Back to Journals » International Journal of Nanomedicine » Volume 19
A Big Prospect for Hydrogel Nano-System in Glioma
Authors Zhang L, Teng F, Xin H, Xu W, Wu W, Yao C, Wang Z
Received 17 April 2024
Accepted for publication 7 June 2024
Published 11 June 2024 Volume 2024:19 Pages 5605—5618
DOI https://doi.org/10.2147/IJN.S470315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Lu Zhang,1– 3,* Fei Teng,1,2,* Huajie Xin,1,2 Wei Xu,1,2 Wei Wu,4 Chenguo Yao,3 Zhiqiang Wang1,2
1Key Laboratory for Biorheological Science and Technology of Ministry of Education (Chongqing University), Chongqing University Cancer Hospital, Chongqing, 400030, People’s Republic of China; 2Center of Thoracic Cancer, Chongqing University Cancer Hospital, Chongqing, 400030, People’s Republic of China; 3The State Key Laboratory of Power Transmission Equipment and System Security and New Technology, College of Electrical Engineering, Chongqing University, Chongqing, 400044, People’s Republic of China; 4College of Biological Engineering, Chongqing University, Chongqing, 400030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Zhang; Zhiqiang Wang, Chongqing University Cancer Hospital, No. 181 Hanyu Road, Shapingba District, Chongqing, 400030, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Patients diagnosed with glioma typically face a limited life expectancy (around 15 months on average), a bleak prognosis, and a high likelihood of recurrence. As such, glioma is recognized as a significant form of malignancy. Presently, the treatment options for glioma include traditional approaches such as surgery, chemotherapy, and radiotherapy. Regrettably, the efficacy of these treatments has been less than optimal. Nevertheless, a promising development in glioma treatment lies in the use of hydrogel nano-systems as sophisticated delivery systems. These nano-systems have demonstrated exceptional therapeutic effects in the treatment of glioma by various responsive ways, including temperature-response, pH-response, liposome-response, ROS-response, light-response, and enzyme-response. This study seeks to provide a comprehensive summary of both the therapeutic application of hydrogel nano-systems in managing glioma and the underlying immune action mechanisms.
Keywords: glioma, hydrogel nano-system, immunotherapy, tumor immune microenvironment
Graphical Abstract:
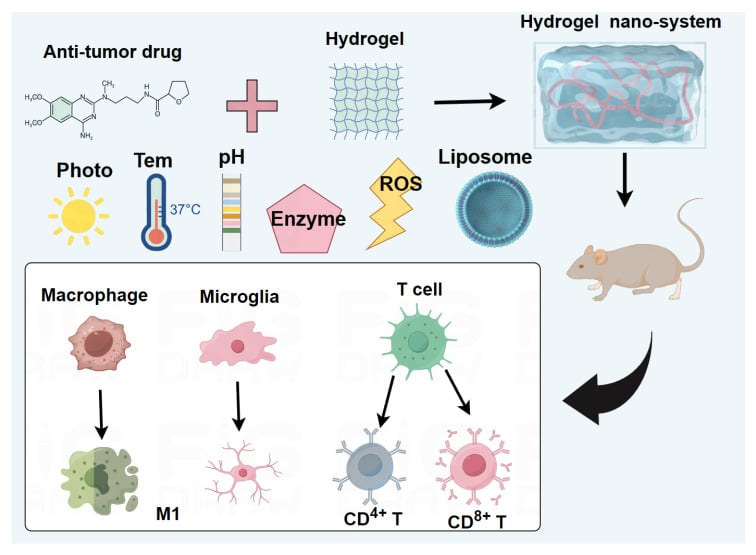
Introduction
Brain tumors, especially glioma, represent a highly malignant form of cancer with a propensity for recurrence, drug resistance, and metastasis.1 Current treatment methods for glioma primarily involve surgery, chemotherapy, and radiotherapy.2 However, the effectiveness of these conventional approaches is limited. Tumor recurrence often follows surgical resection, chemotherapy drugs struggle to penetrate the blood-brain barrier (BBB), leading to significant side effects, and radiotherapy may harm normal brain tissue and impair brain function.3
To address these challenges, researchers have explored various drug carriers, such as liposomes, to improve drug delivery across the BBB. Although liposomes possess the advantage of traversing the BBB, their short half-life in the body hampers their ability to achieve high drug concentrations at the tumor site.4 Another approach involves utilizing biomimetic membranes to prolong the retention of therapeutic agents at the tumor site by evading immune clearance mechanisms. However, previous attempts have been undermined by the tumor microenvironment (TME), which contributes to the frequent recurrence of glioma.5 In addition, there are some conventional nano-materials, which have some evident drawbacks, such as significant side effects,6 poor biocompatibility,7 and lack of targeting specificity.8
A novel nano-material, hydrogel, has emerged as a promising candidate for glioma therapy. Hydrogel-based nano-systems have demonstrated broad applications in the management of brain tumors.9–11 Notably, a polypeptide hydrogel was injected into a mouse glioma model, induced CD8+ T cell responses, promoted M1 macrophage polarization, regulated the expression of hypoxia-inducible factor 1 alpha (HIF1-α), programmed cell death ligand 1 (PDL1), and C-X-C motif chemokine ligand 9 (CXCL9), and reshaped the tumor immune microenvironment (TIME) to inhibit tumor growth.12 Recent research confirms that nano-hydrogel system injected into a mouse glioma model following tumor resection, improved antigen presentation, activated CD8+ T cells and NK cells, reversed the immunosuppressive TME, and inhibited tumor recurrence.9 In addition, studies have shown that utilizing a silk fibroin (SF) hydrogel coupled with a photosensitizer to interfere with a mouse glioma model and observed anti-glioma effects.13
Consequently, the hydrogel nano-system holds tremendous promise as a novel and innovative approach for the treatment of glioma.
BBB Restricts the Direct Use of Anti-Cancer Drugs in Brain
The BBB is a unique tissue structure in the brain, mainly formed by the endothelial cells of blood vessels and glial cells, which acts as a barrier between the blood and brain cells. It can block the entry of foreign substances into the brain and maintain a relatively stable brain environment.14 Primary gliomas originate within the brain, and due to the presence of the BBB, traditional anti-cancer drugs cannot penetrate the BBB to kill glioma cells.15 Therefore, the development of efficient nano-materials to overcome the BBB is crucial for enhancing the effectiveness of brain tumor treatments.
