899 patients aged <20 years and 25,757 patients with data entry errors, including missing data related to emergency admissions, admitting departments, history of falls, Bedriddenness ranks, and information on falls, resulting in an analytical cohort of 124,521 patients (Figure 1). Table 1 shows the patients’ characteristics. Among these patients, 2,986 experienced falls, accounting for an incidence rate of 2.4%. The median age (interquartile range) was 71 years (range, 59–79 years), with 66,283 (53.2%) men, and the median length of hospital stay (interquartile range) was 9 days (range, 4–17 days). The incidence rate of falls was calculated to be 1.71 per 1,000 patient-days.
 |
Table 1 Characteristics of Patients |
 |
Figure 1 Data flow diagram. |
Multivariable Analysis
Multivariable logistic regression analysis showed a significant relationship between falls and the eight items comprising the SFRM2, ie, age, men, emergency admission, admission to neurosurgery, use of hypnotics, history of falls, requiring eating assistance, and Bedriddenness ranks (Table 2). Notably, the odds ratios were 2.1 (95% CI 1.95–2.35, p < 0.001) for having a history of falls, 2.6 (95% CI 1.86–3.58, p < 0.001) for Bedriddenness rank of A, and 3.2 (95% CI 2.31–4.49, p < 0.001) for Bedriddenness rank of B.
 |
Table 2 Results of Multivariate Logistic Regression Analysis |
Performance of Predictive Models
The AUC of SFRM2 as a measure of predictive model performance was 0.687 (95% CI, 0.678–0.697) (Figure 2). The observed incidence of falls was consistent with the predicted incidence calculated using the predictive model, with a shrinkage coefficient of 0.996 (Figure 3). The sensitivity, specificity, PPV, and NPV of SFRM2 are shown in Table 3. The cutoff values for achieving a sensitivity of 90%, the Youden index, and a specificity of 90% were − 3.20, − 2.85, and − 2.16, respectively. The corresponding PPV and NPV values for each cut-off point were 3.0% and 99.1%, 3.9% and 98.8%, and 6.3% and 98.1%, respectively. Additionally, we assessed the validation results using cutoff values from a previous study (Table 3).
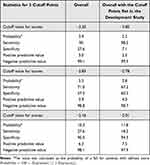 |
Table 3 Validation of the Predictive Model with the Cutoff Points Determined in the Present and Previous Studies |
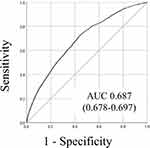 |
Figure 2 Receiver operating characteristic curves and areas under the curves. |
 |
Figure 3 The predicted and observed rates of falls in 10 groups divided into 10 deciles by score using the predictive model. |
Development and Validation of the New Model SFRM2.1
The test set comprised 81,790 patients, of which 1,944 (2.4%) experienced falls (Figure 4). The median age (interquartile range) of the patients was 71 (range, 59–79) years, and 53.3% were men. Similarly, the validation set included 42,731 patients, of which 1,042 (2.4%) patients experienced falls, and the median age (interquartile range) was 71 (range, 59–79), with 53.0% being men (Table S2). Using data from the test set, we conducted a logistic regression analysis using a forced-entry method with the eight factors that comprise SFRM2 (Table 4). Based on the obtained regression coefficients, we developed a new model, SFRM2.1 (Supplementary Material 1). The AUC of SFRM2.1 in the test set was 0.733 (95% CI: 0.723–0.743) and 0.745 (95% CI: 0.731–0.758) in the validation set (Figure 5). In the validation set, the cutoff values for achieving a sensitivity of 90%, the Youden index, and a specificity of 90% were determined to be − 4.35, − 4.02, and − 2.98, respectively. The corresponding PPV and NPV values for each cutoff point were 3.7% and 99.4%, 4.4% and 99.2%, and 7.6% and 98.2%, respectively (Table 5).
 |
Table 4 Results of Multivariate Logistic Regression Analysis in the Test Set |
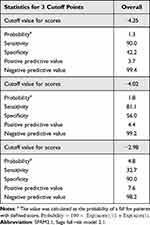 |
Table 5 Validation of the Predictive Model (SFRM2.1) with the Cutoff Points Determined in the Validation Set and Previous Study |
 |
Figure 4 The data flow diagram of the test and validation sets. |
 |
Figure 5 The area under the curves of Saga Fall Risk Model 2.1 in the test and validation sets. |
Subgroup Analyses
The AUC for SFRM2 in subgroups aged <65 years and ≥65 years were 0.707 (0.685–0.729) and 0.651 (0.640–0.662), respectively. For SFRM2.1, the AUC values were 0.782 (0.749–0.815) and 0.705 (0.688–0.721) for the respective subgroups (Figures 6 and 7).