Hydrogel Materials
Currently, hydrogel nano-materials utilized in the treatment of glioma can be categorized into two main types: natural polymers and chemically synthesized polymers. Natural polymer hydrogels encompass a range of materials such as gelatin,16 chitosan,17 alginate,18 hyaluronic acid (HA),19 collagen,20 agarose,21 SF,13 pectin,22 and so on (Table 1).
 |
Table 1 Application of Different Combinations of Hydrogels Materials and Anti-Cancer Drugs in Glioma |
Among them, Gelatin, collagen, and SF are derived from natural protein molecules, offering non-toxicity and excellent biological safety, making them suitable for hydrogel preparation. Chitosan and pectin, both natural polysaccharides albeit from different sources, also exhibit good biocompatibility and can undergo degradation in vivo. Alginate, hyaluronic acid, and agarose can all be extracted from natural sources, which make them suitable choices for hydrogel formulation.
Chemically synthesized polymer hydrogel materials in brain tumor treatment include Polyethylene glycol (PEG),27 Pluronic F-127 (PF127),33 diblockcopolypeptide hydrogels (DCH),30 poly(2-hydroxymethyl) methacrylate (P-HEMA),34 polyacrylic acid (PAA),35 Fmoc-FF,36 and others.
Hydrogels prepared from chemically synthesized polymers offer not only the advantages of natural materials but also possess unique properties. For instance, PEG modified with Polyethylene glycol dimethacrylate can undergo sustained-release of antineoplastic drugs upon UV radiation for glioma treatment.27 PF127 is a temperature-sensitive material that can alter its structure in response to changes in temperature, making it suitable for use in temperature-responsive hydrogel nano-systems.33 P-HEMA is a pH-sensitive polymer that can dissociate in the acidic environment of tumors, enabling it to function as a sustained-release drug carrier for anti-tumor purposes.37 PAA, containing abundant carboxyl groups, has the capability to adsorb proteins, thus serving as a carrier for protein drugs in anti-tumor applications.35 Fmoc-FF, a negatively charged dipeptide, can self-assemble into fiber hydrogels under mild conditions and enhance the anti-tumor immune response.36
In general, chemically synthesized polymer materials offer certain advantages in functional properties compared to natural materials. However, hydrogels prepared from natural materials possess unparalleled advantages in terms of biosafety, biocompatibility, and degradability. They are less prone to immune rejection and other related issues. Additionally, natural materials have a wide range of sources, lower costs, and are generally more readily applicable for commercial conversion and widespread use.
Anti-Cancer Drugs
Currently, anti-cancer drugs used for the treatment of tumors can be classified into several categories, including plant alkaloids, anti-metabolic agents, hormones, antibiotics, alkylating agents, and more. In the context of brain tumor treatment, some commonly used anti-tumor drugs include paclitaxel (PTX),38 temozolomide (TMZ),39 camptothecin (CPT),40 cisplatin (Cis),41 curcumin (Cur),42 doxorubicin (DOX),43 and others (Table 2).
 |
Table 2 The Mode of Action of Nano-Drugs Constructed from Different Materials on Glioma |
Therapeutic Effect of Multiplexresponse Pathways of Hydrogel Nano-Systems on Glioma
Based on the characteristics of different functional molecules and hydrogels, the hydrogel nano-systems can be categorized into various types, including temperature-responsive hydrogel nano-systems, pH-responsive hydrogel nano-systems, liposome-responsive hydrogel nano-systems, ROS-responsive hydrogel nano-systems, light-responsive hydrogel nano-systems, and enzyme-responsive hydrogel nano-systems. The hydrogel nano-systems hold great potential for the treatment of glioma, as depicted in Figure 1.
 |
Figure 1 Schematic diagram of response pathways of hydrogel nano-system in glioma. |
pH-Responsive Hydrogel Nano-System
The TME is characterized by low pH, which is primarily attributed to the rapid proliferation of tumor cells, increased metabolism, and accumulation of metabolic byproducts. Taking advantage of this acidic characteristic of the TME, researchers have developed pH-responsive hydrogel systems for targeted treatment of glioma, yielding promising results (Figure 2).
 |
Figure 2 Establishment of targeted therapy of brain tumors with hydrogel nano-systems based on ROS, pH, liposome, temperature, photo, and enzyme response. |
Hydrogel materials, such as gelatin, PF127, and CS, are particularly suitable for pH-responsive hydrogel formulations, whose structures can undergo changes in response to the acidic TME, resulting in controlled drug release. For instance, a study reported the use of an oligomeric sulfonamide gelatin vehicle for PTX delivery, injected into an in situ glioma nude mouse model. PTX release was delayed in the acidic TME, leading to sustained and slow release of PTX, thereby inhibiting tumor growth.16 Similarly, Turabee et al designed a pH-responsive hydrogel carrier using PF127/trimethyl chitosan, loaded with DOX, and administered it in an in situ glioma nude mouse model. They observed gradual release of DOX within the tumor acidic microenvironment over an extended period of more than one month, effectively achieving tumor suppression.49 And then, it is reported that a multifunctional hybrid hydrogel system (CP&CL@RNPPTX-Gel) injected into a mouse glioma model after resection. Within the tumor’s acidic environment, copper peroxide nanoparticles decomposed into Cu2+ and hydrogen peroxide, exerting a chemotherapeutic effect on the tumor.51 Additionally, some studies have found that hydrogel scaffolds using thermally condensed β-glycerophosphate (BGP), hydroxyethyl cellulose (HEC), chitosan (CS), as well as cellulose nanocrystals (CNCS) encapsulated and preserved the functionality of tumor acidic environmental stem cells, serving as a postoperative treatment for glioma.17
These studies demonstrate the potential of pH-responsive hydrogel systems in targeted therapy for brain tumors by leveraging the specific characteristics of the TME.
Liposome-Responsive Hydrogel Nano-System
Liposomes, which are bilayer lipid molecules with sizes ranging from 10 to 500 nm, are commonly used as drug delivery vehicles for water-insoluble antineoplastic drugs such as PTX. They offer targeted penetration of the BBB, making them suitable for brain tumor treatment. Researchers have combined liposomes with hydrogels to construct liposome hydrogel systems for more effective therapy for brain tumors (Figure 2).
Recently, there is a study that on a liposome hydrogel system where curcumin (Cur) and liposomes (Cur@Lip) were coated with positive chitosan oligosaccharides (Cur@Lip-Cos). This coating improved water solubility and entrapment efficiency, delayed the release of Cur, enhanced stability, cell uptake, and biological activity of the system. The liposomes were then incorporated into an injectable thiochitosan hydrogel, enabling local immobilization and sustained release. This strategy effectively delayed the release of Cur, leading to the inhibition of breast cancer MCF-7 cell growth.52 The researcher developed a liposome hydrogel system containing gold cluster-labeled adriamycin liposomes (DOX) and chitosan hydrogel (CH-HG-GLDOX), which was directly injected into tumor tissue. This approach prolonged the retention time of DOX in the tumor tissue, significantly inhibiting tumor growth while reducing DOX toxicity.53 And then, a new type of core-shell nanostructure, liposome template hydrogel nanoparticles (LHNPs), which utilized a microring DNA technique to target the inhibition of Polo-like kinase 1 (PLK1) expression in brain tumors. This approach effectively inhibited tumor growth and improved the survival rate of tumor-bearing mice.54 Furthermore, a liposome hydrogel incorporating the tumor-targeting photosensitizer IR780 (IR780/lipo/Gels), which efficiently transported IR780 to subcutaneous tumors and deep metastases. Hydrogels were applied either to the skin above the tumor or distant normal skin areas. Remarkably, local application of the hydrogel significantly inhibited tumor growth without causing any toxicity.55
These studies demonstrate the potential of liposome hydrogel systems for targeted drug delivery and enhanced therapeutic outcomes in brain tumor treatment.
Temperature-Responsive Hydrogel Nano-System
Researchers have utilized the thermosensitivity of certain materials to develop hydrogels that exhibit temperature-responsive behavior (Figure 2). These hydrogels can undergo physical changes, such as solid-liquid conversion, when the temperature is controlled in vitro. In vivo, the physical properties of these hydrogels can be regulated by the body’s natural temperature. One example of a thermosensitive material commonly used for constructing temperature-responsive hydrogel systems is Pluronic F-127 (PF127).
In the context of brain tumor treatment, researchers have explored temperature-responsive hydrogel systems with various functionalities. For instance, a hydrogel nano-system, into whichsuperparamagnetic iron oxide nanoparticles (SPION) and doxorubicin (DOX) were co-loaded. Under an alternating magnetic field (AMF), heat was generated, triggering drug release and directly inhibiting the proliferation of glioma cells.56 Similarly, the combination of superparamagnetic iron oxide nanoparticles and hydrogel has been investigated for the treatment of gliomas. an oligosulfonamides grafted gelatin hydrogel system loaded with the anti-cancer drug PTX, which underwent a phase transition from liquid to solid upon stimulation by body temperature, forming a porous structure that continuously released PTX, effectively inhibiting glioma recurrence.16 And, a copolymer poly (N-isopropylacrylamide-co-Jeffamine VR-MMI 1000 acrylamide-hydroxyethyl methacrylate-RGD), referred to as PNJ-RGD, which exhibited reverse gelation in aqueous solution when heated under normal cell culture conditions (37°C). The PNJ-RGD hydrogel system could be potentially employed for the treatment of glioma through its temperature-responsive properties.57 Next, a multifunctional hybrid hydrogel system (CP&CL@RNPPTX-Gel) composed of self-luminescent e6 (Ce6), copper peroxide nanoparticles (CPNDS) loaded with luminol molecules (CL), glioma-targeting nanoparticles, and embedded in a three-dimensional thermosensitive hydroxypropyl chitin hydrogel framework was developed. Upon injection into the postoperative cavity of glioma, the solution transformed into a gel structure as a drug repository under the stimulation of body temperature. This system enabled sustained release of CPNDS for dynamic therapy (CDT) and photodynamic therapy (PDT).51
Furthermore, a liposome hydrogel system called LiP-Gel by embedding near-infrared photothermal agents (DPP-BTz) and chemotherapeutic drugs (GEM) in temperature-sensitive liposomes, combined with a hydrogel precursor solution, which was injected into mouse tumors as an injectable flow solution at room temperature, then transformed into a cross-linked gel structure at physiological temperature. Photodynamic therapy was performed to destroy the thermosensitive liposomes and release GEM to kill tumor cells.58
While some of these studies focus on applications in other types of tumors, they underscore the potential of temperature-responsive hydrogel systems and their broad application Prospects in various fields, including brain tumor treatment.
Photo-Responsive Hydrogel Nano-System
The incorporation of a photosensitizer into hydrogel materials can alter the properties of the hydrogel and enable light-induced effects on the TME or tumor cells, leading to tumor inhibition. Photodynamic therapy (PDT) is an approach that utilizes light energy to induce various effects, including changes in the TIME or direct killing of tumor cells (Figure 2). This strategy holds promise for inhibiting tumor recurrence, which is a significant clinical challenge, particularly in malignant tumors such as glioma.
A lot of literature has been reported, such as an injectable hydrogel system called DNA-UCNP-Au nanoparticle hybrid (DNA-UCNP-Au), which utilized near-infrared light response to convert light energy into chemical energy, effectively killing tumor cells through photodynamic therapy. This approach has potential applications in the treatment of glioma, where inhibiting postoperative tumor recurrence plays a critical role.59 Additionally, photopolymerized hydrogels using polyethylene glycol diacrylate (PEGDA) for drug screening was designed on 3D brain tumor chips. Incorporating photopolymerization techniques into hydrogel fabrication allows for precise control over the gelation process using light exposure, enabling the development of advanced platforms for drug screening in brain tumor models.60
These studies highlight the use of photosensitizers in hydrogel systems to leverage light-based therapies, such as photodynamic therapy and photopolymerization, for tumor inhibition and drug screening applications in brain tumors.
Enzyme-Responsive Hydrogel Nano-System
In vivo, enzymes play a vital role as proteins with catalytic functions. Researchers have combined enzymes with hydrogels to construct enzyme-responsive hydrogel nano-systems for targeted therapy of brain tumors (Figure 2).