 |
Figure 6 Receiver operating characteristic curves and areas under the curves (AUC) for the groups aged <65 years and ≥65 years for SFRM2. |
 |
Figure 7 Receiver operating characteristic curves and areas under the curves (AUC) for the groups aged <65 years and ≥65 years in the validation set for SFRM2.1. |
Discussion
The SFRM2, comprising eight factors including Bedriddenness ranks, demonstrated good discrimination and calibration even in the external validation using data from a diverse set of eight hospitals. Although SFRM2 proved to be valuable in a heterogeneous population encompassing both acute and chronic care settings, its AUC in previous studies (0.787–0.793)18,23 and that reported by Hendrich et al (0.71–0.80)26 were somewhat higher. However, SFRM2.1, which was created by adjusting the model coefficients, showed a higher AUC in the internal validation.
The AUC of SFRM2 was 0.687 for the entire study population, which was lower than that reported previously. This discrepancy in AUC is attributable to substantial variations in patient backgrounds, as this study included patients not only from mid-sized acute care hospitals, such as hospitals where SFRM was initially developed but also from tertiary, chronic care, and general hospitals with several specialized departments. One notable factor contributing to the variance in AUC is the Bedriddenness rank, which is included in the SFRM. In our previous study, the proportion of patients with normal Bedriddenness ranks was 48%,18 whereas in the present study, it significantly decreased to 3.9%. Conversely, the proportion of patients with a Bedriddenness rank of J increased from 9.9% in the previous study18 to 46.9% in this study. These differences indicate substantial disparities in patient backgrounds between the previous and current studies. ADL can fluctuate greatly depending on a patient’s clinical condition after admission.27,28 Additionally, poor initial ADL have been associated with adverse outcomes, such as in-hospital mortality, delirium, and nosocomial infections,29 implying that patients with initially poor ADL may experience more significant changes in ADL during their hospital stay. As the SFRM is a prediction model for in-hospital falls based solely on admission data, the higher prevalence of patients with initially poor ADL and their subsequent ADL fluctuations in this population may have led to the decreased predictive accuracy for in-hospital falls compared to that in the previous study.
An increased AUC was observed in the internal validation of SFRM2.1, in which the same eight items as those in SFRM2 were used with adjusted coefficients. We hypothesized that the differences in population characteristics in this study contributed to the decrease in the AUC of SFRM2. This study included patients from acute care, chronic care, and tertiary hospitals, thereby representing diverse healthcare settings. Despite the heterogeneity of this patient population, the AUC in the internal validation was improved by simply adjusting the model coefficients without altering the evaluation parameters. Furthermore, subgroup analyses of the patients aged ≥65 years demonstrated comparable results. Although external validation was not conducted, this suggests the potential for enhancing predictive accuracy by repeatedly adjusting the model coefficients while keeping the evaluation parameters constant when analyzing new data, even in heterogeneous populations. In other words, while maintaining the simplicity of the SFRM evaluation, a more generalized fall prediction model can be developed by utilizing larger datasets and fine-tuning the coefficients without changing the evaluation criteria. Therefore, we advocate for the optimal utilization of SFRM2.1 across diverse clinical settings.
History of falls and Bedriddenness ranks consistently showed significant associations with in-hospital falls.18,23,30,31 Furthermore, in the multivariate analysis of the present study, odds ratios higher than 2.0 were observed for both history of falls and Bedriddenness ranks A and B, suggesting their potential significance as predictive factors for falls. Data on fall history collected from each hospital included items with vague definitions of the location and timing of falls, and we were unable to establish a clear definition. However, similar results were obtained in previous studies that limited the timeframe for falls to a certain period before admission,23,32,33 suggesting that further clarification of the definition may enhance the accuracy of predicting falls. Moreover, Bedriddenness ranks are a unique public ADL scale widely used in Japanese healthcare and long-term care settings. However, its association with falls has not been examined extensively. Because poor ADL is related to falls.34,35 the Bedriddenness rank is a relevant factor for predicting falls. The well-known Barthel Index, a widely used ADL assessment tool, is time-consuming,36 whereas the Bedriddenness rank is a convenient ADL scale encompassing various activities, including independence of transferring and eating.21,22 Therefore, the Bedriddenness rank, which exhibited a strong association with falls in this study, can potentially serve as a valuable predictive factor for falls. Furthermore, this study showed a significant association between falls and male sex. Similarly, a previous study involving older inpatients in acute care hospitals in Japan found a significant association between falls and male sex.37 However, findings across studies have been inconsistent, with some studies reporting no sex differences and others indicating a significant association between falls and female sex.38 The relationship between falls and sex remains inconclusive, highlighting the need for further research.