Previous reports have highlighted the involvement of matrix metalloproteinases (MMPs) as responsive enzymes in hydrogel nanosystems. MMPs play a significant role in the degradation, remodeling, and regulation of the extracellular matrix (ECM). These enzymes are not only critical for normal physiological processes but also contribute to tumor cell invasion and metastasis. Recently, a self-assembled hydrogel system using polypropylsulfide 60 and triglyceride hydrogel carrier loaded with temozolomide (TMZ) was developed. Upon injection into a mouse glioma model after surgery, this hydrogel system effectively inhibited the growth and recurrence of glioma through a matrix metalloenzyme response. The hydrogel depolymerized in response to matrix metalloproteinases, leading to the release of TMZ and its therapeutic effect on glioma.61 Polyethylene glycol diacrylate hydrogels with different chain lengths complexed with matrix metal oxide substrates (polypeptide conjugates) and encapsulated with cisplatin, a chemotherapeutic drug, which could trigger the release of cisplatin upon contact with MMPs, thereby increasing its biological activity. This approach allows for the slow release of antineoplastic drugs, enabling tumor cell killing through the activity of matrix metalloenzymes in the tumor microenvironment.62 Interestingly, the hydrogel system consists of hyaluronic acid, gelatin, and polyethylene glycol. Gelatin, one of the components of the hydrogel, contains a degradation site that is sensitive to MMPs. This design allows for controlled degradation of the hydrogel by MMPs, enabling precise modulation of the extracellular matrix remodeling process. Upon degradation, the hydrogel induced gliom a cells to overexpress matrix metalloproteinase 2 (MMP-2) and promote the expression of Epidermal Growth Factor Receptor (EGFR), thereby influencing tumor behavior.63
These studies illustrate the use of enzyme-responsive hydrogel nano-systems for targeted therapy in brain tumors, where the presence of specific enzymes in the TME triggers controlled drug release or influences tumor cell behavior.
ROS-Responsive Hydrogel Nano-System
The accelerated metabolism of tumor cells leads to the generation of reactive oxygen species (ROS), including superoxide radicals, hydrogen peroxide, and hydroxyl radicals, among others. Tumor cells often exhibit high expression of GSH to counteract the damaging effects caused by ROS.64 ROS can disrupt cell membranes, induce oxidative stress in cells, and lead to cell apoptosis. However, GSH can undergo redox reactions with ROS, neutralizing the negative effects caused by ROS. Therefore, exploiting the high expression of GSH in the TME, researchers have developed hydrogel nano-systems that provide new avenues for treating the glioma (Figure 2).
In the latest report, a self-assembled hydrogel system using polypropyl sulfide 60 (PPS) and triglyceride hydrogel carrier loaded with TMZ, which was injected into a mouse glioma model after surgery, effectively inhibited the growth and recurrence of glioma through a response to ROS, which can decompose PPS into Poly (pepopylenesulfone)60 and Poly (propylene sulfoxide)60, causing the disintegration of hydrogel nano-systems and the sustained release of TMZ and its therapeutic effect on glioma.61 Zhuo et al developed a hydrogel nano-systems (cRGD/PSDOX-Cur@NPs) that can penetrate the BBB. This system is designed to respond to high levels of GSH in the TME, which triggers the decomposition of a disulfide bond-bridged PEG-SS-DOX prodrug and releases DOX for killing the glioma cells.65
These findings highlight the potential of hydrogel nano-systems that take advantage of the high expression of GSH in the TME, providing a novel approach for the treatment of gliomas. By designing hydrogels capable of responding to ROS, controlled drug release and targeted therapy can be achieved in glioma treatment.
The Effect of Hydrogel Nano-System on Glioma
Researchers have made significant advancements in the field of hydrogel nano-systems for the treatment of brain tumors, including gliomas. By constructing hydrogel nano-systems that respond to various stimuli such as pH, liposomes, temperature, light, enzymes, ultrasound waves, and ROS, and researchers have achieved promising anti-tumor effects.
For instance, a temperature-responsive hydrogel system was developed for the treatment of glioma, which was injected by a liquid solution containing drug-loaded micelles and water-dispersible ferromagnetic nanocubes into the resected glioma site. The gel nano-materials solidified with a change in temperature, and an external magnetic field was applied to induce heat generation from the ferromagnetic nano-materials. This approach facilitated targeted drug delivery, resulting in tumor growth inhibition in situ and improved survival rates in glioma mice.66 Moreover, an injectable pre-injection drug hydrogel delivery system that can be activated by ultrasound, which involved the use of semiconductor polymer nanoparticles (SPN) and an inhibitor of indoleamine 2,3-dioxygenase 1 (NLG919) prodrug connected through 1O2 cleavable connectors. Under ultrasound irradiation, the SPN acted as an ultrasound sensitizer, generating 1O2 for sonodynamic therapy (SDT). The released 1O2 induced immunogenic cell death and activated NLG919 prodrugs, enhancing anti-tumor immunity and inhibiting tumor growth in situ glioma-bearing mice.67
In recent research, double-responsive hydrogel nanosystems have been explored for anti-glioma treatments. A multi-functional hybrid hydrogel system (CP&CL@RNPPTX-Gel) composed of self-luminescent e6 (Ce6) coupled with copper peroxide nanoparticles (CPNDS) loaded with luminol molecules (CL), glioma targeting nanoparticles, and embedded in a thermosensitive hydroxypropyl chitin hydrogel framework, which exhibited sustained-release properties and achieved chemical kinetic therapy (CDT) through the decomposition of CPNDS into Cu2+ and H2O2 in the acidic microenvironment of glioma cells. Furthermore, it induced photodynamic therapy (PDT) using the spontaneous luminescence of CL catalyzed by Cu2+. The prodrug nanoparticles also inhibited the response of intracellular reduced glutathione (GSH) in residual glioma cells. This multi-functional hybrid hydrogel system demonstrated efficacy in postoperative chemotherapy, CDT, and PDT treatment of glioma.51
Furthermore, constructing hydrogel nano-systems that respond to reactive oxygen species (ROS) in the TME has proven to be an effective strategy. After resection of glioblastoma, an in situ dual-sensitive hydrogel drug delivery system loaded with polylactic-glycolic acid nanoparticles (NPs) sensitive to ROS, encapsulating BCNU and TMZ drugs, which was injected into the resection cavity. The median survival time of the experimental group was significantly longer than that of the control group, indicating that in situ the drug delivery system effectively inhibited the tumor recurrence.68
These examples illustrate the potential of hydrogel nano-systems that respond to various stimuli for the treatment of brain tumors, particularly gliomas. By leveraging the unique properties and responses of hydrogels, researchers are advancing targeted therapies with improved anti-tumor effects.
Hydrogel Nano-System Reshapes the TIME in Glioma
Previous studies have indicated a close relationship between the occurrence and development of glioma and the TIME. The microenvironment of glioma contains various immune cells, including microglia cells (specific to the brain), and peripheral lymphoid T cells. Hydrogel nano-systems have shown potential in improving the immune microenvironment of glioma by activating immune cells and inhibiting tumor growth (Figure 3).
Firstly, the hydrogel nano-system activates the immunity of lymphoid T cells. A new study reports that an in situ sustained release hydrogel delivery system, incorporating glioma homing peptide modified paclitaxel targeting nanoparticles (PNPPTX) and mannitol immunoadjuvant CpG targeted nanoparticles (MNPCpG) into PLGA1750-PEG1500-PLGA1750 thermosensitive hydrogel skeletons (PNPPTX and MNPCpG@Gel). When injected into the glioma resection cavity, this targeted nanoparticles-hydrogel hybrid system forms a gel drug repository that releases PNPPTX, which targets residual glioma cells to produce tumor antigens. Simultaneously, MNPCpG targets and activates antigen-presenting cells (APCs), enhancing tumor antigen presentation, activating CD8+ T cells and NK cells, and reversing the TIME in the glioma. This study demonstrates that the PNPPTX&MNPCpG@Gel system can improve glioma treatment through chemical immunotherapy.9 Additionally, hydrogel nano-systems reshape the microenvironment to effectively reduce glioma recurrence. Next, the researchers found that self-assembled paclitaxel (PTX) fiber (PF) hydrogel containing aCD47 can be directly deposited into the tumor resection cavity, seamlessly filling it and releasing two therapeutic drugs over an extended period. PTX and PFS induce an immunostimulatory TME, making the tumor sensitive to aCD47-mediated blocking of “don’t eat me” signals, thus promoting macrophage phagocytosis of tumor cells and triggering anti-tumor T cell responses. Surprisingly, CD47 binds to SIRP α to activate the cGAS/STING signal pathway of CD103+ dendritic cells, and the secreted CXCL9 triggers NK cells to produce IFN- γ and TNF- α, killing tumor cells.69 This approach effectively inhibits primary brain tumor recurrence, prolongs overall survival time, and minimizes non-targeted side effects.70 Intriguingly, a nano-hydrogel system encapsulated with intracavitary sprayable nano-modulator (NR) was used in situ GBM model and postoperative recurrence model, and the results of the study found that degradable NR-mediated 7-dehydrocholesterol reductase (DHCR7) ablation effectively suppresses cholesterol supply and activates T cell immunity. Furthermore, the combined use of Toll-like receptor 7/8 (TLR7/8) agonist significantly promotes the transition from GSM polarization to an anti-tumor phenotype, enhancing the TME and demonstrating improved anti-tumor effects. These findings reveal the role of glioma-supportive macrophages (GSMs) 7-dehydrocholesterol reductase (DHCR7) / cholesterol signaling in TME regulation and suggest a potential therapeutic strategy for future clinical trials.71
Secondly, the hydrogel nano-system activates the immunity of microglia. Microglia, being unique immune cells of the brain, play a crucial role in glioma development. Hydrogel nano-systems can reshape microglial polarization, which may be a key mechanism for inhibiting glioma progression. An injectable nanoparticle-hydrogel system using DOX loaded mesoporous dopamine (MPDA) nanoparticles encapsulated in M1 macrophage-derived nanocapsules (M1Nvs) as effectors, with fibrin hydrogels serving as in situ delivery carriers, which was delivered into the tumor cavity in a subtotal resection model, this hydrogel system triggers photochemical immunotherapy to destroy remaining tumor cells. Simultaneously, M1Nvs reprogram M2-like TAMs to M1-like TAMs, comprehensively improving the TIME. The combination of the hydrogel system and near-infrared laser effectively promotes T cells infiltration, restores effector function, inhibits myeloid suppressor cell and regulatory T cells infiltration, and generates a robust anti-tumor immune responses, significantly suppressing tumor growth.72
Thirdly, the hydrogel nano-system activates the immunity of macrophages (from peripheral macrophages). A temperature-sensitive hydrogel loaded with a novel non-viral vector, G5-BGG, forming a gene complex with an shRNA vector- G5-BGG / shRNA871 complex, which down-regulates CD47 protein expression, enhancing phagocytosis of bone marrow-derived macrophages by U87 MG cells. The G5-BGG / PDNA complex is embedded in a poly (lactide-glycolide)-b-polyethylene glycol-b-poly (lactide-glycolide) (PLGA-PEG-PLGA) hydrogel. Studies have confirmed that the G5-BGG/PDNA complex remains integrated in the hydrogel, releasing continuously for up to 7 days. In an in vivo tumor model following in situ U87 MG surgery, the G5-BGG/shRNA871 hydrogel, combined with temozolomide, down-regulates CD47 protein expression, promotes macrophage invasion into residual tumors, and significantly prolongs mouse survival time.73 Besides, a cavity-injectable nanotube-hydrogel superstructure capable of producing glioma stem cell (GSC)-specific chimeric antigen receptor (CAR) macrophages/microglia around the cavity, which delivers GSC-targeted CAR genes into MΦ nuclei, producing CAR-MΦs in mouse models.10 These CAR-MΦs can locate and phagocytize GSCs, stimulate adaptive anti-tumor immune responses within the TME, and prevent postoperative glioma recurrence by inducing long-term anti-tumor immunity in mice. In an in situ patient-derived glioblastoma humanized mouse model, the combination of nano-hydrogel ultrastructure and CD47 antibody increases the frequency of positive immunoreactive cells, inhibits negative immunomodulatory cells, generates strong anti-tumor immunity in the postoperative cavity, and hinders glioblastoma recurrence.10
Finally, the hydrogel nano-system activates the immunity of lymphoid T cells and macrophages. An effective anti-glioblastoma (GBM) hydrogel nano-system encapsulated C-novyi spores with melittin-RADA32 nanofiber hybrid peptides and loading them with the immunomodulator metformin is developed based on activation time, resulting in MRM-encapsulated spores. MRM-coated spores exhibit prolonged release and enhanced killing effects on GBM in vitro and in vivo. Furthermore, these MRM-coated spores educate the innate and adaptive immune systems by inducing continuous CD8+ T cell responses, promoting M1 macrophage polarization, and regulating the timely expression of HIF1-α, PDL1, and CXCL9.12
Prospect
The hydrogel nano-system, as the name suggests, is composed of hydrogels and nanoparticles. It possesses the advantages of both hydrogels, such as biocompatibility, and nanoparticles, such as targeted drug delivery. In the context of brain tumors, which present challenges like the BBB, drug targeting, and biosafety, the hydrogel nano-system shows great potential as an intervention strategy. Hydrogels can be classified into natural polymers and synthetic polymer materials, each with its own unique characteristics. Nanoparticles, on the other hand, can carry various anti-tumor drugs to target tumor cells. By combining and optimizing these two components in different ways (as shown in Table 1), the anti-tumor advantages of hydrogel nano-systems can be fully utilized. Furthermore, the incorporation of functional molecules into hydrogels, such as pH-sensitive, liposome-based, temperature-responsive, photo-responsive, enzyme-responsive, ROS-responsive, and other response, can enhance their therapeutic efficacy. One key focus of future research is to utilize the characteristics of the tumor microenvironment, more responsive treatment strategies can be proposed. Furthermore, an urgent issue that needs to be addressed is the administration route of hydrogel nano-systems. Currently, it should be noted that most in situ hydrogel nano-systems are administered via intratumoral injection, limiting their ability to circulate systemically and precisely target tumor cells when administered intravenously. Therefore, further improvements are needed for the implementation of hydrogel nano-systems.