Limitations
There are several limitations in this study. First, this was a retrospective study. There is a possibility of inadequate data accuracy, even if patients were independent in ADL before admission, which may have been classified as having Bedriddenness ranks other than normal, such as Bedriddenness ranks J or A, owing to the need for monitoring. Additionally, because data from multiple facilities were used, the data quality may have been heterogenous across different hospitals. Furthermore, the excluded data of 25,757 patients with missing information on items included in the SFRM2, such as the Bedriddenness rank, may have potentially impacted the results, as a significant proportion of these individuals may have had normal Bedriddenness ranks. Finally, interventions for fall prevention were not assessed.
Conclusion
The SFRM2, that includes the history of falls and the Bedriddenness rank, which are particularly useful in predicting falls, showed good ability to predict falls in the validation in diverse populations with significant background variations. Furthermore, it is possible to improve the accuracy by adjusting the coefficients while keeping the model parameters fixed. Validation with big data encompassing more diverse populations is necessary to further generalize the model.
Abbreviations
ADL, activity of daily living; AUC, area under the receiver operating characteristic curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; SFRM2, Saga Fall Risk Model 2.
Data Sharing Statement
The datasets generated and analyzed during the current study are available in the UMIN-ICDR repository, https://center6.umin.ac.jp/cgi-bin/icdr_e/ctr_view.cgi?recptno=R000050831.
Ethics Approval and Informed Consent
This study conforms to Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Japanese Ministry of Health, Labour and Welfare and the Ministry of Education, Culture, Sports, Science, and Technology. This study was approved by the Ethics Committee of Saga University Hospital (approval ID: 2021-07-R-07). The study was registered at the University Hospital Medical Information Network (UMIN) at www.umin.ac.jp (UMIN ID: UMIN000045420). We obtained consent from all patients using the hospital’s comprehensive agreement method, and anonymity of patients was protected. We disclosed research information on the hospital’s website and allowed patients to opt out of participation.
Acknowledgments
We thank Miho Hayashida and Naoko Otsubo from Saga University Hospital; Kenta Yamaguchi, Yuka Hisamoto, Yasuhiro Chibu, and Toshinobu Eguchi from Yuai-Kai Foundation and Oda Hospital; Tomokazu Ichibakase from National Hospital Organization Ureshino Medical Center; Yoshihiko Nakashima and Kaori Hamai from Karatsu Municipal Hospital; and Yuriko Takao, Mika Tokushima, Yoshiro Nakayama, and Dr. Kozo Naito from Saga-Ken Medical Centre Koseikan for assistance with data acquisition.
Author Contributions
All authors made a significant contribution to the work reported with respect to the conception, study design, execution, acquisition of data, analysis, and interpretation. All authors took part in drafting, revising, or critically reviewing the article and gave their final approval of the version submitted for publication. All the authors have agreed on the journal for submission and agree to be accountable for all aspects of the work.
Funding
This work was supported by JSPS KAKENHI (Grant Number JP21H03166). The sponsor of the study had no role in the preparation of the manuscript.
Disclosure
Masaki Tago is supported by grants from the Japan Society for the Promotion of Science, JSPS KAKENHI (Grant Number JP18K17322 and JP21H03166). Naoko E. Katsuki is supported by grants from the Japan Society for the Promotion of Science, JSPS KAKENHI (Grant Number JP23K16257). The authors report no other conflicts of interest in this work.