Conclusion
The hydrogel nano-system represents a groundbreaking and promising strategy in the treatment of gliomas, a category of brain tumors. By merging the attributes of hydrogels and nanoparticles via various methodologies, researchers have successfully harnessed the strengths of both components. This has led to considerable advances in modulating the TIME, curbing the proliferation of glioma, averting postoperative relapse, and ultimately enhancing survival rates in animal models. These compelling findings underscore the potential of the hydrogel nano-system as a promising avenue for therapeutic intervention in clinical scenarios involving gliomas.
Abbreviations
HIF1-α, hypoxia-inducible factor 1 alpha; PDL1, programmed cell death ligand 1; CXCL9, C-X-C motif chemokine ligand 9; TIME, Tumor immune microenvironment; TME, tumor microenvironment; BBB, blood-brain barrier; TX, paclitaxel; TMZ, temozolomide; CPT, camptothecin; Cis, cisplatin; Cur, curcumin; DOX, doxorubicin; HA, hyaluronic acid; SF: silk fibroin; APCs: antigen-presenting cells.
Acknowledgments
This research project is supported by Scientific and Technological Research Program of Chongqing Municipal Education Commission (Grant No. KJQN202300127) and the Natural Scinence Foundation of Chongqing (CSTB2022NSCQ-MSX1143). We thank for the help from Figdraw.com for drawing illustration.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Barthel L, Hadamitzky M, Dammann P, et al. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022;41(1):53–75. doi:10.1007/s10555-021-09997-9
2. Mou Y, Zhang P, Lai W-F, et al. Design and applications of liposome-in-gel as carriers for cancer therapy. Drug Deliv. 2022;29(1):3245–3255. doi:10.1080/10717544.2022.2139021
3. Afshari AR, Mollazadeh H, Henney NC, et al. Effects of statins on brain tumors: a review. Semin Cancer Biol. 2021;73:116–133. doi:10.1016/j.semcancer.2020.08.002
4. Zamani P, Penson PE, Barreto GE, et al. Recent advancements in liposome-based strategies for effective drug delivery to the brain. Curr Med Chem. 2021;28(21):4152–4171. doi:10.2174/0929867328666201218121728
5. Dong CY, Huang Q-X, Cheng H, et al. Neisseria meningitidis Opca Protein/MnO 2 hybrid nanoparticles for overcoming the blood–brain barrier to treat glioblastoma. Adv Mater. 2022;34(12):e2109213. doi:10.1002/adma.202109213
6. Pogribna M, Hammons G. Epigenetic effects of nanomaterials and nanoparticles. J Nanobiotechnology. 2021;19(1):2. doi:10.1186/s12951-020-00740-0
7. Kus-Liśkiewicz M, Fickers P, Ben Tahar I. Biocompatibility and cytotoxicity of gold nanoparticles: recent advances in methodologies and regulations. Int J Mol Sci. 2021;22(20):10952. doi:10.3390/ijms222010952
8. Ferreira-Faria I, Yousefiasl S, Macário-Soares A, et al. Stem cell membrane-coated abiotic nanomaterials for biomedical applications. J Control Release. 2022;351:174–197. doi:10.1016/j.jconrel.2022.09.012
9. Wang X, Ye L, He W, et al. In situ targeting nanoparticles-hydrogel hybrid system for combined chemo-immunotherapy of glioma. J Control Release. 2022;345:786–797. doi:10.1016/j.jconrel.2022.03.050
10. Chen C, Jing W, Chen Y, et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med. 2022;14(656):eabn1128. doi:10.1126/scitranslmed.abn1128
11. Tang M, Xie Q, Gimple RC, et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020;30(10):833–853. doi:10.1038/s41422-020-0338-1
12. Zhu L, Liu J, Qiu M, et al. Bacteria-mediated metformin-loaded peptide hydrogel reprograms the tumor immune microenvironment in glioblastoma. Biomaterials. 2022;288:121711. doi:10.1016/j.biomaterials.2022.121711
13. Yao Q, Lan Q-H, Jiang X, et al. Bioinspired biliverdin/silk fibroin hydrogel for antiglioma photothermal therapy and wound healing. Theranostics. 2020;10(25):11719–11736. doi:10.7150/thno.47682
14. Alahmari A. Blood-brain barrier overview: structural and functional correlation. Neural Plast. 2021;2021:6564585. doi:10.1155/2021/6564585
15. Wang J, Wang Z, Zhang G, et al. Blood-brain barrier-crossing dendrimers for glioma theranostics. Biomater Sci. 2024;12(6):1346–1356. doi:10.1039/D4BM00043A
16. Kang JH, Turabee MH, Lee DS, et al. Temperature and pH-responsive in situ hydrogels of gelatin derivatives to prevent the reoccurrence of brain tumor. Biomed Pharmacother. 2021;143:112144. doi:10.1016/j.biopha.2021.112144
17. King JL, Maturavongsadit P, Hingtgen SD, et al. Injectable pH thermo-responsive hydrogel scaffold for tumoricidal neural stem cell therapy for glioblastoma multiforme. Pharmaceutics. 2022;14(10):2243. doi:10.3390/pharmaceutics14102243
18. Solano AG, Dupuy J, Therriault H, et al. An alginate-based macroporous hydrogel matrix to trap cancer cells. Carbohydr Polym. 2021;266:118115. doi:10.1016/j.carbpol.2021.118115
19. Safarians G, Sohrabi A, Solomon I, et al. Glioblastoma spheroid invasion through soft, brain-like matrices depends on hyaluronic Acid-CD44 interactions. Adv Healthc Mater. 2023;12(14):e2203143. doi:10.1002/adhm.202203143
20. Clark CC, Yoo KM, Sivakumar H, et al. Immersion bioprinting of hyaluronan and collagen bioink-supported 3D patient-derived brain tumor organoids. Biomed Mater. 2022;18(1):015014.
21. Ravi M, Ramesh A, Pattabhi A. Human brain malignant glioma (BMG-1) 3D aggregate morphology and screening for cytotoxicity and anti-proliferative effects. J Cell Physiol. 2017;232(4):685–690. doi:10.1002/jcp.25603
22. McCrorie P, Mistry J, Taresco V, et al. Etoposide and olaparib polymer-coated nanoparticles within a bioadhesive sprayable hydrogel for post-surgical localised delivery to brain tumours. Eur J Pharm Biopharm. 2020;157:108–120. doi:10.1016/j.ejpb.2020.10.005
23. Ngo MT, Harley BAC. Perivascular signals alter global gene expression profile of glioblastoma and response to temozolomide in a gelatin hydrogel. Biomaterials. 2019;198:122–134. doi:10.1016/j.biomaterials.2018.06.013
24. Puente P, Fettig N, Luderer MJ, et al. Injectable hydrogels for localized chemotherapy and radiotherapy in brain tumors. J Pharm Sci. 2018;107(3):922–933. doi:10.1016/j.xphs.2017.10.042
25. Dai X, Ma C, Lan Q, et al. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication. 2016;8(4):045005. doi:10.1088/1758-5090/8/4/045005
26. Zhao M, Danhier F, Bastiancich C, et al. Post-resection treatment of glioblastoma with an injectable nanomedicine-loaded photopolymerizable hydrogel induces long-term survival. Int J Pharm. 2018;548(1):522–529. doi:10.1016/j.ijpharm.2018.07.033
27. Fourniols T, Randolph LD, Staub A, et al. Temozolomide-loaded photopolymerizable PEG-DMA-based hydrogel for the treatment of glioblastoma. J Control Release. 2015;210:95–104. doi:10.1016/j.jconrel.2015.05.272
28. Wanjale MV, Sunil Jaikumar V, Sivakumar KC, et al. Supramolecular hydrogel based post-surgical implant system for hydrophobic drug delivery against glioma recurrence. Int J Nanomed. 2022;17:2203–2224. doi:10.2147/IJN.S348559
29. Hu X, Yang F, Liao Y, et al. Docetaxel-Loaded Cholesterol-PEG Co-Modified Poly (n-Butyl) cyanoacrylate nanoparticles for antitumor drug pulmonary delivery: preparation, characterization, and in vivo evaluation. Int J Nanomed. 2020;15:5361–5376. doi:10.2147/IJN.S249511
30. Garrett MC, O’Shea TM, Wollenberg AL, et al. Injectable diblock copolypeptide hydrogel provides platform to deliver effective concentrations of paclitaxel to an intracranial xenograft model of glioblastoma. PLoS One. 2020;15(7):e0219632. doi:10.1371/journal.pone.0219632
31. Adhikari B, Li J, Brandel MG, et al. The use of TMZ embedded hydrogels for the treatment of orthotopic human glioma xenografts. J Clin Neurosci. 2017;45:288–292. doi:10.1016/j.jocn.2017.07.027
32. Cieśluk M, Piktel E, Wnorowska U, et al. Substrate viscosity impairs temozolomide-mediated inhibition of glioblastoma cells’ growth. Biochim Biophys Acta Mol Basis Dis. 2022;1868(11):166513. doi:10.1016/j.bbadis.2022.166513
33. Yang J, Chen Z, Pan D, et al. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomed. 2020;15:5911–5926. doi:10.2147/IJN.S249129
34. Horák D, Švec F, Adamyan A, et al. Hydrogels in endovascular embolization. V. Antitumour agent methotrexate-containing p(HEMA). Biomaterials. 1992;13(6):361–366. doi:10.1016/0142-9612(92)90041-L
35. Sun J, Li Y, Wang X, et al. Entropy-driven quick loading of functional proteins in nanohydrogels for highly efficient tumor targeting therapy. ACS Appl Mater Interfaces. 2021;13(11):12888–12898. doi:10.1021/acsami.0c23124
36. Xing R, Li S, Zhang N, et al. Self-assembled injectable peptide hydrogels capable of triggering antitumor immune response. Biomacromolecules. 2017;18(11):3514–3523. doi:10.1021/acs.biomac.7b00787
37. Tarawneh O, Abu Mahfouz H, Hamadneh L, et al. Assessment of persistent antimicrobial and anti-biofilm activity of p-HEMA hydrogel loaded with rifampicin and cefixime. Sci Rep. 2022;12(1):3900. doi:10.1038/s41598-022-07953-3
38. McSheehy PMJ, Forster-Gross N, El Shemerly M, et al. The fibroblast growth factor receptor inhibitor, derazantinib, has strong efficacy in human gastric tumor models and synergizes with paclitaxel in vivo. Anticancer Drugs. 2023;34(4):532–543. doi:10.1097/CAD.0000000000001469
39. Hotchkiss KM, Sampson JH. Temozolomide treatment outcomes and immunotherapy efficacy in brain tumor. J Neurooncol. 2021;151(1):55–62. doi:10.1007/s11060-020-03598-2
40. Wang Z, Wang X, Yu H, et al. Glioma-targeted multifunctional nanoparticles to co-deliver camptothecin and curcumin for enhanced chemo-immunotherapy. Biomater Sci. 2022;10(5):1292–1303. doi:10.1039/D1BM01987B
41. Zou Y, Wang Y, Xu S, et al. Brain co-delivery of temozolomide and cisplatin for combinatorial glioblastoma chemotherapy. Adv Mater. 2022;34(33):e2203958. doi:10.1002/adma.202203958
42. Peter K, Kar SK, Gothalwal R, et al. Curcumin in combination with other adjunct therapies for brain tumor treatment: existing knowledge and blueprint for future research. Int J Mol Cell Med. 2021;10(3):163–181. doi:10.22088/IJMCM.BUMS.10.3.163
43. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
44. Sukumar UK, Bose RJC, Malhotra M, et al. Intranasal delivery of targeted polyfunctional gold-iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials. 2019;218:119342. doi:10.1016/j.biomaterials.2019.119342
45. Zhao J, Yao L, Nie S, et al. Low-viscosity sodium alginate combined with TiO(2) nanoparticles for improving neuroblastoma treatment. Int J Biol Macromol. 2021;167:921–933. doi:10.1016/j.ijbiomac.2020.11.048
46. Cao Y, Jin L, Zhang S, et al. Blood-brain barrier permeable and multi-stimuli responsive nanoplatform for orthotopic glioma inhibition by synergistic enhanced Chemo-/Chemodynamic/Photothermal/Starvation therapy. Eur J Pharm Sci. 2023;180:106319. doi:10.1016/j.ejps.2022.106319