References
1. Dykes PC, Curtin-Bowen M, Lipsitz S, et al. Cost of inpatient falls and cost-benefit analysis of implementation of an evidence-based fall prevention program. JAMA Health Forum. 2023;4(1):e225125. doi:10.1001/jamahealthforum.2022.5125
2. Lyu H, Dong Y, Zhou W, et al. Incidence and clinical characteristics of fall-related injuries among older inpatients at a tertiary grade a hospital in Shandong province from 2018 to 2020. BMC Geriatr. 2022;22(1):632. doi:10.1186/s12877-022-03321-y
3. Mele F, Leonardelli M, Duma S, et al. Requests for compensation in cases involving patients’ falls in healthcare settings: a retrospective analysis. Healthcare. 2023;11(9):1290. doi:10.3390/healthcare11091290
4. Hendrich AL, Bender PS, Nyhuis A. Validation of the Hendrich II fall risk model: a large concurrent case/control study of hospitalized patients. Appl Nurs Res. 2003;16(1):9–21. doi:10.1053/apnr.2003.016009
5. Morse JM, Black C, Oberle K, Donahue P. A prospective study to identify the fall-prone patient. Soc Sci Med. 1989;28(1):81–86. doi:10.1016/0277-9536(89)90309-2
6. Aranda-Gallardo M, Morales-Asencio JM, Canca-Sanchez JC, et al. Instruments for assessing the risk of falls in acute hospitalized patients: a systematic review and meta-analysis. BMC Health Serv Res. 2013;13(1):122. doi:10.1186/1472-6963-13-122
7. Bravo J, Rosado H, Tomas-Carus P, et al. Development and validation of a continuous fall risk score in community-dwelling older people: an ecological approach. BMC Public Health. 2021;21(Suppl S2):808. doi:10.1186/s12889-021-10813-w
8. Oliver D, Britton M, Seed P, Martin FC, Hopper AH. Development and evaluation of evidence based risk assessment tool (STRATIFY) to predict which elderly inpatients will fall: case-control and cohort studies. BMJ. 1997;315(7115):1049–1053. doi:10.1136/bmj.315.7115.1049
9. Chen LC, Shen YC, Ho LH, Shih WM. The fall risk screening scale is suitable for evaluating adult patient fall. Healthcare. 2022;10(3):510. doi:10.3390/healthcare10030510
10. Jung H, Yoo S, Kim S, et al. Patient-level fall risk prediction using the observational medical outcomes partnership’s common data model: pilot feasibility study. JMIR Med Inform. 2022;10(3):e35104. doi:10.2196/35104
11. Wong Shee A, Phillips B, Hill K. Comparison of two fall risk assessment tools (FRATs) targeting falls prevention in sub-acute care. Arch Gerontol Geriatr. 2012;55(3):653–659. doi:10.1016/j.archger.2012.05.003
12. Vratsistas-Curto A, Tiedemann A, Treacy D, Lord SR, Sherrington C. External validation of approaches to prediction of falls during hospital rehabilitation stays and development of a new simpler tool. J Rehabil Med. 2018;50(2):216–222. doi:10.2340/16501977-2290
13. Tapper EB, Nikirk S, Parikh ND, Zhao L. Falls are common, morbid, and predictable in patients with cirrhosis. J Hepatol. 2021;75(3):582–588. doi:10.1016/j.jhep.2021.04.012
14. McLay R, Kirkwood RN, Kuspinar A, et al. Validity of balance and mobility screening tests for assessing fall risk in COPD. Chron Respir Dis. 2020;17:1479973120922538. doi:10.1177/1479973120922538
15. Wabe N, Siette J, Seaman KL, et al. The use and predictive performance of the Peninsula Health Falls Risk Assessment Tool (PH-FRAT) in 25 residential aged care facilities: a retrospective cohort study using routinely collected data. BMC Geriatr. 2022;22(1):271. doi:10.1186/s12877-022-02973-0
16. Thapa R, Garikipati A, Shokouhi S, et al. Predicting falls in long-term care facilities: machine learning study. JMIR Aging. 2022;5(2):e35373. doi:10.2196/35373
17. Soyano A, Suzuki M, Harada A, Okada S, Kaminai T. Fall risk assessment tool usage and issues: results of a survey of Japanese Society for Fall Prevention members. Jpn J Fall Prev. 2018;5(1):41–49. Japanese.