47. Liang Q, Zhuo Y, Wu X, et al. Curcumin combining temozolomide formed localized nanogel for inhibition of postsurgical chemoresistant glioblastoma. Nanomedicine. 2023;18(12):907–921. doi:10.2217/nnm-2023-0058
48. Chen ZA, Wu C-H, Wu S-H, et al. Receptor ligand-free mesoporous silica nanoparticles: a streamlined strategy for targeted drug delivery across the blood-brain barrier. ACS Nano. 2024;18(20):12716–12736. doi:10.1021/acsnano.3c08993
49. Turabee MH, Jeong TH, Ramalingam P, et al. N,N,N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr Polym. 2019;203:302–309. doi:10.1016/j.carbpol.2018.09.065
50. Chen Y, Liu P, Sun P, et al. Oncogenic MSH6-CXCR4-TGFB1 feedback loop: a novel therapeutic target of photothermal therapy in glioblastoma multiforme. Theranostics. 2019;9(5):1453–1473. doi:10.7150/thno.29987
51. Cao X, Li S, Chen W, et al. Multifunctional hybrid hydrogel system enhanced the therapeutic efficacy of treatments for postoperative glioma. ACS Appl Mater Interfaces. 2022;14(24):27623–27633. doi:10.1021/acsami.2c05147
52. Li R, Lyu Y, Luo S, et al. Fabrication of a multi-level drug release platform with liposomes, chitooligosaccharides, phospholipids and injectable chitosan hydrogel to enhance anti-tumor effectiveness. Carbohydr Polym. 2021;269:118322. doi:10.1016/j.carbpol.2021.118322
53. Won JE, Wi TI, Lee CM, et al. NIR irradiation-controlled drug release utilizing injectable hydrogels containing gold-labeled liposomes for the treatment of melanoma cancer. Acta Biomater. 2021;136:508–518. doi:10.1016/j.actbio.2021.09.062
54. Chen Z, Liu F, Chen Y, et al. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv Funct Mater. 2017;27(46). doi:10.1002/adfm.201703036
55. Chen G, Ullah A, Xu G, et al. Topically applied liposome-in-hydrogels for systematically targeted tumor photothermal therapy. Drug Deliv. 2021;28(1):1923–1931. doi:10.1080/10717544.2021.1974607
56. Shi D, Mi G, Shen Y, et al. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an in vitro blood–brain barrier. Nanoscale. 2019;11(32):15057–15071. doi:10.1039/C9NR03931G
57. Heffernan JM, Overstreet DJ, Srinivasan S, et al. Temperature responsive hydrogels enable transient three-dimensional tumor cultures via rapid cell recovery. J Biomed Mater Res A. 2016;104(1):17–25. doi:10.1002/jbm.a.35534
58. Kong Y, Dai Y, Qi D, et al. Injectable and Thermosensitive Liposomal Hydrogels for NIR-II Light-Triggered Photothermal-Chemo Therapy of Pancreatic Cancer. ACS Appl Bio Mater. 2021;4(10):7595–7604. doi:10.1021/acsabm.1c00864
59. Liu B, Sun J, Zhu J, et al. Injectable and NIR-Responsive DNA-Inorganic hybrid hydrogels with outstanding photothermal therapy. Adv Mater. 2020;32(39):e2004460. doi:10.1002/adma.202004460
60. Fan Y, Nguyen DT, Akay Y, et al. Engineering a brain cancer chip for high-throughput drug screening. Sci Rep. 2016;6(1):25062. doi:10.1038/srep25062
61. Zhu Y, Jia J, Zhao G, et al. Multi-responsive nanofibers composite gel for local drug delivery to inhibit recurrence of glioma after operation. J Nanobiotechnology. 2021;19(1):198. doi:10.1186/s12951-021-00943-z
62. Tauro JR, Gemeinhart RA. Matrix metalloprotease triggered delivery of cancer chemotherapeutics from hydrogel matrixes. Bioconjug Chem. 2005;16(5):1133–1139. doi:10.1021/bc0501303
63. Pedron S, Becka E, Harley BA. Regulation of glioma cell phenotype in 3D matrices by hyaluronic acid. Biomaterials. 2013;34(30):7408–7417. doi:10.1016/j.biomaterials.2013.06.024
64. Niu B, Liao K, Zhou Y, et al. Application of glutathione depletion in cancer therapy: enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110. doi:10.1016/j.biomaterials.2021.121110
65. Zhuo W, Wang W, Zhou W, et al. A targeted and responsive nanoprodrug delivery system for synergistic glioma chemotherapy. Small. 2024:e2400630. doi:10.1002/smll.202400630
66. Kang T, Cha GD, Park OK, et al. Penetrative and sustained drug delivery using injectable hydrogel nanocomposites for postsurgical brain tumor treatment. ACS Nano. 2023;17(6):5435–5447. doi:10.1021/acsnano.2c10094
67. Zhu L, Wang X, Ding M, et al. Prodrug-loaded semiconducting polymer hydrogels for deep-tissue sono-immunotherapy of orthotopic glioblastoma. Biomater Sci. 2023;11(20):6823–6833. doi:10.1039/D3BM00585B
68. Chen S, Qiu Q, Wang D, et al. Dual-sensitive drug-loaded hydrogel system for local inhibition of post-surgical glioma recurrence. J Control Release. 2022;349:565–579. doi:10.1016/j.jconrel.2022.07.011
69. Wang S, Wu Q, Chen T, et al. Blocking CD47 promotes antitumour immunity through CD103(+) dendritic cell-NK cell axis in murine hepatocellular carcinoma model. J Hepatol. 2022;77(2):467–478. doi:10.1016/j.jhep.2022.03.011
70. Wang F, Huang Q, Su H, et al. Self-assembling paclitaxel-mediated stimulation of tumor-associated macrophages for postoperative treatment of glioblastoma. Proc Natl Acad Sci U S A. 2023;120(18):e2204621120. doi:10.1073/pnas.2204621120
71. Dong Y, Zhang J, Wang Y, et al. Intracavitary spraying of nanoregulator-encased hydrogel modulates cholesterol metabolism of glioma-supportive macrophage for postoperative glioblastoma immunotherapy. Adv Mater. 2023;36(13):e2311109.
72. Zhang R, Ye Y, Wu J, et al. Immunostimulant in situ fibrin gel for post-operative glioblastoma treatment by macrophage reprogramming and photo-chemo-immunotherapy. ACS Appl Mater Interfaces. 2023;15(14):17627–17640. doi:10.1021/acsami.3c00468
73. Song J, Zhang H, Wang D, et al. Hydrogel loading functionalized PAMAM/shRNA complex for postsurgical glioblastoma treatment. J Control Release. 2021;338:583–592. doi:10.1016/j.jconrel.2021.08.052
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