18. Tago M, Katsuki NE, Oda Y, Nakatani E, Sugioka T, Yamashita SI. New predictive models for falls among inpatients using public ADL scale in Japan: a retrospective observational study of 7858 patients in acute care setting. PLoS One. 2020;15(7):e0236130. doi:10.1371/journal.pone.0236130
19. Aihara H, Tago M, Oishi T, Katsuki NE, Yamashita SI. Visual impairment, partially dependent ADL and extremely old age could be predictors for severe fall injuries in acute care settings. Int J Gerontol. 2018;12(3):175–179. doi:10.1016/j.ijge.2018.02.014
20. Yokobayashi K, Matsushima M, Watanabe T, Fujinuma Y, Tazuma S. Prospective cohort study of fever incidence and risk in elderly persons living at home. BMJ Open. 2014;4(7):e004998. doi:10.1136/bmjopen-2014-004998
21. Tago M, Hirata R, Katsuki NE, et al. Criterion-related validity of bedriddenness rank with other established objective scales of ADLs, and cognitive function score with those of cognitive impairment, both are easy-to-use official Japanese scales: a prospective observational study. PLoS One. 2022;17(11):e0277540. doi:10.1371/journal.pone.0277540
22. Tago M, Katsuki NE, Yaita S, et al. High inter-rater reliability of Japanese bedriddenness ranks and cognitive function scores: a hospital-based prospective observational study. BMC Geriatr. 2021;21(1):1–10. doi:10.1186/s12877-021-02108-x
23. Tago M, Katsuki NE, Nakatani E, et al. External validation of a new predictive model for falls among inpatients using the official Japanese ADL scale, Bedriddenness ranks: a double-centered prospective cohort study. BMC Geriatr. 2022;22(1):331. doi:10.1186/s12877-022-02871-5
24. Parkkari J, Kannus P, Palvanen M, et al. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int. 1999;65(3):183–187. doi:10.1007/s002239900679
25. Hagino H, Nakamura T, Sakamoto K; Committee for Osteoporosis Treatment of The Japanese Orthopaedic Association. Nationwide survey of hip fractures in Japan. J Orthop Sci. 2004;9(1):1–5. doi:10.1007/s00776-003-0741-8
26. Kim EAN, Mordiffi SZ, Bee WH, Devi K, Evans D. Evaluation of three fall-risk assessment tools in an acute care setting. J Adv Nurs. 2007;60(4):427–435. doi:10.1111/j.1365-2648.2007.04419.x
27. Liu Z, Han L, Leo-Summers L, Gahbauer EA, Allore HG, Gill TM. The subsequent course of disability in older persons discharged to a skilled nursing facility after an acute hospitalization. Exp Gerontol. 2017;97:73–79. doi:10.1016/j.exger.2017.08.004
28. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451–458. doi:10.1046/j.1532-5415.2003.51152.x
29. Avelino-Silva TJ, Farfel JM, Curiati JA, Amaral JR, Campora F, Jacob-Filho W. Comprehensive geriatric assessment predicts mortality and adverse outcomes in hospitalized older adults. BMC Geriatr. 2014;14(1):129. doi:10.1186/1471-2318-14-129
30. Hirata R, Tago M, Katsuki NE, et al. History of falls and bedriddenness ranks are useful predictive factors for in-hospital falls: a single-center retrospective observational study using the Saga Fall Risk Model. Int J Gen Med. 2022;15:8121–8131. doi:10.2147/IJGM.S385168
31. Tago M, Hirata R, Katsuki NE, et al. Validation of predictive model for inpatient falls (Saga Fall Risk Model 2) in a university hospital: a retrospective single-center study. J Hosp Gen Med. 2023;5(3):53–61.
32. Chu LW, Chi I, Chiu AY. Incidence and predictors of falls in the Chinese elderly. Ann Acad Med Singap. 2005;34(1):60–72. doi:10.47102/annals-acadmedsg.V34N1p60
33. Najafpour Z, Godarzi Z, Arab M, Yaseri M. Risk factors for falls in hospital in-patients: a prospective nested case control study. Int J Health Policy Manag. 2019;8(5):300–306. doi:10.15171/ijhpm.2019.11
34. Mamikonian-Zarpas A, Laganá L. The relationship between older adults’ risk for a future fall and difficulty performing activities of daily living. J Aging Gerontol. 2015;3(1):8–16. doi:10.12974/2309-6128.2015.03.01.2
35. Hayakawa T, Hashimoto S, Kanda H, et al. Risk factors of falls in inpatients and their practical use in identifying high-risk persons at admission: fukushima medical university hospital cohort study. BMJ Open. 2014;4(8):e005385. doi:10.1136/bmjopen-2014-005385
36. Galeoto G, Lauta A, Palumbo A, et al. The Barthel index: Italian translation, adaptation and validation. Int J Neurol Neurother. 2015;2:2378–3001.
37. Ishida Y, Maeda K, Nonogaki T, et al. Malnutrition at admission predicts in-hospital falls in hospitalized older adults. Nutrients. 2020;12(2):541. doi:10.3390/nu12020541
38. Lee YS, Choi EJ, Kim YH, Park HA. Factors influencing falls in high- and low-risk patients in a tertiary hospital in Korea. J Patient Saf. 2020;16(4):e376–e382. doi:10.1097/PTS.0000000000000593
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

